Abstract
Acerophagus texanus (Howard) (Hymenoptera: Encyrtidae) is a primary endoparasitoid of Ferrisia virgata Cockerell (Hemiptera: Pseudococcidae). The parasitoid was described in 1898. It has been little studied and much of its biology remained unexplored. In the present investigation, several biological aspects of this species, such as the life cycle, fecundity, and longevity were studied under laboratory conditions. The parasitoids were collected from rambutan trees infested with F. virgata in Chiapas, Mexico, and transferred to the laboratory to establish a colony. Insects used in the experiments were obtained from a mass rearing of this colony. The results indicated that A. texanus is a koinobiont, gregarious endoparasitoid, with a pre-ovipositional period of 3 days. The life cycle from egg to adult, was 15 ± 0.4 days at 26.5 ºC. The mean longevity of females fed on bee honey was 9.5 ± 1.2 days, whereas unfed females survived for two days only. Hosts parasitized by A. texanus died four days after parasitization, just when entering the 2nd larval instar of the parasitoid. The average progeny produced by mated females was 12.6 individuals per host, and the sex-ratio was biased to females in a proportion of 5:1. Unmated females can reproduce parthenogenetically, producing only males as progeny.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rambutan (Nephelium lappaceum L.) is one of the most important cash crops in the South of Chiapas, Mexico (Castillo-Vera et al. 2017). A wide array of insect pests threatens rambutan production. Mealybugs (Hemiptera: Pseudococcidae) are among the most serious pests, as they feed on the sap and may transmit viruses, as the pineapple wilt–associated virus transmitted by two species of mealybugs (Sether et al. 1998). The invasive species, Ferrisia virgata Cockerell is widely distributed and can be found in high densities in rambutan orchards (Villatoro-Moreno et al. 2016). Mealybugs are difficult to control with conventional insecticides, since they have cryptic habits and are protected by waxy hydrophobic hairs covering their bodies (McCorquodale and Hodges 2017). Use of other control methods is needed, such as biological control, which represents an important alternative against mealybugs in rambutan orchards. In this sense, encyrtids are the most efficient parasitoids for mealybug management in the Neotropics (Colmenarez et al. 2018). The parasitic wasp Anagyrus kamali Moursi (Hymenoptera: Encyrtidae), for example, has been successfully used against the pink hibiscus mealybug, Maconellicoccus hirsutus Green (Kairo et al. 2000). Similarly, the papaya mealybug, Paracoccus marginatus Williams & Granara de Willink has been efficiently controlled by the hymenopteran parasitoid Acerophagus papayae Noyes & Schauff as biological control agent (Amarasekare et al. 2009).
Many parasitoids are recorded to parasitize F. virgata (Watson 2016). From them, Acerophagus texanus (Howard) (Hymenoptera: Encyrtidae) is the most promising natural enemy because it is easy to rear in the laboratory, reproduces quickly, and has presented the highest levels of parasitism in the field (Noyes 2010). In Mexico, relatively few studies focussed on mealybugs natural enemies, particularly on A. texanus parasitizing F. virgata (Debach and Warner 1969). Recently, this parasitoid was reported parasitizing F. virgata in Chiapas, Mexico in rambutan orchards as a member of a complex of several parasitoids (Castillo-Vera et al. 2021). The objective of the present study was to investigate the biological aspects, longevity, and fertility of A. texanus parasitizing F. virgata under laboratory conditions.
Material and methods
Biological material
Mealybug mummie’s were collected from a rambutan orchard in Frontera Hidalgo, Chiapas (14°45′7.47" N, 92°11′23.53" W; 52 m asl). Specimens were collected during several days and transferred to the laboratory to establish a laboratory colony. In the laboratory, pupae were placed into plastic containers (2 L) for adults´ emergence. Newly emerged parasitoids were fed with bee honey and subsequently transferred to rearing cages containing F. virgata. Nymph and adult individuals of this mealybug were obtained from a colony established on courgette (Cucurbita pepo L.). The taxonomic keys described by Rosen (1969) were used to identify A. texanus and voucher specimens were deposited in the Natural History Museum, London (UK). Both rearing and bioassays were performed at 26.5 ± 2 °C, 70 ± 10% relative humidity, and a 10 h light/14 h dark photoperiod.
