Abstract
Selectivity of pesticides to the natural enemies in an agroecosystem is required for more effective integrated pest management. Trichogramma chilonis (Ishii) is an important natural enemy of lepidopteran pests, and is often exposed to pesticides. Effects of selected pesticides on acute mortality and parasitism when applied to parasitoids in egg, larval and pupal stages in their hosts were evaluated at 1x (field dose in Pakistan), 2x (double field dose) and 0.5x (half of field dose) doses. The parasitized host eggs were dipped in formulated solutions of pesticides when parasitoids were in different life stages. Parasitoid emergence from hosts treated with acetamiprid, fipronil and abamectin in the egg treatment and with spinetoram in all immature treatments were ≤ 33.9%. Treatment with acetamiprid (≤ 83.1 and ≤ 60.9%), fipronil (≤ 42.3and ≤ 72.7%), and abamectin (≤ 17.2 and ≤ 52.6%) yielded emergence in larval and pupal stage treatments, respectively. Spirotetramat, chlorantraniliprole, spiromesifen, haloxyfop-p-methyl, bispyribac sodium, nicosulfuron, chlorothalonil + procymidone, myclobutanil, pyraclostrobin + metiram, and trifloxystrobin + tebuconazole produced parasitoid emergence ≥ 80.1%.
Parasitoids emerged from hosts treated with spirotetramat, chlorantraniliprole, spiromesifen, bispyribac sodium, pyraclostrobin + metiram and trifloxystrobin + tebuconazole (except at 2 × dose in egg treatment) produced ≥ 84% parasitism in all treatments. Myclobutanil treatment of egg, and nicosulfuron and haloxyfop-p-methyl treatments of larvae and pupae, yielded > 90% parasitism. Acetamiprid and fipronil treatment of larvae and pupae, and abamectin treatment of pupae produced ≤ 78.46% and ≤ 10.76% parasitism, respectively. Over half of the pesticides caused no significant mortality to immature stages or exhibited little to no adverse impacts on parasitism and are promising for integration with these parasitoids.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Characterizing pesticide effects on beneficial insects is important for developing pest management strategies that integrate pesticides and natural enemies (van den Bosch and Stern 1962; Fishel 2013; You et al. 2016; de Paiva et al. 2020), and seek to minimize the adverse effects of pesticides by using selective compounds, or altering the dosage or schedule of pesticide application (Way 1986; Hassan et al. 1994; Martinson et al. 2001; Khan et al. 2014). Chemicals and biological control agents are being successfuly integrated under integrated pest mangement (IPM) programs (Akhtar et al. 2021).
Pesticides contribute to pest control in agroecosystems (Engindeniz and Engindeniz 2006). Efficient.
integration of chemical and biological controls lessens the input of chemicals in agroecosystem (Jiang et al. 2018). Selective pesticides contribute to maintain pest population under economic threshold levels (de Paiva et al. 2020). Pesticides with selective modes of action contribute to 1) enhanced ecosystem services by conserving natural enemies (Jacas and Urbaneja 2009; Cheng et al. 2018; Tores and Bueno 2018), 2) reduced environmental degradation from broad spectrum insecticides (Gurr et al. 2000; Landis et al. 2000; Snyder 2019), 3) reduced likelihood of pest resurgence, (Hutchison et al. 2004; Mahankuda et al. 2019), and 4) potentially delayed development of insecticide resistance in arthropod pests (Ruberson et al. 1998; Stark et al. 2007; Khan et al. 2015b; Khan 2017).
The safe, effective and sustainable management of insect pests demands use of strategies that encourage biological control (Lou et al. 2013). Biological control agents or natural enemies play a key role in agricultural production and can help to minimize the use of synthetic chemicals (Petersen 1993; Thomson and Hoffmann 2010). Parasitoids are important and often play critical roles as natural enemies in crop production due to their capacity to suppress various host populations (Wajnberg and Hassan 1994; Bale et al. 2008; Bompard et al. 2013; Bellows 2001; Jiang et al. 2019). However, pesticides adversely affect parasitoids’ efficacy against their hosts (Brown 1989; Desneux 2004, 2006; Biondi et al. 2013, 2015; Wang et al. 2016, 2017; Parreira et al. 2018). Both parasitoids and predators are typically more susceptible to pesticides than the pest insects (Gill and Garg 2014). Nevertheless, parasitoids are generally more susceptible to pesticides compared to predators (Croft 1990; Hassan 1989, 1992; Biondi et al. 2012; Khan and Ruberson 2017). Insect parasitoids come into contact with insecticides through direct exposure spray droplets or residues on crop foliage (Sheng et al. 2021). Pesticides may induce direct mortality of natural enemies or inflict sublethal effects on their reproduction, behaviour, foraging or movement (Jepson 1989; Croft 1990; Desneux 2007; de Paiva et al. 2018; Wahengbam et al. 2018).
Trichogramma species play an important role in IPM of many crops by parasitizing eggs of many lepidopterans worldwide (Hassan et al. 1998; Hassan and Abdelgader 2001; Khan et al. 2015b; Cheng et al. 2018; Willow et al. 2019). They are successfully used in inundative and inoculative biological control programs worldwide for management of insect pests in corn, rice, cotton, sugar beet, tomatoes, vegetables, and orchards (Hassan 1993; Smith 1996; Wang et al. 2012; Nascimento et al. 2018). There are around 210 described species of Trichogramma distributed globally, of which 25 species are used for pest management in 34 crops in 30 countries, which manage more than 400 pest species (Wajnberg and Hassan 1994; Parra and Zucchi 1997; Hassan et al. 1998; Pinto 2006; Zucchi et al. 2010; Polaszek 2010, Goulart et al. 2011).
Trichogramma chilonis has been reared and successfully used in augmentation worldwide (Pinto and Stouthamer 1994; Lingathurai et al. 2015) for controlling several lepidopteran insect pests on corn (Zea mays L.), cotton (Gossypium hirsutum L.), and vegetables (Chang et al. 2001; Ballal et al. 2009; Wang et al. 2012). It is an important egg parasitoid of lepidopteran pests (Sattar et al. 2011; Khan et al. 2014), including rice leaf folder Cnaphalocrocis medinalis (Guenée) in Pakistan (Sagheer et al. 2008).
