Abstract
Malaria is a widespread vector-borne disease in the tropics and subtropics causing nearly half a million deaths every year. Malaria vector control intervention mainly rely on the control of adults using Indoor residual sprayings (IRS) and long lasting insecticidal nets (LLINs). The purpose of this study was to assess the species composition of Anopheles mosquitoes and determine the environmental and physicochemical parameters of their breeding habitats in Bambasi district, Benshangul Gumuz regional state, northwestern Ethiopia. Three major Anopheles breeding habitats were identified in three Kebeles namely; drainage ditch (Keshmando), swamp (Amba 46), and stagnant water (Amba 47). Anopheles mosquito larvae were sampled twice a month from September 2020 to November 2020. A total of 2185 Anopheles mosquito larvae were collected. Of those collected larvae three Anopheles species (Anopheles gambiae s.l. An. funestus and An. coustani complex) were identified. Anopheles gambiae s.l was the most abundant whereas An. funestus and An. coustani were the least in all the study kebeles. Of the three kebeles, Amba 47 was found the most productive for Anopheles followed by Amba 46 and Keshmando. The highest mean density of larvae per dip was sampled in September in all the study sites. The three sampling sites varied in physicochemical characteristics. The findings of this study showed that dissolved oxygen (DO) was highest (7.07 \(\pm\) 0.55 mg/L) in the swamps and lowest (0.32 \(\pm\) 0.04 mg/L) in the drainage ditches. Conductivity across different habitats showed wide variations. There were slight variations in temperature between different habitats. Higher total dissolved solids (TDS) 12.19 \(\pm\) 0.26 mg/L was recorded from the drainage ditches; whereas TDS 9.49 \(\pm\) 1.62 mg/L was recorded from the swamp. Salinity in the drainage ditches and stagnant water was 5.54 \(\pm\) 1.00PSU and 3.30 \(\pm\) 0.97 PSU respectively. There were negative strong correlation between the larval density with temperature and EC but positive correlation between larval density with salinity. However, there was no significant correlation between Anopheles larval density with TDS and DO. In conclusion this study suggested that environmental and physicochemical factors could play an important role in the development of mosquito larvae. Therefore, characterizing mosquito larval habitats is important for targeted control of malaria vectors in Ethiopia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Background of the study
Malaria, is transmitted to humans by the bite of adult female Anopheles mosquitoes (Cox 2010). In 2019, worldwide there were an estimated 229 million cases of malaria and 409 000 deaths of which 94% of the cases were reported from Africa (WHO 2020). In Ethiopia, even though there has been steady progress in the reduction of malaria cases (Deribew et al. 2017; Taffese et al. 2018; WHO 2020) over one million cases of malaria has been reported in 2019 (WHO 2020).
The genus Anopheles mosquito is the most studied genera among medically important insects. Of the total 465 Anopheles species globally described, about 70 species are known to transmit human malaria (Sinka et al. 2012). Forty one Anopheles mosquito species are known to transmit human Plasmodium parasites, of which 20 species are the dominant malaria vectors in Africa (Sinka et al. 2012). The major malaria vectors in Africa included An. gambiae s.l., An. funestus, An. nili, An. pharoensis and An. moucheti (Sinka et al. 2012, 2011).
In Ethiopia, 47 Anopheles mosquito species are documented (Gaffigan et al. 2018) of which An. arabiensis, An. funestus, An. pharoensis and An. nili are recognized malaria vectors. Anopheles arabiensis is the primary malaria vector in Ethiopia (Abose et al. 1998; Lulu et al. 1999) while, An. funestus, An. pharoensis and An. nili are secondary vectors occurring with varying densities, limited distribution and vector competence (White 1982; FMoH 2014). Moreover, very recently a new invasive Anopheles species, An. stephensi, has been documented in Ethiopia (Carter et al. 2018) which might complicate the malaria elimination effort of the country.
Anopheles mosquitoes undergo egg, larval, pupal and adult stages in their life cycle. The egg, larval and pupal stages are aquatic and have very small spatial dispersion. Mosquito breeding habitats are significantly higher in the rainy season than the dry season (Mahgoub et al. 2017). In the dry season, the number and size of larval habitats are generally limited and contribute to a low population of adult Anopheles mosquitoes transmitting malaria (Himeidan et al. 2009). Even though, Anopheles mosquito species exploit diverse breeding habitats that considerably vary in size, altitude, vegetation cover and topography (Minakawa et al. 2012), the majority of malaria vectors may emerge from prolific habitats, which could account only for a small proportion of the habitats. Small size breeding habitats have many advantages over larger permanent ones which increase the developmental rate or survivorship of the aquatic stage (Gu et al. 2008). Therefore, mosquito larval habitat ecology is important in determining larval densities and designing mosquito control programs (Overgaard et al. 2002; Simisek 2004).
Indoor residual spraying (IRS) and long-lasting insecticidal nets (LLINs) are the main pillars of the malaria vector control strategy in Ethiopia. In the last decade, both IRS and LLINs significantly reduced malaria incidence and prevalence in the country (Girum et al. 2019; Taffese et al. 2018; WHO 2020). However, the wide spread of insecticide resistance in the major malaria vector, An. arabiensis (Alemayehu et al. 2017; Messenger et al. 2017; Yewhalaw et al. 2010) could compromise the effort of malaria vector control and elimination efforts. Moreover, the plasticity in feeding, biting and resting behavior of An. arabiensis contributed to the existing malaria transmission in the country despite intensified malaria intervention, the scaled up of LLINs, high coverage of IRS, and improved malaria diagnosis and treatment (Degefa et al. 2015; Kenea et al. 2016; Yohannes and Boelee 2012). Therefore, besides targeting adult Anopheles populations it is equally important to control the immature stages of Anopheles mosquitoes with appropriate larval control tools. Different interventions targeting larvae of Anopheles mosquitoes successfully suppressed malaria vector population density and the risk of malaria infection in some African countries including Ethiopia (Castro et al. 2009; Kibret et al. 2018; Utzinger et al. 2001; Wamae et al. 2010; Mekonnen Yohannes et al. 2005).