Life cycle
In order to study the life cycle of A. texanus, 98 three-day-old female parasitoids were individually placed into 7.5 × 1.5 cm vials with a 3rd instar nymph of F. virgata for 6–8 h. The parasitoid development was monitored for 15 consecutive days by dissecting 7 parasitized hosts, which were considered replicates, each 24 h after parasitism. Dissected specimens were examined on excavated slides using entomological pins to separate the parasitoid from the host's tissues. The preparation was stained with Giemsa (1%, one drop) to facilitate the observation of the parasitoid’s immature stages. Larval development was recorded by capturing images with a digital camera (Canon EOS Rebel T6) mounted on a compound microscope (Olympus CX31). Larval growth was measured daily with a micrometre ruler. Images were processed with the ImageJ software program (version 1.52a) to determine the body size of the parasitoid (Rueden et al. 2017). Individuals were then treated with Karnovsky's fixative (3% glutaraldehyde, 2% formaldehyde in 0.1 M phosphate buffer, pH 7.4) and labelled for analysis by scanning electron microscopy (SEM).
Parasitoid specimens for SEM examination were prepared according to the following protocol: samples were washed by distilled water in an ultrasonic cleaning bath (5 times, 5 min. per wash). Next, the samples were dehydrated in increasing series of ethanol concentrations (30, 50, 70, 90, and 100%; for 30 min. at a time). The final concentration (100%) was repeated once. After passing the specimens for the critical point drying (Tousimis, Samdri-795), they were secured on aluminium mounting stubs with a double sided carbon tape. Next, the samples were coated with gold–palladium in a sputter coater (Denton Vacuum Desk II) and immediately examined under SEM (JEOL, NeoScope JCM-7000).
Adult survival
The survival of A. texanus adult females was studied under three different feeding regimes: (i) deprived of food, (ii) fed with ten µl of water, and (iii) fed with ten µl of bee honey, offered as drops inside the vial. Each treatment included 50 individuals, which were considered replicates. Each treatment was set up with newly emerged females that were individually placed into 7.5 × 1.5 cm vials. The number of living and dead individuals was recorded daily for each treatment and the experiment was completed until the death of the last individual.
Fecundity
The reproduction of A. texanus was studied in mated and virgin females. In the first case, females were kept with males for 72 h to favour mating, and in the second, females were kept alone, without any contact with males. Females of both groups were 3 days old and were fed with bee honey. F. virgata hosts were individually offered to each parasitoid female for six hours for oviposition, which was used once. Subsequently, the hosts were isolated for daily observation until the emergence of the parasitoid’s offspring. The clutch size, sex, and development time from parasitization to adult emergence were recorded.
Statistical analysis
Descriptive statistics were used to analyse the parasitoid size and developmental time. Principal component analysis was applied to determine the instars of the parasitoid. Kaplan–Meier survival analysis was performed for female parasitoids longevity. All analyses were conducted using the R project software package (version 3.6.2; R Core Team 2019).
Results
Life cycle
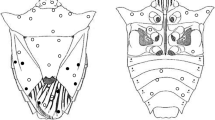
The life cycle from egg to adult of A. texanus averaged 15 (± 0.40) days (Fig. 1). The parasitoid's external morphology is presented in Fig. 2. Different developmental stages of the parasitoid are shown in Fig. 3 and described below.
Acerophagus texanus eggs are 1 mm (± 0.1) long, slightly oval, translucent, with a short pedicel. Because of the eggs' small size and translucent shell, observations were complicated. The mean incubation time was two days (n = 235). Egg colour changed to light orange 2 days after oviposition as approached the eclosion. The mature egg remained encyrtiform and exhibited a rounded pedicel with an intense orange colour (Fig. 3b). Various changes in the parasitized hosts were also observed during this 2-day period. On the second day after parasitization, a marked decrease in the host mobility was noted compared to the first day.
Total larval stage lasted 5 days on average. Principal component analysis indicated that larval size could be grouped in 2 instars. The first and second instars were 0.34 mm (± 0.14) and 1 mm (± 0.1) in length, respectively (Fig. 2). The first instar larva has a translucent, slightly elongated, and rounded body (different from the typical egg shape) with a yellow-orange interior. The second instar larva had well-differentiated larval segments (Fig. 2c) with yellow external borders that allowed to observe its brown-coloured interior. The first instar lasted 2 days, during which time, the larva remained attached to the filamentous tissue of its host. On the third day after parasitization, the host stopped moving, and on the fourth day -which coincided with the beginning of the second larval instar- the host died.
The pupa is white but changes to yellow as it develops. The eyes also displayed colour changes during pupal development: they were initially white, but subsequently became yellow-orange, and finally turned brown. By that time, the pupa’s appendices were clearly defined. The size of the larva was slightly reduced (10%) when transformed to pupa. The pupal stage lasted about 7 days.