Trichogramma spp. wasps are highly susceptible to most broad-spectrum pesticides (Bull and Coleman 1985; Wang et al. 2012), which reduce their efficacy against host pests (Brar et al. 1991; Consoli et al. 1998; Schuld and Schmuck 2000; Wang et al. 2012; Khan et al. 2015b; Pontes et al. 2020), creating compatibility problems in integrated pest management. Therefore, the current study is focused on the integration of Trichogramma chilonis with pesticides for more effective control of lepidopteran pests.
The current study evaluated effects of selected pesticides, including insecticides, miticides, herbicides and fungicides on 1) the emergence of T. chilonis from hosts treaed when parasitoids were in different life stages, and 2) parasitism of eggs of the angoumois grain moth, Sitotroga cerealella Olivier by female T. chilonis emerged from eggs of S. cerealella treated with pesticides when parasitoids were in the egg, larval and pupal stages. S. cerealella has typically been used for rearing Trichogramma species because of low rearing costs and ease of mass production (Hassan 1997). Pesticides were selected because 1) they are commonly and widely used chemicals worldwide, and have novel chemistries, and 2) almost all of these chemicals specifically target Lepidopteran in the agroecosystem.
Materials and methods
Rearing of Sitotroga cerealella
An electric suction apparatus was used to collect adults of Sitotroga cerealella Olivier from infested wheat grains and transfer them into a plastic jar (10 × 15 cm) with mesh (size 35 to 40) affixed to the bottom for holding wheat flour to allow moths to lay eggs for 24 h. Subsequently, eggs were collected from the flour using sieves (mesh no 50, 70). All moth stages were reared on wheat grains in plastic jars (10 × 15 cm) and was maintained in the Entomology laboratory of Nuclear Institute for Food and Agriculture (NIFA), Tarnab, Peshawar, under ambient laboratory conditions of 24 ± 6 °C, 65 ± 10% RH, and 16:8 h (L:D), until adult emergence.
Rearing of Trichogramma chilonis
Approximately 800 to 1000 fresh eggs of S. cerealella (< 24 h old) were glued (with Arabic gum) on paper card (4 × 7 cm), and were exposed to newly emerged T. chilonis (approximately 30 to 50 pairs) in a glass jar (5 × 12 cm) for 24 h under the same laboratory conditions used for rearing of S. cerealella. The likely parasitized exposed egg card was then transferred to another glass jar and was incubated at the 23 ± 3οC, 70 ± 10% (RH), and 14:10 (L:D) until adult parasitoid emergence.
Experimental design and setup/toxicity testing
Commercial formulations of 14 pesticides (Table 1) were mixed with water to prepare three doses, namely the field dose (x) used in Pakistan, double the field dose (2x), and half the field dose (0.5x) to determine their effects on the immature stages of T. chilonis in the experiments conducted in the Entomology laboratory (NIFA) under the same laboratory conditions used for rearing S. cerealella.
Eggs of S. cerealella containing eggs (24 h after exposure to parasitoids), larvae (72 h after exposure to parasitoids) and pupa (144 h after exposure to parasitoids) of T. chilonis were treated with pesticides by dipping 10 card strips (0.8 × 8 cm), each containing 10–15 parasitized host eggs, in each of the pesticide solutions or in water (untreted control) for 1–2 s. One complete treatment of pesticide used approximately 300–450 host eggs. Each card was air dried at room temperature for 1 h, and subsequently transferred to a glass vial (1 × 10 cm), was incubated at laboratory conditions used for rearing of T. chilonis until adult emergence.
After parasitoids emerged, single cards containing approximately 250 to 300 fresh eggs of S. cerealella were each exposed to 4–6 female T. chilonis (depending on the number of host eggs on the card) in a vial for 24 h. Each exposed card was transferred to a separate vial and was incubated under the stated conditions. The number of parasitoid adults emerged as well as numbers of pupae that failed to yield adults (blackened host eggs containing parasitoid puipae) were counted and percentage of emergence and parasitism relative to controls calculated.
Data calculation and statistical analysis
The raw data obtained were converted into percent emergence/parasitism and mean parasitism for each treatment before analysis.
The Shapiro–Wilk tests for all data analyzed indicated that none of these data were normally distributed, with all p-values (< 0.001). Therefore data were analyzed with a rank-based nonparametric tests: Aligned ranks transformation ANOVA (ART-ANOVA: one way, see, e.g., Beasley and Zumbo 2009; or Higgins and Tashtoush 1994). The R package (R statistical software: R Core team 2013) called ARTool (Kay and Wobbrock 2015) is developed by Wobbrok et al. (2011) is used to cover ART-ANOVA. For all tests the alpha level was set at α = 0.001 (also denoted as ***). Multiple comparison between pesticides (Table 1: Active ingredients), by doses were conducted using post hoc Kruskal–Wallis tests based on the R agricolae (de Mendiburu 2020) package. The alpha level was adjusted with the Benjamin & Hochberg method (Benjamini and Hochberg 1995) (which is much less conservative than the Bonferroni correction). Multiple comparison between doses, by pesticides were conducted using the aligned Friedman rank test from the scmamp R packages (Calvo and Santafe 2016).
The percentage reduction in emergence or parasitism compared to controls was calculated by the formula: E (%) = (1 − Et/Ec) × 100, where “E” is measured as either reduction of parasitism rate or adult emergence rate compared to controls. “Et” is the parasitism or emergence rate observed in each pesticide treatment, and “Ec” is the parasitism or emergence rate observed in the untreated control (Manzoni et al. 2007).