In Ethiopia, few studies assessed the habitat of mosquito larvae and some environmental factors that affect mosquito abundance (Dejenie et al. 2011; Getachew et al. 2020; Hawaria et al. 2020; Mereta et al. 2013). However, to our knowledge, there is no study reported from Bambasi district (woreda), one of the malaria sentinel sites in Ethiopia, to characterize and assess Anopheles mosquito larval breeding habitats. Hence, this study aimed at characterizing Anopheles mosquito larval breeding habitats and determining Anopheles mosquito fauna in Bambasi district, Ethiopia.
Materials and methods
Description of the study area
Bambasi district is located in Assosa Zone, Benishangul-Gumuz Regional state Northwestern Ethiopia. Assosa town is the capital of the Assosa district and Benishangul-Gumuz Regional state, which is located about 600 km West of Addis Ababa. Bambasi town is 45 km East of Assosa town. It is part of the Assosa Zone, bordered by the Mao-Komo special district on the Southwest, Assosa in the Northwest, Oda Buldigilu in the Northeast and by the Oromia Region in the Southeast. Bambasi has a longitude and latitude of 9°45′N 34°44′E with an elevation of 1668 m above sea level and is known malarious area. The district experiences mean annual rainfall and temperature values of 1381.42 mm and 28.37 °C, respectively. It is characterized by unimodal rainfall distribution with the rainy season extending from March to November while reaching a peak during the months of June to September (Mossisa et al. 2019). Therefore, the area falls within the lowland (moist Kolla) agro-ecology (Hurni et al. 2016).
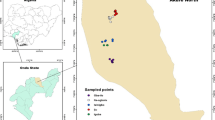
The study was conducted in three Kebeles namely Keshmando, Amba 46 and Amba 47 (Fig. 1). Amba 46 and Amba 47 are characterized by having agricultural fields whereas, Keshmando is mainly a human settlement. Keshmando is located 9°36.257 ‘N and 34°41.2008 ‘E 1399 meters above sea level (masl). Amba 46 is located 9°50.88 ‘N and 34°41.316 ‘E 1446 masl. Amba 47 is located 9°52.087 ‘N and 34°41.145 ‘E 1423 masl.
Mosquito larval sampling
Mosquito larvae were sampled twice a month from September 2020 to November 2020 after the long rainy season. Different breeding habitat types such as drainage ditch, swamp and stagnant water were surveyed and searched for the presence of Anopheles larvae and their productivity (Fig. 2). During each survey, a habitat was first inspected for the presence of mosquito larvae and then larvae were collected using a standard dipper (350 ml). From each larval habitat, about 20 dips were taken at intervals along the edge of each larval habitat. Sampling was done by the same individual in the morning (10:00–12:00 h) or afternoon (2:00–4:00 h) for about 30 minutes. The larvae were then picked using pipette and transferred to collecting containers. From each habitat, larvae were always transferred into containers with water from the site of collections. Containers were labeled with relevant information such as date, site and number of larvae collected, and were kept in a room of the field insectary and allowed to rear in to adults. Larvae were fed with brewery yeast once per day, and exposed to sunlight twice a day for maximum 30 minutes. Pupae were collected and transferred into a beaker, placed in a cage for adult emergence.
Identification of adult Anopheles mosquitoes
Adult Anopheles mosquito samples were morphologically identified to the species level under a light microscope using standard taxonomic keys (Coetzee 2020; Gillies and Coetzee 1987) in Biology laboratory, Assosa University, Assosa. Afterwards, the samples were kept individually in Eppendorf tubes over silica gel and transported to Jimma University for further identification.
Larval habitat characterization
Environmental and physicochemical characteristics of each larval habitat were observed, measured and recorded during the larval collection. The environmental variables included intensity of light, being natural or artificial, water current, vegetation covering, the presence of algae, substrate type, turbidity and permanence of the habitat were recorded during the study. The physicochemical parameters such as salinity, dissolved oxygen (DO), electrical conductivity (EC), total dissolved solid (TDS), and water temperature were measured on site. The measurements were done two times following the larval collection period using a multi-probe meter (Palintest micro 800; Tyne and Wear, UK, NE11 0NS). The multi- probe meter was calibrated for each parameter following the standard procedure of user manual for the Palintest micro 800.
Each habitat was visually inspected for the presence or absence of algae. The proportion of vegetation cover estimated visually and expressed as the presence or absence of vegetation. Turbidity was measured by putting water sample in a clear glass container and placing it against a white background and recorded as either slightly turbid or turbid (Minakawa et al. 1999; Shililu et al. 2003). The substrate type was categorized as mud, stone if the pool was lined with stones that were large in size (rocks generally larger than 10 cm in diameter) and gravel when the stones were small in size but larger than sand. The proportion of the water surface exposed to sunlight was estimated visually by assessing the proportion of the water surface shadowed at midday (Sattler et al. 2005). Light intensity was visually categorized as sunlit (the habitat that received full sunlight throughout the day) or shaded. Water current was categorized as still or flow. Habitats were categorized as artificial or natural.