Emergence of adult parasitoids occurred at 13–17 days post-oviposition. Adults of A. texanus exhibited an obvious sexual dimorphism. Females were larger than males: females measured 1.09 mm (± 0.1, n = 10), while males were 0.8 (± 0.1, n = 8). Moreover, the female body had a dark yellow appearance (Fig. 3g), whereas for the males were light-yellow (Fig. 3h). Females had a small typical encyrtid ovipositor that is retracted into the abdomen.
Adult survival
Type of food had a significant effect on the survival of A. texanus. Females fed on bee honey had a significantly longer survival than the other treatments (χ2 = 124; df = 2, p < 0.0001). The mean survival for bee honey-fed females was 9.5 ± 1.2 days, whereas individuals deprived of food and fed with only water, lived 2.0 ± 0.2 and 2.5 ± 0.2 days, respectively. The longest survival (18 days) was registered for females fed with bee honey.
Fecundity
Dissection of virgin females 3 days after the emergence as adults revealed the presence of a large number of eggs (about 40) in the ovaries. Mated females produced 12.6 ± 0.7 individuals per host (maximum recorded: 17 individuals per host). The progeny sex ratio was 5:1 (female: male). Unmated females produced only male progeny. Males emerge from the puparium a few seconds earlier than females.
Discussion
In this study, aspects of the life cycle of A. texanus that hitherto have not been reported we described. Our findings indicate that A. texanus is a koinobiont and gregarious endoparasitoid with a life cycle of about 15 days. Females require a diet of bee honey to live for a maximum of 18 days. A. texanus had a preoviposition period of 3 days and can reproduce by arrhenotokous parthenogenesis. This wasp has 2 larval instars and exhibited a marked sexual dimorphism.
The life cycle of A. texanus was similar to other encyrtids of the same genus, such as Acerophagus papayae, which had the same number of instars as A. texanus (Nisha and Kennedy 2017). The number of instars in encyrtids varies from two to five, although 3 are more commonly reported (Pandey and Johnson 2006). The life cycle of A. texanus was shorter than that of its host F. virgata, whose duration can last up to 64 days (Lapis 1970).
Longevity for adult females of A. texanus was similar to the reported value of 20 days for honey-fed Acerophagus nr. coccois (Beltrà et al. 2013). Female parasitoids, in general, require a sugar-rich diet to enhance their longevity (Villa et al. 2017). Another study indicated that females of Anagyrus nr. sinope attained the highest longevity when 50% of a bee honey solution was provided (Chong and Oetting 2006). In the case of Anagyrus ananatis Gahan, longevity increased up to 28 days when females were fed on a 50% bee honey solution (González-Hernández et al. 2005). In nature, it has been proved that parasitoids generally feed on honeydew, nectar, or sugar-rich substances (Wäckers et al. 2008). The increased longevity of A. texanus could therefore influence host-searching efficiency, which is an important attribute for natural enemies (Royer et al. 1999).
Newly emerged A. texanus females explored their host and even attempted to oviposit. These attempts, however, failed to produce progeny, since females emerged from the puparium without egg load. Our preliminary observations indicate that females are synovigenic and have a preoviposition period of several days.
The most common mode of asexual reproduction in parasitoids is arrhenotokous parthenogenesis, in which biparental females and uniparental males are produced through haplodiploidy (Heimpel and De Boer 2008). Arrhenotoky offers an evolutionary advantage by producing male offspring at times when females are rare, and thus overcomes the need to spend time and energy in searching for a mate (Lanteri et al. 2010). Genetic variability is lower in parthenogenetically produced males because the offspring derived from the same individual, which could lead to a reduced adaptability to environmental factors (Heimpel and De Boer 2008).
Several encyrtid species prefer to parasitize completely developed nymphs rather than adults (Kapranas and Tena 2015) because the reproductive activity of the latter limits their suitability as hosts (Cadée and van Alphen 1997). Females of A. texanus, however, can parasitize both, nymphs and adults of F. virgata. Therefore, its potential as a biological pest control agent is greater compared to parasitoids that restrict their host range to one stage only. Further research is necessary, however, to characterise and quantify the preference of A. texanus for each development stage of the host, especially considering that the larval development (greater longevity, reduced development time, smaller body) of several parasitoids is influenced by host stage (Badshah et al. 2016).