Rates of emergence or parasitism relative to the controls were characterized using toxicity categories of International Organization for Biological Control (IOBC)/West Palaearctic Regional Section (WPRS) (Hassan 1994; Sterk et al. 1999): 1 = harmless (E < 30% emergence or parasitism); 2 = slightly harmful (30 ≤ E ≤ 79%); 3 = moderately harmful (79 < E ≤ 99%); 4 = harmful (> 99% emergence or parasitism), where “E” is the effect of the pesticide on the biological control agent being measured as the reduction in percentage of emergence or parasitism compared to the control.
Results
Effect of pesticides on parasitoid emergence
Egg stage treatment
The R package called Artool (ART-ANOVA) indicated a significant main effects for pesticides (F = 427.24, df = 13/126, p < 0.001) and doses (F = 843.80, df = 3/378, p < 0.001), as well as interaction between pesticides and doses (F = 139.28, df = 39/378, P < 0.001).
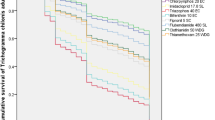
Table 2 presents the mean percentage emergence of T. chilonis from host eggs treated at egg stage of parasitoids with different insecticides, miticides, herbicides and fungicides at 2x, x and 0.5x doses and from untreated (control) eggs. Figure 1 shows the mean percentage parasitoid emergence relative to the controls for all pesticide treatments of parasitoids in the egg stage. Emergence in the treatments with the insecticides acetamiprid, spinetoram and fipronil and the miticide abamectin was ≤ 42.1% (Fig. 1). The field dose (x) of fipronil was harmful, those of spinetoram and abamectin were moderately harmful and acetamiprid at dose x was slightly harmful for emergence of parasitoids from host eggs (IOBC classification, Table 2). Spiromesifen, with 68.1%–81.5% emergence relative to the controls (Fig. 1), was harmless at both x and 0.5x, but slightly harmful for emergence at 2x dose (IOBC classification, Table 2). Spirotetramat and chlorantraniliprole, with ≥ 88.5% emergence relative to the controls, along with all herbicides and fungicides tested against the parasitoid egg stage were harmless for emergence of parasitoids at used all doses (Fig. 1; IOBC classification, Table 2).
Larval stage treatment
The ART-ANOVA indicated a significant main effects for pesticides (F = 594.46, df = 12/117, p < 0.001) and doses (F = 1184.74, df = 3/351, p < 0.001), as well as an interaction between pesticides and doses (F = 167.71, df = 36/351, p < 0.001).
Table 3 shows the mean percentage emergence of T. chilonis from host eggs treated at larval stage of.
parasitoids with different insecticides, miticides, herbicides and fungicides at 2x, x and 0.5x doses and from untreated (control) eggs. Figure 2 shows the mean percentage parasitoid emergence relative to the controls for all pesticide treatments of parasitoids in the larval stage. Emergence in the treatments with the insecticides acetamiprid, spinetoram and fipronil and the miticide abamectin was ≤ 68.7%, except 0.5x dose of acetamiprid (Fig. 2). The field doses of spinetoram, fipronil and abamectin were moderately harmful, and acetamiprid at x dose was slightly harmful for emergence of parasitoids from host eggs (IOBC classification, Table 3). The remaining pesticides including spirotetramat, chlorantraniprole, 2x dose of acetamiprid and spiromesifen were harmless, as were all doses of the herbicides and fungicides with > 73% emergence relative to the controls (Fig. 2; IOBC classification, Table 3). The data regarding effect of chlorothalonil + procymidone on emergence in the larval stage treatments are not included due to fungus attack on the parasitized cards.
Pupal stage treatment
The ART-ANOVA showed a significant main effects for pesticides (F = 221.18, df = 12/117, p < 0.001) and doses (F = 1777.87, df = 3/351, p < 0.001) as well as an interaction (F = 227.51, df = 36/351, p < 0.001) between pesticides and doses.
Table 4 indicates the mean percentage emergence of T. chilonis from host eggs treated at pupal stage of parasitoids with different insecticides, miticides and herbicides at 2x, x and 0.5x dose rates and from untreated (control) eggs. Figure 3 shows the mean percentage parasitoid emergence relative to the controls for all pesticide treatments of parasitoids in the pupal stage. Emergence in the treatments with the insecticides spinetoram, acetamiprid, and fipronil and the miticide abamectin were ≤ 32.4% at 2x dose, and the latter three products led to emergence ranging from 61% to 82.8% at 0.5x dose (Fig. 3). The field dose (x) of acetamiprid and abamectin were slightly harmful, those of spinetoram and fipronil were moderately harmful and harmless for emergence, respectively (IOBC classification, Table 4). Abamectin and acetamiprid at dose x were slightly harmful (Table 4) for emergence of parasitoids with ≤ 33.9% emergence relative to control (Fig. 3). Spirotetramat, chlorantraniliprole and spiromesifen with ≥ 91.4% emergence relative to the controls, and all the herbicides and fungicides (except chlorothalonil + procymidone) tested were harmless for emergence at used all doses, (Fig. 3; IOBC classification, Table 4). Data on chlorothalonil + procymidone were not included because of fungal attack on the egg cards.
Effect of pesticides on parasitism
Parasitism by females treated in the egg stage
The ART-ANOVA demonstrated a significant main effects for pesticides (F = 183.386, df = 10/99, p < 0.001) and doses (F = 684.198, df = 3/297, p < 0.001), as well as an interaction (F = 48.046, df = 30/297, p < 0.001) between pesticides and doses.
Table 5 presents the mean percentage parasitism by female T. chilonis emerged from host eggs treated with various insecticides, miticides, herbicides and fungicides at x, 2x and 0.5x doses when parasitoids were in the egg stage and from control eggs. Figure 4 shows the percentage parasitism relative to controls by female T. chilonis emerged from host eggs treated when parasitoids were in the egg stage. Spinetoram, fipronil and abamectin were toxic to parasitoids in the egg stage with ≤ 28.6% emergence (Table 2) at all doses. Consequently, very few adults emerged or those that emerged were unable to parasitize the host eggs. These products are considered harmful for parasitism and are, therefore, are not included in Table 5 and Fig. 4. According to Fig. 4 and the IOBC classification in Table 5, acetamiprid was harmful for parasitism at 2x and x (0 parasitism), and moderately harmful at 0.5x (2 mean parasitism) dose. Nicosulfuron and chlorothalonil + procymidone were slightly harmful for parasitism (Fig. 4; IOBC, Table 5) at 2x, while the other pesticides were harmless for parasitism at all doses.