Data analysis
Anopheles mosquito species composition, spatial distribution and mean larval density data were analyzed using SPSS version 20. Percentile scores were used to compare abundance of Anopheles larvae among habitat types and distribution of their species within the habitats. Variations in larval mean density of the collected larvae among habitat types and environmental factors (parameters) of the larval habitats were analyzed using ANOVA. Mean larval density in all breeding habitats and study kebeles was calculated as the number of Anopheles mosquito larvae (early or late) per dip divided by the number of dips taken from each larval habitat (Sattler et al. 2005). Correlation analysis was employed to analyze the association between temperature, dissolved oxygen, total dissolved solids and conductivity to the larval density of Anopheles and P values < 0.05 considered significant during the analysis.
Results
Species composition of Anopheles mosquitoes
A total of 2185 Anopheles mosquitoes larvae were collected from three aquatic habitats during the study period. Of the total, 595, 765 and 823 larvae were collected from Keshmando, Amba 46 and Amba 47 sites respectively. The total number of mosquito larvae collected from Amba 47 (Fig. 3) was higher than Anopheles mosquito collected from the other two sites (P < 0.05).
A total of 1786 adults emerged from larval collections and morphologically identified as Anopheles gambiae s.l., An. funestus and An. coustani. An. gambiae s.l. was greater in number than the other two species (Table 1). Anopheles gambiae s.l. was more abundant in all study sites and breeding habitats. The highest density of An. gambiae s.l was collected throughout the study period at Amba 47 as compared to Keshmando and Amba 46. Overall, the number of adult An. funestus and An. coustani that emerged from the larval collections were low compared to An. gambiae s.l. In Keshmando, the abundance of An. gambiae s.l. was found to be 99.80% whereas An. coustani was 0.2%. At Amba 46 and Amba 47 the density of An. gambiae s.l. was 99.5% and 98.85% while An. funestus was 0.5% and 1.15% respectively (Table 1).
Anopheles larval productivity
Three habitat types (drainage ditch, swamp and stagnant water) were identified as breeding sites in the study area. Anopheles mosquito larvae were collected and identified from different breeding sites as shown in Fig. 4. There was variation in larval productivity across breeding habitats. The most productive habitat for Anopheles mosquito was a stagnant water from Amba 47 with 21.24 larvae per dip followed by a swamp from Amba 46 and drainage ditch from Keshmando with a mean larval density of 19.75 and 14.83 larvae per dip, respectively (Table 2).
The highest number of Anopheles mosquito larvae was recorded in September whereas the lowest was recorded in November in all the study sites (Table 3).
Abundance and distribution of Anopheles mosquitoes in breeding sites
Table 2 depicts the abundance and spatial distribution of Anopheles species in different breeding habitats during the study period. An. gambiae s.l. adult reared from larvae were obtained most abundantly from stagnant water (690) and swamps (592). Higher number of the total An. funestus adults reared were obtained from stagnant water. Anopheles coustani was the only species sampled from the drainage ditch and generally absent from the other two habitat types. The abundance of larvae per number of dips of sampling from the different habitat types revealed that An. gambiae s.l. were the most abundant species in stagnant water (p < 0.05).
Distribution of Anopheles larvae density during the study period
During the study period, September was the most productive month for Anopheles mosquito in all breeding sites. In September, the highest number of larvae per dip was sampled from Amba 46 followed by Amba 47. In October and November the trend was changed and Amba 47 was found to be more productive followed by Amba 46. The lowest larval density was sampled in November in all the study sites (Table 3).
Habitat characteristics and distribution of Anopheles larvae
Habitat characteristics and abundance of Anopheles species in each breeding site are depicted in Table 4. Anopheles gambiae s.l. was greater in larval density in turbid and natural habitats than that of slightly turbid and artificial habitats. Similarly, its density was higher in sunlit habitats and mud than in shaded and mudy habitats with little gravel. This species preferred temporary, still and turbid water with full sunlight and mud substrates. Anopheles funestus were greater in sunlit turbid water. Similarly, An. funestus obtained from temporary and natural habitats with mudy and with emergent vegetation and algae. Anopheles coustani were recorded from temporary habitats with turbid, water, shaded, mudy with little gravel and in artificial habitats. Anopheles coustani was absent from habitats with muddy substrate types.
The drainage ditches located close to the houses were turbid. They contain a lot of debris from vegetation and plants. They were formed by road construction authority. They were temporary because dried up during the dry season. Some of the habitats were with partial sunlight as there were large plants at the edge of habitats whereas some of the habitats were completely exposed to sunlight. Others were muddy with little gravel, still water and man-made habitats. The swamp and stagnant water were located in farmlands (natural and still habitats). The swamp was slightly turbid. The stagnant water was turbid because it contains mud and other organic debris. There was a lot of vegetation and algal bloom. Breeding sites were temporary because they dried during the dry season. Temporary and natural habitats with sunlit, still and turbid water were the most productive breeding sites for An. gambiae s.l. (Table 4).
Physicochemical characteristics of the habitats
The three sampling sites varied in physicochemical characteristics. The findings showed that dissolved oxygen (DO) was highest (7.07 \(\pm\) 0.55 mg/L) in swamp and lowest (0.32 \(\pm\) 0.04 mg/L) in drainage ditch. Conductivity across different habitats showed wide variations, 18.46 \(\pm\) 0.05μS/cm for drainage ditch and (14.13 \(\pm\) 1.98μS/cm) for swamp. There were slight variations in temperature between different habitats. TDS in drainage ditch was higher (12.19 \(\pm\) 0.26 mg/L); whereas it was lowest (9.49 \(\pm\) 1.62 mg/L) in swamp. Salinity in drainage ditches and stagnant water was 5.54 \(\pm\) 1.00PSU and 3.30 \(\pm\) 0.97 PSU respectively (Table 5).