Effectiveness of a parasitoid as natural enemy derives from biological and ecological attributes such as host-searching efficiency, fertility, larval longevity, and sex ratio (Waage and Hassell 1982). The use of encyrtids as biological control agents of mealybugs has yielded some impressive results. For example, the control of cassava mealybug (Phenacoccus manihoti Matile-Ferrero) outbreaks with the parasitic wasp Anagyrus lopezi De Santis (Neuenschwander 2001). However, there is no information about the parasitism level of A. texanus to control mealybugs. For instance, the impact of releases of A. texanus to control F. virgata outbreaks in California was never evaluated (Bartlett 1978). In light of these results, A. texanus could be considered a promising candidate to control F. virgata in rambutan orchards of Chiapas, Mexico. Further field studies are still needed to confirm the efficacy of the parasitoid.
References
Amarasekare KG, Mannion CM, Epsky ND (2009) Efficiency and establishment of the three introduced parasitoids of the mealybug, Paracoccus marginatus (Hemiptera:pseudococcidae). Biol Control 51:91–95. https://doi.org/10.1016/j.biocontrol.2009.07.005
Badshah H, Ullah F, Calatayud PA, Crickmore N (2016) Host stage preference and parasitism behaviour of Aenasius bambawalei an encyrtid parasitoid of Phenacoccus solenopsis. Southwest Entomol 26:1605–1616. https://doi.org/10.1080/09583157.2016.1223836
Bartlett BR (1978) Pseudococcidae. In: Clausen CP (ed) Introduced Parasites and Predators of Arthropod Pests and Weeds: A World Review. Agriculture Handbook no. 480, USDA, Washington, 137–170
Beltrà A, Tena A, Soto A (2013) Reproductive strategies and food sources used by Acerophagus n. sp. near coccois, a new successful parasitoid of the invasive mealybug Phenacoccus peruvianus. J Pest Sci 86:253–259. https://doi.org/10.1007/s10340-012-0475-5
Cadée N, van Alphen JJ (1997) Host selection and sex allocation in Leptomastidea abnormis, a parasitoid of the citrus mealybug Planococcus citri. Entomol Exp Appl 83:277–284. https://doi.org/10.1046/j.1570-7458.1997.00182.x
Castillo-Vera A, López-Guillén G, Sandoval-Esquivez A (2017) La historia del cultivo de rambután (Nephelium lapacceum L.) en México. Agroproductividad 10:53–57. https://historia-cultivo-rambutan.pdf (https://fec-chiapas.com.mx). Acceseed May 2021
Castillo-Vera A, Villatoro-Moreno H, Cisneros J, Rodríguez-Vélez B (2021) Ferrisia virgata and Dysmicoccus brevipes as Hosts of Cirrhencyrtus spp. in Southern Chiapas, Mexico. Southwest Entomol 46:725–729
Chong JH, Oetting RD (2006) Influence of temperature, nourishment, and storage period on the longevity and fecundity of the mealybug parasitoid, Anagyrus sp. nov. nr. sinope Noyes and Menezes (Hymenoptera: Encyrtidae). Environ Entomol 35:1198–1207. https://doi.org/10.1093/ee/35.5.1198
Colmenarez Y, Corniani N, Mundstock S, Sampaio M, Vásquez C (2018) Use of parasitoids as a biocontrol agent in the neotropical region: Challenges and potential. In: Baymei HK, Hamamouch N, Kolombia WA (eds) Horticultural Crops. IntechOpen, London, pp 171–185. https://doi.org/10.5772/intechopen.80720
Debach P, Warner SC (1969) Importation and colonization of natural enemies of the striped mealybug, Ferrisia virgata, in California. Ann Entomol Soc Am 62:1117–1119. https://doi.org/10.1093/aesa/62.5.1117
González-Hernández H, Pandey RR, Johnson MW (2005) Biological characteristics of adult Anagyrus ananatis Gahan (Hymenoptera: Enncyrtidae), a parasitoid of Dysmicoccus brevipes (Cockerell) (Hemiptera: Pseudococcidae). Biol Control 35:93–103. https://doi.org/10.1016/j.biocontrol.2005.07.014
Heimpel GE, De Boer JG (2008) Sex determination in the Hymenoptera. Ann Rev of Entomol 53:209–230. https://doi.org/10.1146/annurev.ento.53.103106.093441
Kairo MTK, Pollard GV, Peterkin DD, Lopez VF (2000) Biological control of the hibiscus mealybug, Maconellicoccus hirsutus Green (Hemiptera: Pseudococcidae) in the Caribbean. Integr Pest Manag Rev 5:241–254. https://doi.org/10.1023/A:1012997619132
Kapranas A, Tena A (2015) Encyrtid parasitoids of soft scale insects: Biology, behavior, and their use in biological control. Ann Rev Entomol 60:195–211. https://doi.org/10.1146/annurev-ento-010814-021053