Parasitism by females treated in the larval stage
The ART-ANOVA indicated a significant main effects for pesticides (F = 106.773, df = 10/99, p < 0.001) and doses (F = 346.785, df = 3/297, p < 0.001), as well as an interaction (F = 31.734, df = 30/297, p < 0.001) between pesticides and doses.
Table 6 presents the mean percentage parasitism by female T. chilonis emerged from host eggs treated with various insecticides, miticides, herbicides and fungicides at x, 2x and 0.5x doses when parasitoids were in the larval stage and from control eggs. Figure 5 shows the percentage parasitism relative to controls by female T. chilonis emerged from host eggs treated when parasitoids were in the larval stage. Spinetoram and abamectin were toxic to parasitoid larval stage with ≤ 17.2% emergence from the treated host eggs at all doses. Very few adults emerged or those that emerged were unable to parasitize the host eggs. These products are considered harmful for parasitism and are, therefore, are not included in Table 6 and Fig. 5. Acetamiprid was slightly harmful for parasitism at 2x and x (≤ 69.9% parasitism relative to control) doses (Fig. 5, Table 6), and harmless at 0.5x. Female wasps emerged from myclobutanil treated eggs parasitized hosts at a rate of 65%–78.2% at 2x and x doses. The remaining pesticides yielded parasitism of ≥ 85.6% at all doses.
Parasitism by females treated in the pupal stage
The ART-ANOVA indicated a significant main effects for pesticides (F = 212.166, df = 11/108, p < 0.001) and doses (F = 417.209, df = 3/324, p < 0.001), as well as an interaction (F = 51.223, df = 33/324, p < 0.001) between pesticides and doses.
Table 7 presents the mean percentage parasitism by female T. chilonis emerged from host eggs treated at pupal stage of parasitoids with various insecticides, miticides, herbicides and fungicides at x, 2x and 0.5x doses and from control eggs. Figure 6 shows the percentage parasitism relative to controls by female T. chilonis emerged from host eggs treated at the pupal stage of parsitoids. Acetamiprid, fipronil and abamectin were toxic to the parasitoid pupal stage with ≤ 56.8% parasitism relative to control at all doses. Few adults emerged (≤ 6.4%) from the spinetoram treated host eggs, and those that emerged were unable to parasitize the host eggs. These products are considered harmful for parasitism and are, therefore, not included in Table 7 and Fig. 6. The other pesticides showed ≥ 81% parasitism relative to control and were harmless at all doses (Fig. 6, Table 7).
Discussion
Most effective IPM programmes require that biological control agents and applied pesticides be compatible with each other (Stark et al. 2007; Desneux et al. 2007) so that the chemicals have minimal influence on the activity of natural enemies (Singh and Varma 1986; Guedes et al. 1992; Suinaga et al. 1996). In the current study pesticides were tested against the egg, larval, and pupal stages of T. chilonis to evaluate adverse effects of selected pesticides on parasitoid emergence, and females that emerged from treated eggs were evaluated for their parasitism efficacy. The pesticides used (Table 1) are relatively novel chemistries with novel modes of action compared to older, conventional broad-spectrum pesticides. They are commercially available and formulated products of inert materials and active ingredients, so there may be a contribution of inert materials in the overall effects of pesticides. More than half of tested pesticides were relatively selective and caused no significant.
x adverse impact on emergence of and parasitism by the parasitoids.
All herbicides tested were post emergence products and therefore are sprayed during the cropping season.
Insecticides, miticides, herbicides and fungicides were included in the study to achieve more effective integration of chemicals and biological controls to address multiple pest problems in the agroecosystem.
Very limited information is available on potential effects of pesticides on natural enemies (Firake et al. 2017), and on the effect of many of the newer pesticides on Trichogramma spp. (Lingathurai et al. 2015). The present results could not exclude harmful lethal effects of certain pesticides in general, but only at doses that are likely to get into contact with the parasitoid. Acetamiprid, spinetoram, fipronil, and abamectin caused high mortality of immature parasitoids and more adversely affected parasitism compared to the remaining pesticides used in the present study. Nevertheless, acetamiprid, fipronil, and abamectin were less adverse for emergence when treatments shifted from the egg stage toward the pupal stage, as well as from 2x toward 0.5x dose (Tables 2, 3 and 4; Figs. 1, 2 and 3). The tendency among those exhibiting stage-dependent effects regarding emergence was for treatment of larval and pupal stages to yield greater parasitoid emergence (Tables 3 and 4). Acetamiprid (all stages treated within the host eggs), fipronil (both larval and pupal stages treated), and abamectin (pupal stage treated) adversely affected parasitism and showed stage variable and dose dependent effect, as parasitism rate decreased when dose shifted from the 0.5x through 2x dose (Tables 5, 6 and 7; Figs. 4, 5 and 6). The high rate of toxicity of such pesticides for emergence and parasitism are due to the high rates of penetration into the cuticle of host eggs (Yu 1998; de Paiva 2018). Stage-dependent effects suggest that the parasitoid egg and larval stages were more sensitive/susceptible to these pesticides compared to the pupal stage (Varma and Singh 1987; Consoli et al. 1998; Biondi et al. 2015; Khan and Ruberson 2017), and moreover, dose affects the level of adverse effects on the organism as the dose makes the poison.