A negative strong correlation exists between the larval densities with temperature and EC while moderate correlation with Salinity. However, there was no significant correlation for Anopheles larval density with TDS and DO (Table 6).
Discussion
Of the forty seven species and subspecies of Anopheles mosquitoes in Ethiopia (Gaffigan et al. 2018), three of them (An. gambiae s.l.., An. funestus and An. coustani) were documented in the present study. The most abundant species was An. gambiae s.l. while, very few An. funestus and An. coustani were recorded. Unlike our findings large numbers of An. coustani was observed from adult collection in Bambasi (PMI 2020). The variation might be due to differences in collection, sampling period and also these species rarely feature in larval surveys of Anopheles species. The current study revealed differences in the abundance and distribution of Anopheles breeding habitats in the different sampling sites.
Anopheles larvae breed in various types of habitats, varying from large permanent to small temporary water bodies (Service 2008). Three larval habitat types were identified as breeding sites in this study, namely swamp, drainage ditch and stagnant water. All habitats were the most common breeding sites in the area. Hawaria and his co-workers also reported similar habitat types from Arjo-Didessa, Ethiopia (Hawaria et al. 2020). In this study, different Anopheles species were collected from various habitat types but, the most abundant species was An. gambiae s.l. Stagnant water was the most prolific habitat for Anopheles larvae.
This study revealed that Anopheles gambiae s.l. was the most predominant in stagnant water and least abundant in the drainage ditches. Anopheles coustani were collected only from the drainage ditch. On the other hand An. funestus had its highest number in stagnant water and the lowest were collected from the swamp. In contrast An. funestus group mainly prefers swamps (Dida et al. 2018). Habitat type also influences the abundance of An. funestus. Anopheles funestus mainly prefer to breed in permanent water bodies; this could be the reason for very rare occurence in swamps, drainage ditch and stagnant water. The major reason for this was, An. funestus larvae are associated with larger, semi-permanent water bodies with emergent vegetation and algal bloom (Gimmg et al. 2001). This study was conducted following the long rainy season but, for An. funestus dry season was favorable than the wet season. Thus, the findings of this study agrees with (Umar 2014), which reported that An. gambiae s.l. was responsible for malaria transmission during the wet season while An. funestus has been confirmed to be responsible for the transmission of malaria during the dry season.
The physical characteristics of the breeding sites documented during the study were; turbidity, presence and absence of vegetation and algae, habitat permanence, water condition, the origin of habitat and exposure to sunlight. The larvae occurred in a wide range of habitats, but most species prefer turbid water. Anopheles gambiae s.l. abundance was associated with turbid water. Similarly, the previous findings reported by Munga et al., 2005 suggested that An. gambiae s.l. exploit turbid water for oviposition. This indicates that during the rainy season, An. gambiae s.l. prefers turbid water. The other observation contradicts the present findings by Shililu et al. (2003) who recorded higher anopheline larval densities from clear aquatic habitats. Also, An. gambiae s.l. was documented from a slightly turbid habitat (Teklu et al. 2010). Anopheles gambiae s.l. was collected from a slightly turbid habitats with emergent vegetation and open sunlit conditions. Few An. funestus was collected, where the habitats were turbid. This indicated that An. funestus prefers clear water to turbid water. A similar finding was reported from Tanzania that An. funestus prefer clear water (Nambunga et al. 2020).
Highest An. gambiae s.l. was collected in the study area. The three habitats identified in this study were temporary and they dried at the end of the study period. This finding agrees with the previous report that An. gambiae s.l. prefers temporary breeding sites (Kenea et al. 2011).
Moreover, it was observed that the characteristic substrate type for anopheline larva habitat in the study area was mud as high anopheline larvae were observed to occur in larval habitats than other substrate types of mud with little gravel. Soil provides nutrients for the enrichment of bacteria that serve as food sources for larvae and possibly oviposition attractants. This observation is in agreement with previous reports by Minakawa et al., 1999 who described that anopheline larvae generally do not prefer habitats such as water tanks without soil substrates.
Vegetation was also an important predictor for Anopheles larvae presence and abundance. A similar finding was reported for An. gambiae s.l. larvae by Mwangangi et al., 2007. The presence of vegetation could help the larvae to hide from their predators. Algae was an important factor in the abundance of An. gambiae s.l. larvae in swamp, drainage ditch and stagnant water body; that algae favored the abundance of An. gambiae s.l. as it was the main source of its food. The presence of algae with anopheline larval occurrence or abundance is also in agreement with the finding of Gimnig and his coworkers who reported that algal food is a key for larval abundance (Gimnig et al. 2002).
The present study showed that anopheline larvae were collected from still waters. Similar findings were reported from Eritrea, which showed that still water is the main larval habitats for anopheline mosquitoes (Shililu et al. 2003). The main reason for the high abundance of anopheline larvae in still water could be the favorable situation in which larvae can stay close to the surface with their spiracle open to the air for breathing. Moreover, high water current and flooding are detrimental to Anopheles larval survival as a result of the physical harm to the larvae and reduction in their oxygen tension (Okogun 2005).