Lanteri AA, Rodriguero MS, Confalonieri VA (2010) La partenogenesis en el reino animal. Ciencia Hoy 20:19–28
Lapis EB (1970) The biology of the grey mealybug, Ferrisia virgata (Cockerell)(Pseudococcidae, Homoptera). Philipp Entomol 1:397–405
McCorquodale A, Hodges A (2017) Striped Mealybug Ferrisia virgata Cockerell (Insecta: Hemiptera: Pseudococcidae). EDIS 2017:4–4
Neuenschwander P (2001) Biological control of the cassava mealybug in Africa: a review. Biol Control 21:214–229. https://doi.org/10.1006/bcon.2001.0937
Nisha R, Kennedy JS (2017) Life cycle of the parasitoid Acerophagus papayae Noyes and Schauff on papaya mealybug Paracoccus marginatus Williams and Granara de Willink vis-a-vis local adaptation with coevolutionary “Arms Race.” J Entomol Zool Stud 5:1711–1719. https://doi.org/10.5958/0974-4576.2017.00025.1
Noyes JS (2010) Encyrtidae of Costa Rica (Hymenoptera: Chalcidoidea), 3. Subfamily Encyrtinae: Encyrtini, Echthroplexiellini, Discodini, Oobiini and Ixodiphagini, parasitoids associated with bugs (Hemiptera), insect eggs (Hemiptera, Lepidoptera, Coleoptera, Neuroptera) and ticks (Acari). Mem Amer Ent Inst 281–358
Pandey RR, Johnson MW (2006) Physiological and morphological development of Anagyrus ananatis at constant temperatures. Biocontrol 51:585–601. https://doi.org/10.1007/s10526-005-2156-2
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed Nov 2021
Rosen D (1969) A systematic study of the genus Acerophagus E. Smith with descriptions of new species (Hymenoptera: Encyrtidae). Hilgardia 40(2):41–72
Royer L, Fournet S, Brunel E, Boivin G (1999) Intra-and interspecific host discrimination by host-seeking larvae of coleopteran parasitoids. Oecologia 118(1):59–68. https://springerlink.bibliotecabuap.elogim.com/content/pdf/10.1007/s004420050703.pdf
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf 18:1–26. https://imagej.nih.gov/ij/download.html. Accessed Nov 2021
Sether DM, Ullman DE, Hu JS (1998) Transmission of pineapple mealybug wilt-associated virus by two species of mealybug (Dysmicoccus spp.). Phytopathology 88:1224–1230. https://doi.org/10.1094/PHYTO.1998.88.11.1224
Villa M, Santos SA, Mexia A, Bento A, Pereira JA (2017) Wild flower resources and insect honeydew are potential food items for Elasmus flabellatus. Agron Sustain Dev 37:15. https://doi.org/10.1007/s13593-017-0423-0
Villatoro-Moreno H, Cisneros J, Gómez J, Infante F, Castillo A (2016) Mealybugs (Hemiptera: Pseudococcidae) Associated with Rambutan (Nephelium lappaceum L.) in Chiapas, México. J Kans Entomol Soc 89:289–296. https://doi.org/10.2317/0022-8567-89.4.289
Waage JK, Hassell MP (1982) Parasitoids as biological control agents–a fundamental approach. Parasitology 84:241–268. https://doi.org/10.1017/S003118200005366X
Wäckers FL, van Rijn PCJ, Heimpel GE (2008) Honeydew as a food source for natural enemies: making the best of a bad meal?. Biol Control 45:176–184. https://doi.org/10.1016/j.biocontrol.2008.01.007
Watson G (2016) The CABI Invasive Species Compendium. Detailed coverage of invasive species threatening livelihoods and the environment worldwide. California Department of Food & Agriculture, Sacramento, California, USA. https://www.cabi.org/isc/datasheet/23981. Accessed Nov 2021
Acknowledgements
Sergio A. Mejía-Ortíz would like to acknowledge support from a master's scholarship awarded by Consejo Nacional de Ciencia y Tecnología (CONACyT). We are very grateful to Hernán Villatoro-Moreno for his dedicated effort to collect field samples and to Eduardo Chamé for his support taking photos.
Funding
Funding was provided by El Colegio de la Frontera Sur (ECOSUR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors have declared that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mejía-Ortíz, S.A., Noyes, J.S., Infante, F. et al. Biology of Acerophagus texanus (Howard, 1898) (Hymenoptera: Encyrtidae), a parasitoid of Ferrisia virgata Cockerell (Hemiptera: Pseudococcidae). Int J Trop Insect Sci 42, 2523–2529 (2022). https://doi.org/10.1007/s42690-022-00782-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-022-00782-x