The toxicity of the aforementioned pesticides to the immature stages of T. chilonis was supported by previous findings on lethal effects of pesticides on the immature stages of Trichogramma pretiosum (Khan and Ruberson 2017). The findings also concur with previous results on adult T. chilonis (Khan 2020) that acetamiprid, spinetoram, fipronil and abamectin were very harmful pesticides for adult T. chilonis exposed to the dried residues on glass, as well as subsequent parasitism by the exposed female parasitoids. Acetamiprid was less adverse for emergence and parasitism when dose declined from 2x to 0.5x dose for all immature stages. The toxic effects of acetamiprid on emergence are supported by Hewa-Kapuge et al. (2003), who reported that acetamiprid was toxic for emergence of T. chilonis. Moura et al. (2006) also treated (sprayed) host eggs (Anagasta kuehniella) with acetamiprid and found acetamiprid harmless to larvae, but slightly harmful to pupae of T. pretiosum. However, the current study demonstrated that acetamiprid is considerably more damaging to both larval and pupal stages of T. chilonis. This may be due to different methodology: Moura et al. (2006) sprayed parasitized eggs, whereas in the current experiment the parasitized eggs were immersed in the solution for 1–2 s, which would have increased coverage and possibly permeation into the host egg. Similarly, both acetamiprid and abamectin were slightly to moderately toxic to adult parasitoids T. dendrolimi, T. ostriniae and T. chilonis when exposed to dry residues in glass vials (Cheng et al. 2018).
Abamectin had a greater adverse effect when parasitoids were treated in the egg and larval stages (Tables 2, 3; Figs. 1, 2) than in the pupal stage (Table 4; Fig. 3). Similar observations were made by Cônsoli et al. (1998), who reported that abamectin was harmful to slightly harmful for emergence of T. chilonis, when parasitoids were treated in different immature stages. Hussain et al. (2010) concluded that abamectin significantly adversely affected emergence of T. chilonis from the treated host eggs of S. cerealella. Carvalho et al. (2003) also found abamectin adversely affected emergence of T. pretiosum from host eggs treated when parasitoids were in egg, larval and pupal stages. Similarly, Carvalho et al. (2003) found that abamectin-treated host eggs at the parasitoids’ pupal stage led to significantly reduced parasitism by emerged female T. pretiosum.
Fipronil significantly reduced emergence of and parasitism by parasitoids. Fipronil was slightly to moderately harmful for emergence of T. chilonis at field recommended concentration (FRC) in the larval and pupal stage treatments, respectively (Tables 3, 4). This result was supported by Ghorbani et al. (2016), who observed that FRC of fipronil was slightly harmful for emergence of T. brassicae from the treated larvae, prepupae and pupae. Similarly, fipronil negatively affected parasitism by females treated in the pupal stage at both x and 0.5x doses.
Spinetoram was moderately harmful for parasitoids emerging after treatment in larval and pupal stages at all used doses (Tables 3, 4). The adverse effect of spinetoram in the egg treatment declined from harmful to slightly harmful as the dose decreased from 2x to 0.5x (Table 2). However, parasitoids treated in host eggs at egg, larval and pupal stages with spinetoram did not parasitize host eggs (Tables 5, 6 and 7). The current study is supported by Khan and Ruberson (2017) who found spinetoram adversely affected immature stages of T. pretiosum. Similarly, Khan et al. (2015a) found abamectin was moderately harmful, while spinetoram, fipronil and acetamiprid were slightly harmful for parasitism at field dose by T. chilonis of previously treated host eggs of Sitotroga cerealella.
The remaining pesticides (spirotetramat, chlorantraniliprole, spiromesifen, haloxyfop-p-methyl, bispyribac sodium, nicosulfuron, chlorothalonil + procymidone, myclobutanil, pyraclostrobin + metiram, and trifloxystrobin + tebuconazole) were harmless for parasitoid emergence at all doses and all parasitoid stages treated, except spiromesifen, which was slightly harmful for emergence when host eggs were treated at parasitoid egg stage at 2x dose (Table 2). The miticide spiromesifen generally did not adversely affect emergence of parasitoids. The pesticides found to be harmless to the parasitoids in immature stages might be due to that fact that 1) the pesticide could not penetrate through the egg-skin of S. cerealella, or their mode of action simply implies no harmful interaction with the parasitoids within the host, for example herbicides and fungicides or/and 2) the immature stages of parasitoids could easily degrade the pesticides so that pesticides have no adverse impact on the parasitoids (Yu 1998; de Paiva 2018). This result corresponded with previous results with immature stages of T. pretiosum (Khan and Ruberson 2017). Chlorantraniliprole was found harmless for emergence of T. chilonis, which was confirmed by Hussain et al. (2012), who demonstrated that the same chemical resulted in maximum emergence of T. chilonis from the host eggs treated 8 days after parasitism and showed minimum effect on parasitoid emergence from eggs treated 1, 3, 5 and 7 days after parasitism. Wahengbam et al. (2018) found both chlorantraniliprole and spiromesifen did not adversely affect T. chilonis and T. pretiosum when they were treated in the pupal stage. Chlorantraniliprole was harmless for parasitism by T. pretiosum (de Paiva et al. 2018).
Spirotetramat demonstrated ≥ 82.8% emergence and is classified as harmless for emergence of T. chilonis at all doses and all stages treated, except 2 × dose in larval treatment (Table 3). This is supported by Tabebordbar et al. (2020) who concluded the mean emergence rate of T. evanescens when exposed to the recommended field concentrations of spirotetramat was 84%. The rating of spirotetramat as harmless for parasitism is supported by Bruck et al. (2009), who found that spirotetramat was harmless for parasitism by T. cryptophlebiae in citrus. According to Moens et al. (2012), limited research has been conducted to assess the side effects of spirotetramat on natural enemies. Furthermore, an earlier finding by Khan and Ruberson (2017) demonstrated that both chlorantraniliprole and spirotetramat caused no significant mortality to immature stages of T. pretiosum.