Anopheline larval abundance was highly associated with natural habitats compared to artificial habitats. This indicates that environmental variables of larval breeding habitats regulate the abundance of mosquitoes (Kenawy et al. 2013; Godwin R.A. Okogun et al. 2003; Paaijmans et al. 2008).
The findings of the present study indicated that various physicochemical parameters in mosquito breeding sites at various levels had some influence on mosquito vector oviposition, survival and spatial distribution. High salinity was were recorded in drainage ditch, due to higher turbid breeding sites. Large number of An. gambiae s.l. were collected in stagnant water which had low salinity compared to drainage ditch habitats. This indicates that high salinity influences the oviposition of mosquito larvae. Low salinity was observed in swamps, which An. gambiae s.l. and An. funestus larvae can survive, this suggests that An. gambiae s.l. larvae can survive in water with either high or and low salinity.
High EC and TDS were recorded in the drainage ditch that might be due to high turbidity and high salinity in the area. Anopheles larvae abundance was lower in water with high levels of salinity and conductivity. A similar study reported that high water conductivity due to high salinity and other dissolved ions have a negative impact on the primary production of mosquito larvae (Closs et al. 2003). This findings are inconsistent with a study conducted from Nigeria that conductivity and TDS appeared to have no influence on Anopheles larval density (Imam and Deeni 2015). Also, this finding disagrees with Anopheles larvae abundance in water with high salinity and conductivity (Emidi et al. 2017).
Temperature is one of the most important water quality parameters. It affects water chemistry and the functions of aquatic organisms. It influences the amount of oxygen that can be dissolved in water, the rate of photosynthesis by algae and other aquatic plants and the metabolic rates of organisms. In this study slightly varied temperatures were recorded ranging from 29.08 ºC – 29.45 ºC. It was reported from a study conducted in Ghana that a temperature range between 30 ºC – 36.2 ºC found the most suitable conditions for the development of the Anopheles larvae (Opoku and Ansa-Asare 2009). Previous studies reported that moderate temperatures were necessary for the optimum growth of Anopheles larvae; they further described that high temperatures usually accelerated their growth (Minakawa et al. 1999). Additionally, other studies observed that warm water also allowed more microorganisms to grow, which is food sources for mosquito larvae (Sunahara et al. 2002).
Anopheles mosquito larvae were abundantly collected from stagnant water which has a tolerable DO (4 mg/L or less) required by most mosquito species. This finding is in agreement with the study reported by Mereta et al. 2013. High DO record in swamp site might be due to the abundance of vegetation which increased photosynthetic activity. Low dissolved oxygen, in drainage ditch habitats, might have been caused by organic pollution resulting from high input of solid and liquid waste from households as the site is in close proximity to human habitation.
Conclusion and recommendation
Among the three Anopheles species, Anopheles gambiae s.l. was the most abundant species in three of the larval habitat sites (drainage ditch, swamp and stagnant water) identified in the study area. The density of Anopheles gambiae s.l. was high in temporary, turbid, muddy, still water, with sunlight and some vegetations and algal bloom. Each habitat in Bambasi had different physicochemical characteristics that were key determinants of the presence of Anopheles larvae. The abundance of the three identified mosquito species larvae was found to vary according to different environmental and physicochemical factors which determined the quality of water of the breeding habitats. Thus, combinations of these factors contributed to the differential abundance of the larval mosquitoes observed at the sampling sites. Therefore, it is highly important to clearly identify the breeding sites of Anopheles mosquito and the contributing factors for their productivity so as to implement larval control strategies together with the current IRS and LLINs malaria vector control interventions. Future work should focus on longitudinal sampling including other breeding sites such as hoof cattle prints, artificial containers and ponds to properly identify productive mosquito larval habitats and implement integrated malaria vector management.
Availability of data and material
The datasets supporting the conclusions of this article are included within the article.
Code availability
Not applicable.
Abbreviations
- DO:
-
Dissolved Oxygen
- EC:
-
Electrical Conductivity
- FMoH:
-
Federal Ministry of Health
- IRS:
-
Indoor Residual Spraying
- LLIN:
-
Long-lasting Insecticidal Net
- pH:
-
Hydrogen ion concentration
- PMI:
-
President’s Malaria Initiative
- SPSS:
-
Statistical package for social sciences
- TDS:
-
Total dissolved solids
- WHO:
-
World Health Organization
References
Abose T, Ye-ebiyo Y, Olana D, Alamirew D, Beyene Y, Regassa L (1998) Re-orientation and definition of the role of malaria vector control in Ethiopia. WHO/MAL/98.1085 World Health Organization
Alemayehu E, Asale A, Eba K, Getahun K, Tushune K, Bryon A, Morou E, Vontas J, Van Leeuwen T, Duchateau L, Yewhalaw D (2017) Mapping insecticide resistance and characterization of resistance mechanisms in Anopheles arabiensis (Diptera: Culicidae) in Ethiopia. Parasit Vectors 10(1):1–11. https://doi.org/10.1186/s13071-017-2342-y
Carter TE, Yared S, Gebresilassie A, Bonnell V, Damodaran L, Lopez K, Ibrahim M, Mohammed S, Janies D (2018) First detection of Anopheles stephensi Liston, 1901 (Diptera: culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop 188(September):180–186. https://doi.org/10.1016/j.actatropica.2018.09.001
Castro MC, Tsuruta A, Kanamori S, Kannady K, Mkude S (2009) Community-Based Environmental Management for Malaria Control : Evidence from a Small-Scale Intervention in Dar Es Salaam 11:1–11. https://doi.org/10.1186/1475-2875-8-57
Closs G, Downes B, Boulton A (2003) Freshwater Ecology, Blackwell Publishing
Coetzee M (2020) Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J 19(1):1–20. https://doi.org/10.1186/s12936-020-3144-9
Cox FE (2010) History of the discovery of the malaria. Parasit Vectors 3(5):1–9. https://doi.org/10.4018/jec.2013010101
Degefa T, Zeynudin A, Godesso A, Michael YH, Eba K, Zemene E, Emana D, Birlie B, Tushune K, Yewhalaw D (2015) Malaria incidence and assessment of entomological indices among resettled communities in Ethiopia: A longitudinal study. Malar J 14(1):1–11. https://doi.org/10.1186/s12936-014-0532-z
Dejenie T, Yohannes M, Assmelash T (2011) Characterization of Mosquito Breeding Sites in and in the Vicinity of Tigray Microdams. Ethiop J Health Sci 21(1):57–66. https://doi.org/10.4314/ejhs.v21i1.69045
Deribew A, Dejene T, Kebede B, Tessema GA, Melaku YA, Misganaw A, Gebre T, Hailu A, Biadgilign S, Amberbir A, Yirsaw BD, Abajobir AA, Shafi O, Abera SF, Negussu N, Mengistu B, Amare AT, Mulugeta A, Mengistu B, Stanaway JD (2017) Incidence, prevalence and mortality rates of malaria in Ethiopia from 1990 to 2015: Analysis of the global burden of diseases 2015. Malar J 16(1):1–7. https://doi.org/10.1186/s12936-017-1919-4
Dida GO, Anyona DN, Abuom PO, Akoko D, Adoka SO, Matano AS, Owuor PO, Ouma C (2018) Spatial distribution and habitat characterization of mosquito species during the dry season along the Mara River and its tributaries, in Kenya and Tanzania. Infect Dis Poverty 7(1):1–16. https://doi.org/10.1186/s40249-017-0385-0
Emidi B, Kisinza WN, Mmbando BP, Malima R, Mosha FW (2017) Effect of physicochemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza Tanzania. Parasit Vectors 10(1):1–12. https://doi.org/10.1186/s13071-017-2238-x
FMoH (2014) An Epidemiological Profile of Malaria in Mali. Programme National de Lutte Contre Le Paludisme (PNLP), February, 1–88. http://www.inform-malaria.org/wp-content/uploads/2015/03/Mali-Malaria-Epi-Profile-Report_030315.pdf
Gaffigan TV, Wilkerson RC, Pecor JE, Stoffer JA, Anderson T (2018) Systematic Catalog of Culicidae. Walter Reed Biosystematics Unit, Division of Ecology, Walter Reed Army institute of Research, Silver Spring. Accessed on: 3/22/2021. From https://www.mosquitocatalog.org
Getachew D, Balkew M, Tekie H (2020) Anopheles larval species composition and characterization of breeding habitats in two localities in the Ghibe River Basin, southwestern Ethiopia. Malar J 19(1):1–13. https://doi.org/10.1186/s12936-020-3145-8
Gimmg JE, Ombok M, Kamau L, Hawley WA (2001) Characteristics of larval anopheline (Diptera: Culicidae) habitats in western Kenya. J Med Entomol 38(2):282–288. https://doi.org/10.1603/0022-2585-38.2.282
Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED (2002) Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. J Med Entomol 39(1):162–172. https://doi.org/10.1603/0022-2585-39.1.162
Gillies MT, Coetzee M (1987) A supplement to the Anophelinae of Africa South of the Sahara (Afrotropical Region). Johannesburg publications of the South African Institute for Medical Research, Johannesburg
Girum T, Shumbej T, Shewangizaw M (2019) Burden of malaria in Ethiopia, 2000–2016: Findings from the Global Health Estimates 2016. Tropical Diseases, Travel Medicine and Vaccines 5(1):5–11. https://doi.org/10.1186/s40794-019-0090-z
Gu W, Utzinger J, Novak RJ (2008) Habitat-based larval interventions: A new perspective for malaria control. Am J Trop Med Hyg 78(1):2–6. https://doi.org/10.4269/ajtmh.2008.78.2
Hawaria D, Demissew A, Kibret S, Lee MC, Yewhalaw D, Yan G (2020) Effects of environmental modification on the diversity and positivity of anopheline mosquito aquatic habitats at Arjo-Dedessa irrigation development site Southwest Ethiopia. Infect Dis Poverty 9(1):1–11. https://doi.org/10.1186/s40249-019-0620-y
Himeidan YE, Zhou G, Yakob L, Afrane Y, Munga S, Atieli H, El-Rayah EA, Githeko AK, Yan G (2009) Habitat stability and occurrences of malaria vector larvae in western Kenya highlands. Malar J 8(1):1–6. https://doi.org/10.1186/1475-2875-8-234