Herbicides and fungicides were harmless for emergence (Tables 2, 3 and 4), nevertheless, they have some dose based adverse impacts on parasitism in the present study: all the herbicides, namely haloxyfop-p-methyl, bispyribac sodium, nicosulfuron, and fungicides, namely myclobutanil, chlorothalonil + procymidone, pyraclostrobin + metiram and trifloxystrobin + tebuconazole did not adversely affect emergence or parasitism at all doss and all stages treated, except the 2x dose of both nicosulfuron and chlorothalnil + pyraclostrobin which adversely affected parasitism by parasitoids treated at egg stage (Table 5), and the 2x dose of myclobutanil adversely affected parasitism by parasitoids treated at larval stage (Table 6). Thus, dose wise effects of pesticides were observed for both groups of pesticides in some cases.
There are few studies of the effects of herbicides and fungicides on Trichogramma. However, the literature demonstrated fungicides are generally harmless for emergence of Trichogramma (Hagley and Laing 1989; Stark and Banken 1999; Jalali and Singh 1993; Vieira et al. 2001), particularly, when applied to parasitoid pupae (Hassan 1994). In the current study, the herbicide bispyribac sodium was harmless for emergence as well as for parasitism at all doses and immature stages treated. This was supported by Khan et al. (2015a) who concluded that bispyribac sodium was harmless for parasitism by T. chilonis of previously treated host eggs at x, 2x and 0.5x doses. Moreover, Khan (2020) found that bispyribac sodium was slightly harmful for adult T. chilonis exposed to dried field dose residues 1 and 5 days after application, and harmless after 10 and 15 days of drying in glass vials. Khan (2020) also rated bispyribac sodium as slightly harmful for parasitism by females exposed to the 1-day dried residue in glass vials and harmless in older residual treatments. The difference in effects of the bispyribac sodium between the current study and previous studies is likely due to methodology differences, and stages of parasitoid tested.
The mixture of trifloxystrobin + tebuconazole and of pyraclostrobin + metiram did not adversely affect either emergence of, or parasitism by T. chilonis in the current study. Similarly, myclobutanil was deemed harmless for emergence of, as well parasitism by T. chilonis (expect for parasitism at 2x dose in the larval treatment: Table 6). Khan and Ruberson (2017) found that myclobutanil, pyraclostrobin, and trifloxystrobin + tebuconazole did not adversely affect immature stages of T. pretiosum. Similarly, the results of Carmo et al. (2010) also supports that many fungicides, including trifloxystrobin, pyraclostrobin and tebuconazole do not significantly affect emergence of and parasitism by Telonomus remus compared to an untreated control. Bueno et al. (2008) treated egg, larval and pupal stages of T. pretiosum with myclobutanil under laboratory conditions and found myclobutanil harmless for emergence of T. pretiosum, Literature on the effect of myclobutanil on parasitism by Trichogramma emerged from host eggs treated with the myclobutanil is not available. However, Khan and Ruberson (2017) found the same chemical had a negligible impact on the foraging behavior, including stinging of host eggs, of T. pretiosum.
The present result revealed that pyraclostrobin + metiram, and chlorothalonil + procymidone (except 2 × dose in egg treatment: Table 5) had no adverse impact on parasitism by T. chilonis, supporting Petersen (1995), who concluded that metiram had no significant effect on percent reduction in egg production or hatchability by rove beetle Alleochara bilineata, and further concluded that chlorothalonil and procymidone had no adverse impact on predation by the beetle. Haloxyfop-p-methyl was harmless for parasitism by parasitoids in the current study. This is supported by Peterson (1995) that haloxyfop-R was found safe for egg production (%) of the beetle Alleochara bilineata. Khan et al. (2015a) found that pyraclostrobin + metiram, chlorothalonil + procymidone and haloxyfop-p-methyl were harmless for parasitism by T. chilonis of previously treated host eggs at x, 2x and 0.5x doses. Furthermore, Khan (2020) also demonstrated that both chlorothalonil + procymidone and haloxyfop-p-methyl were harmless for parasitism by T. chilonis exposed to the dried residues at field dose in a glass vial 1, 5, 10, and 15 days after treatment of the glass.
Harmlessness of nicosulfuron and chlorothalonil + procymidone for emergence of T. chilonis is supported by earlier findings that nicosulfuron had no negative impact on the development and emergence of T. pretiosum (Khan and Ruberson 2017). Nicosulfuron and chlorothalonil + procymidone showed increased toxicity for parasitism from harmless at x dose to slightly harmful at 2x dose in the parasitoid egg stage (Table 5), while the same chemicals were found harmless for parasitism at all used doses when host eggs were treated at larval and pupal stages of parasitoids (Tables 6 and 7). Leite et al. (2017) also described nicosulfuron as "harmless" for parasitism by Trichogramma of previously treated host eggs of Anagasta kuehniella.
Products that are harmful at field rate (x) and even at half dose (0.5x) are not compatible with IPM. Further, the compatibilty of field rate/dose to all stages of parasitoids is required for more successful integration of biological and chemical control in agroecosystem. Even if parasitoids larvae or pupae were slightly less susceptible, the high toxicity to eggs of parasitoids means that such products should preferably not be used in IPM as at any given time, there will be a mixture of parasitoid eggs, larvae and pupa in the host eggs and one cannot time pesticide application to only expose larvae or pupae.
The pesticides tested in the current study have different modes of action. Pesticides found harmless in the laboratory will most probably be harmless to the natural enemies in the field. However, pesticides evaluated and found harmful in the laboratory may be less harmful in the field and therefore should be further tested (Hassan 1977; Steiner 1977), because of possible environmental degradation of pesticides or coverage differences.
Conclusion
Selected pesticides, including insecticides, miticides, herbicides, and fungicides were tested against the immature stages (eggs, larvae and pupae) of Trichogramma chilonis to determine their effects on emergence of and parasitism by parasitoids emerged from the treated eggs of Sitotroga cerealella. Acetamiprid, spinetoram, fipronil and abamectin exhibited relatively high adverse effects on emergence of T. chilonis from hosts exposed at field recommended dose, and at 2x and 0.5x doses for all immature stages. However, the impact of fipronil and abamectin varied with parasitoid life stage and pesticide dose. Most of the remaining pesticides yielded > 80% parasitoid emergence at all doses and stages treated.