Hurni H, Berhe WA, Chadhokar P, Daniel D, Gete Z, Grunder M, Kassaye G (2016) Soil and Water Conservation in Ethiopia: Guidelines for Development Agents. Second revised edition. Bern, Switzerland: Centre for Development and Environment (CDE), University of Bern, with Bern Open Publishing (BOP). 134pp
Imam A, Deeni Y (2015) Larval Productivity and Detoxification Enzymes Profile in Response to Physico-chemical Environmental Factors of Anopheles gambiae Breeding Ecologies in Nigeria. British Journal of Applied Science & Technology 5(6):595–612. https://doi.org/10.9734/bjast/2015/13268
Kenawy MA, Ammar SE, Abdel-Rahman HA (2013) Physico-chemical characteristics of the mosquito breeding water in two urban areas of Cairo Governorate Egypt. Journal of Entomological and Acarological Research 45(3):17. https://doi.org/10.4081/jear.2013.e17
Kenea O, Balkew M, Gebre-Michael T (2011) Environmental factors associated with larval habitats of anopheline mosquitoes (diptera: Culicidae) in irrigation and major drainage areas in the middle course of the rift valley, central ethiopia. J Vector Borne Dis 48(2):85–92
Kenea O, Balkew M, Tekie H, Gebre-Michael T, Deressa W, Loha E, Lindtjørn B, Overgaard HJ (2016) Human-biting activities of Anopheles species in south-central Ethiopia. Parasit Vectors 9(1):1–12. https://doi.org/10.1186/s13071-016-1813-x
Kibret S, Wilson GG, Ryder D, Tekie H, Petros B (2018) Can water-level management reduce malaria mosquito abundance around large dams in sub-Saharan Africa? 1–12
Lulu M, Hadis M, Makonnen Y, Asfaw T (1999) Inversion polymorphisms on anopheles arabiensis chromosomes from several regions of ethiopia. Insect Science and Its Application 19(2–3):207–210. https://doi.org/10.1017/s1742758400019482
Mahgoub MM, Kweka EJ, Himeidan YE (2017) Characterisation of larval habitats, species composition and factors associated with the seasonal abundance of mosquito fauna in Gezira Sudan. Infect Dis Poverty 6(1):1–10. https://doi.org/10.1186/s40249-017-0242-1
Mereta ST, Yewhalaw D, Boets P, Ahmed A, Duchateau L, Speybroeck N, Vanwambeke SO, Legesse W, De Meester L, Goethals PL (2013) Physico-Chemical and biological characterization of anopheline mosquito larval habitats Diptera: Culicidae: Implications for malaria control. Parasit Vectors 6(1):1–16. https://doi.org/10.1186/1756-3305-6-320
Messenger LA, Shililu J, Irish SR, Anshebo GY, Tesfaye AG, Ye-Ebiyo Y, Chibsa S, Dengela D, Dissanayake G, Kebede E, Zemene E, Asale A, Yohannes M, Taffese HS, George K, Fornadel C, Seyoum A, Wirtz RA, Yewhalaw D (2017) Insecticide resistance in Anopheles arabiensis from Ethiopia (2012–2016): A nationwide study for insecticide resistance monitoring. Malar J 16(1):1–14. https://doi.org/10.1186/s12936-017-2115-2
Minakawa N, Dida GO, Sonye GO, Futami K, Njenga SM (2012) Malaria vectors in Lake Victoria and adjacent habitats in Western Kenya. PLoS ONE, 7(3). https://doi.org/10.1371/journal.pone.0032725
Minakawa N, Mutero CM, Githure JI, Beier JC, Yan G (1999) Spatial distribution and habitat characterization of anopheline mosquito larvae in western Kenya. Am J Trop Med Hyg 61(6):1010–1016. https://doi.org/10.4269/ajtmh.1999.61.1010
Mosissa D, Mohammed A, Tesfaye Y (2019) The Effectiveness of Soil and Water Conservation as Climate Smart Agricultural Practice and Its Contribution to Smallholder Farmers’ Livelihoods. The Case of Bambasi District Benishangul Gumuz Regional State, Northwest of Ethiopia. World Journal of Agriculture and Soil Science (WJASS) 2 (4), 17pages. https://doi.org/10.33552/WJASS.2019.02.000542
Munga S, Minakawa N, Zhou G, Barrack OOJ, Githeko AK, Yan G (2005) Oviposition site preference and egg hatchability of Anopheles gambiae: Effects of land cover types. J Med Entomol 42(6):993–997. https://doi.org/10.1093/jmedent/42.6.993
Mwangangi JM, Mbogo CM, Muturi EJ, Nzovu JG, Githure JI, Yan G, Minakawa N, Novak R, Beier JC (2007) Spatial distribution and habitat characterisation of Anopheles larvae along the Kenyan coast. J Vector Borne Dis 44(1):44–51
Nambunga IH, Ngowo HS, Mapua SA, Hape EE, Msugupakulya BJ, Msaky DS, Mhumbira NT, McHwembo KR, Tamayamali GZ, Mlembe SV, Njalambaha RM, Lwetoijera DW, Finda MF, Govella NJ, Matoke-Muhia D, Kaindoa EW, Okumu FO (2020) Aquatic habitats of the malaria vector Anopheles funestus in rural south-eastern Tanzania. Malar J 19(1):1–11. https://doi.org/10.1186/s12936-020-03295-5
Okogun GRA, Nwoke BEB, Okere AN, Anosike JC, Esekhegbe AC (2003) Epidemiological implications of preferences of breeding sites of mosquito species in Midwestern Nigeria. Ann Agric Environ Med 10(2):217–222
Okogun GR, Anugboba. (2005) Life-table analysis of Anopheles malaria vectors: Generational mortality as tool in mosquito vector abundance and control studies. J Vector Borne Dis 42(2):45–53
Opoku A, Ansa-Asare O (2009) The occurrences and habitat characteristics of mosquitoes in Accra, Ghana. West Afr J App Ecol 11(1). https://doi.org/10.4314/wajae.v11i1.45730
Overgaard HJ, Tsuda Y, Suwonkerd W, Takagi M (2002) Characteristics of Anopheles minimus (Diptera: Culicidae) larval habitats in northern Thailand. Environ Entomol 31(1):134–141. https://doi.org/10.1603/0046-225X-31.1.134
Paaijmans KP, Takken W, Githeko AK, Jacobs AFG (2008) The effect of water turbidity on the near-surface water temperature of larval habitats of the malaria mosquito Anopheles gambiae. Int J Biometeorol 52(8):747–753. https://doi.org/10.1007/s00484-008-0167-2
PMI (2020) Project Final Entomology Report. May 2018
Sattler MA, Mtasiwa D, Kiama M, Premji Z, Tanner M, Killeen GF, Lengeler C (2005) Habitat characterization and spatial distribution of Anopheles sp. mosquito larvae in Dar es Salaam (Tanzania) during an extended dry period. Malar J 4:1–15. https://doi.org/10.1186/1475-2875-4-4
Service M (2008) Medical entomology for students, fourth edition. In Medical Entomology for Students, Fourth Edition. https://doi.org/10.1017/CBO9780511811012
Shililu J, Ghebremeskel T, Mengistu S, Fekadu H, Zerom M, Mbogo C, Githure J, Gu W, Novak R, Beier JC (2003) Distribution of anopheline mosquitoes in Eritrea. Am J Trop Med Hyg 69(3):295–302. https://doi.org/10.4269/ajtmh.2003.69.295
Simisek FM (2004) Seasonal Larval and Adult Population Dynamics and Breeding Habitat Diversity of Culex theileri Theobald, 1903 (Diptera : Culicidae) in the Gölbafl › District, Ankara, Turkey *. Turkish Journal of Zoology 28:337–344
Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Coetzee TCM, Mbogo CM, Charles M, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, Burkot TR, Harbach RE, Hay SI (2012) A global map of dominant malaria vectors. Parasit Vectors 5(69). https://doi.org/10.1017/S0007485300047945
Sinka ME, Rubio-Palis Y, Manguin S, Patil AP, Temperley WH, Gething PW, Van Boeckel T, Kabaria CW, Harbach RE, Hay SI (2011) Erratum: The dominant Anopheles vectors of human malaria in the Americas: Occurrence data, distribution maps and bionomic précis (Parasit Vectors (2010) 3 (72)). Parasit Vectors 4(1):1–26. https://doi.org/10.1186/1756-3305-4-210
Sunahara T, Ishizaka K, Mogi M (2002) Habitat size: a factor determining the opportunity for encounters between mosquito larvae and aquatic predators. J Vector Ecol 27:8–20
Taffese HS, Hemming-Schroeder E, Koepfli C, Tesfaye G, Lee MC, Kazura J, Yan GY, Zhou GF (2018) Malaria epidemiology and interventions in Ethiopia from 2001 to 2016. Infect Dis Poverty 7(1):1–9. https://doi.org/10.1186/s40249-018-0487-3
Teklu BM, Tekie H, Mccartney M, Kibret S (2010) The effect of water physical quality and water level changes on the occurrence and density of larvae of Anopheles mosquitoes around the shoreline of the Koka reservoir, Central Ethiopia. Hydrol Earth Syst Sci Discuss 7(4):6025–6055. https://doi.org/10.5194/hessd-7-6025-2010
Umar AM (2014) Characterisation of Anopheles Mosquitoes, Breeding Sites and Retrospective Study of Malaria in Katsina State, Nigeria
Utzinger È, Tozan Y, Singer BH (2001) Ef® cacy and cost-effectiveness of environmental management for malaria control. 6(9):677–687
Wamae PM, Githeko AK, Menya DM, Takken, W (2010) Shading by Napier Grass Reduces Malaria Vector Larvae in Natural Habitats in Western Kenya Highlands. 485–497. https://doi.org/10.1007/s10393-010-0321-2
White G (1982) Malaria Vector Ecology and Genetics. Br Med Bull 38
WHO (2020) World Malaria Report 2020. In WHO (Vol. 73, Issue 1). https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2020
Yewhalaw D, Van Bortel W, Denis L, Coosemans M, Duchateau L, Speybroeck N (2010) First evidence of high knockdown resistance frequency in Anopheles arabiensis (Diptera: Culicidae) from Ethiopia. Am J Trop Med Hyg 83(1):122–125. https://doi.org/10.4269/ajtmh.2010.09-0738
Yohannes M, Boelee E (2012) Early biting rhythm in the afro-tropical vector of malaria, Anopheles arabiensis, and challenges for its control in Ethiopia. Med Vet Entomol 26(1):103–105. https://doi.org/10.1111/j.1365-2915.2011.00955.x
Yohannes M, Haile M, Ghebreyesus TA, Witten KH, Getachew A (2005) Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia? 10(12), 1274–1285. https://doi.org/10.1111/j.1365-3156.2005.01512.x
Acknowledgements
The authors are grateful to the department of Biology, Jimma University for the financial support of the study. We are also grateful to the Department of Biology, Assosa University for the logistic support.
Funding
The study was financially supported by Department of Biology, Jimma University, Ethiopia.
Author information
Authors and Affiliations
Contributions
HK, DE, DY and EA conceived and designed the study. HK performed the field and laboratory experiments and drafted the manuscript. DY and EA supervised the experiments. GM did statistical analysis. TN, MW, GN and DY critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Keno, H., Ejeta, D., Negisho, T. et al. Characterization of Anopheles mosquito larval habitats and species composition in Bambasi District, Northwestern Ethiopia. Int J Trop Insect Sci 42, 2325–2336 (2022). https://doi.org/10.1007/s42690-022-00755-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-022-00755-0