Acetamiprid, fipronil and abamectin adversely affected parasitism by parasitoids emerging from treated. host eggs. Nevertheless, the adverse impact declined when dose was reduced from 2x through 0.5x. Field trials are recommended to determine if their negative effect on parasitoid emergence and parasitism persist under field conditions. The remaining pesticides yielded ≥ 84% parasitism at field dose across all parasitoid stages. Therefore, most of the used pesticides exhibited good compatibility with parasitoids for both emergence and parasitism. The current results should be largely extrapolatable to other lepidopteran pests and real world situations.
References
Akhtar ZR, Tariq K, Handler AM, Ali A, Ullah F, Ali F, Zang L-S, Gulza A, Ali S (2021) Toxicological risk assessment of some commonly used insecticides on Cotesia flavipes, a larval parasitoid of the spotted stem borer Chilo partellus. Ecotoxico 30(3):448–458
Bale J, van Lenteren J, Bigler F (2008) Biological control and sustainable food production. Philos Trans R Soc Lond 363:761–776
Ballal CR, Srinivasan R, Jalali SK (2009) Evaluation of an endosulfan tolerant strain of Trichogramma chilonis on cotton, BioContr l54: 723–732
Beasley TM, Zumbo DB (2009) Aligned rank tests for interactions in split-plot designs: Distributional assumptions and stochastic homogeneity. J Mod Appli Statist Meth 8:16–50
Bellows T (2001) Restoring population balance through natural enemy introduction. Biol Cont 21:199–205
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statisti Socie Seri B 57:289–300
Biondi A, Campolo O, Desneux N, Siscaro G, Palmeri V, Zappala` L (2015) Life stage-dependent susceptibility of Aphytis melinus DeBach (Hymenoptera: Aphelinidae) to two pesticides commonly used in citrus orchards. Chemosph 128:142–147
Biondi A, Mommaerts V, Smagghe G, Viñuela E, Zappalà L, Desneux N (2012) The non-target impact of spinosyns on beneficial arthropods. Pest Manag Sci 68:1523–1536
Biondi A, Zappala` L, Stark JD, Desneux N (2013) Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE 8:e76548
Bompard A, Amat I, Fauvergue X, Spataro T (2013) Host-parasitoid dynamics and the success of biological control when parasitoids are prone to allee effects. PLoS ONE 8(10):e76768. https://doi.org/10.1371/journal.pone.0076768
Brar KS, Varma GC, Shenhmar M (1991) Effect of insecticides on Trichogramma chilonis Ishii (hymenoptera: Trichogrammatidae), an egg parasitoid of sugarcane borers and cotton bollworms. J Entomon 16:43–48
Brück E, Elbert A, Fischer R, Krueger S, Kühnhold J, Klueken AM, Nauen R, Niebes J-F, Reckmann U, Schnorbach H-J, Steffens R, van Waetermeulen X (2009) Movento®, an innovative ambimobile insecticide for sucking insect pest control in agriculture: Biological profile and field performance. Crop Prot 28:838–844
Brown RA (1989) Pesticides and non-target terrestrial invertebrates: an industrial approach. In: Jepson PC (ed) Pesticides and nontarget invertebrates. Intercept Ltd, Wimborne 19–42
Bueno A. de F, Bueno RCO de F, Parra JRP, Vieira SS (2008) Effects of pesticides used in soybean crops to the egg parasitoid Trichogramma pretiosum. Ciência Rural. Santa Maria 38(6):1495–1503
Bull DL, Coleman RJ (1985) Effects of pesticides on Trichogramma spp. Southwest Entomol (supplement) 8:156–168
Calvo B, Stantafe G (2016) Scmamp: An R software package for statistical comparison of multiple algorithms in multiple problems. https://www.rdocumentation.org/packages/scmamp/versions/0.2.55 Version 0.2.55
Carmo EL, Bueno AF, Bueno RCOF (2010) Pesticide selectivity for the insect egg parasitoid Telenomus remus. BioCont 55:455–464
Carvalho GA, Reis PR, Rocha LCD, Moraes JC, Fuini LC, Ecole CC (2003) Side-effects of insecticides used in tomato fields on Trichogramma pretiosum (Hymenoptera: Trichogrammatidae). Maringa 25(2):275–279
Chang SC, Hu NT, Hsin CY, Sun CN (2001) Characterization of differences between two Trichogramma wasps by molecular markers. Biol Cont 21:75–78
Cheng S, Lin R, Wang L, Qiu Q, Qu M, Ren X, Zong F, Jiang H, Yu C (2018) Comparative susceptibility of thirteen selected pesticides to three different insect egg parasitoid Trichogramma species. Ecotoxic Environm Safety 166:86–91
Consoli FL, Parra JRP, Hassan SA (1998) Side-effects of insecticides used in tomato fields on the egg parasitoid Trichogramma pretiosum Riley (Hym., Trichogrammatidae), a natural enemy of Tuta absoluta (Meyrick) (Lep., Gelechiidae). J Appl Entomol 122:43–47
Croft BA (1990) Arthropod biological control agents and pesticides. John Wiley and Sons, New York
de Mendiburu F (2020) Agricolae: An R software package for statistical procedures for agricultural research. https://tarwi.lamolina.edu.pe/~fmendiburu/ Version 1.2–8
de Paiva ACR, Beloti VH, Yamamoto PT (2018) Sublethal effects of insecticides used in soybean on the parasitoid Trichogramma pretiosum. Ecotoxico 27(4):448–456
de Paiva ACR, Filho FHI, Parro EA, Barbosa DPL, Yamamoto PT (2020) Do Ready-Mix Insecticides Cause Lethal and Sublethal Effects on Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) Pupa?. J Eco Entomol XX(XX) 1–7
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Desneux N, Pham-Dele`gue MH, Kaiser L (2004) Effects of sub-lethal and lethal doses of lambda-cyhalothrin on oviposition experience and host-searching behaviour of a parasitic wasp, Aphidius ervi. Pest Manag Sci 60:381–389
Desneux N, Ramirez-Romero R, Kaiser L (2006) Multistep bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ Toxicol Chem 25:2675–2682
Engindeniz S, Engindeniz DY (2006) Economic analysis of pesticide use on greenhouse cucumber growing: A case 493 study for Turkey. J Plant Dis Prot 113:193–198
Firake DM, Thubru DP, Behere GT (2017) Eco-toxicological risk and impact of pesticides on important parasitoids of cabbage butterflies in cruciferous ecosystem. Chemosph 168:372–383
Fishel FM (2013) The EPA conventional reduced risk pesticide program. PI-224. University of Florida Institute of Food and Agricultural Sciences, Gainesville
Ghorbani M, Saber M, Bagheri M, Vaez N (2016) Effects of diazinon and fipronil on different developmental stages of Trichogramma brassicae Bezdenko (Hym.; Trichogrammatidae). J Agric Sci Technol 18:1267–1278
Gill HK, Garg H (2014) Pesticide: Environmental Impacts and Management Strategies. Pesticides-Toxic Effects. Intech. Rijeka, Croatia, 187–230
Goulart RM, Volpe HX, Vacari AM, Thuler RT, De Bortoli SA (2011) Insecticide selectivity to two species of Trichogramma in three different hosts, as determined by IOBC/WPRS methodology. Pest Manag Sci 68(2):240–244
Guedes RN, Lima JOG, Zanuncio JC (1992) Seletividade dos inseticidas deltametrina, fenvalerato e fenitrotion para Podisus connexivus Bergroth, 1891 (Heteroptera: Pentatomidae). An Soc Entomol Bras 21:339–346
Gurr GM, Wratten SD, Barbosa P (2000) Success in conservation biological control of arthropods. In: Gurr G, Wratten S (eds) Biological control: measures of success. Springer, Dordrecht, pp 105–132
Hagley EAC, Laing JE (1989) Effect of pesticides on parasitism of artificially distributed eggs of the codling moth Cydia pomonella (Lepidoptera: Tortricidae) by Trichogramma sp. (Hymenoptera: Trichogrammatidae). Proc Entomol Soc Ont 120:25–33
Hassan SA (1977) Standardized techniques for testing side-effects of pesticides on beneficial arthropods in the laboratory. J Plant Dis Prot 84:158–163
Hassan SA (1989) Testing methodology and the concept of the IOBC/WPRS Working Group, in Pesticides and Non-Target Invertebrates, ed. By Jepson PC. Intercept, Andover, 1–18
Hassan SA (1992) Guideline of the side effects of plant protection products on Trichogramma chilonis. In guideline for testing the effects of pesticides on beneficial organisms: description of test method. Bull IOBC/WPRS 15:18–39
Hassan SA (1993) The mass rearing and utilization of Trichogramma to control lepidopterous pests: achievements and outlook. Pestic Sci 37(4):387–391
Hassan SA (1994) Strategies to select Trichogramma species for use in biological control. In: Wajnberg E, Hassan SA (eds) Biological Control With Egg Parasitoids. CAB International, Oxon, 55–71
Hassan SA (1997) Seleção de espécies de Trichogramma para uso em programas controle biológico. In: Parra JRP, Zucchi RA (eds) Trichogramma e o controle biológico aplicado. pp. 183–206
Hassan SA, Abdelgader H (2001) A sequential testing program to assess the side effects of pesticides on Trichogramma cacoeciae Marchal (Hym.Trichogrammatidae). IOBC/WPRS Bull 24:71–81
Hassan SA, Biglert H, Bogenschutz H, Boller E, Brun J, Callis JNM, CPelseneer J, Duso C, Grove A, Heimbach U, Helyer N, Hokkanen H, Lewis GB, Mansour F, Moreth L, Polgar L, S-Petersen L, Suphanor B, Staubli A, Stern G, Vainio A, Veire VDM, Viggiani G, Vogt H (1994) Results of the sixth joint pesticide testing programme of the IOBC/WPRS-working group “Pesticides and Beneficial Organisms.” Entomopha 39(1):107–119
Hassan SA, Hafes B, Degrande PE, Herai K (1998) The side-effects of pesticides on the egg parasitoid Trichogramma cacoeciae Marchal (Hym., Trichogrammatidae), acute dose response and persistence tests. J Appl Ent 122(1–5):569–57
Hewa-Kapuge S, Mcdougall S, Hoffmann AA (2003) Effects of methoxyfenozide, indoxacarb, and other insecticides on the beneficial egg parasitoid Trichogramma nr. Brassicae (Hymenoptera: Trichogrammatidae) under laboratory and field conditions. J Econ Entomol 96:1083–1090
Higgins JJ, Tashtoush S (1994) An aligned rank transform test for interaction. Nonline Wor 1:201–211
Hussain D, Akram M, Iqbal Z, Ali A, Saleem M (2010) Effect of insecticides on Trichogramma chilonis Ishii (Hymenoptera: Trichogrammatidae) immature and adult survival. J Agric Res 48:531–537
Hussain D, Ali A, M-ul-Hassan M, Ali S, Saleem M, Nadeem S (2012) Evaluation of Toxicity of Some New Insecticides against Egg Parasitoid Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae). Pakistan J Zool 44(4):1123–1127
Hutchison WD, Flood B, Wyman JA (2004) Advances in United States sweet corn and snap bean insect pest management. In: Insect pest management Springer, Berlin, Heidelberg, pp. 247–278
Jacas J, Urbaneja A (2009) Origen de las plagas e historia del control Biológico. Control Biológico De. Plagas Agric. Phytoma, Esp. 3–13
Jalali SK, Singh SP (1993) Susceptibility of various stages of Trichogrammatoidea armigera Nagaraja to some pesticides and effect of residues on survival and parasitizing ability. Biocont Sci Technol 3:21–27
Jepson PC (1989) Pesticides and non-target invertebrates. (ed.) Intercept. Wimborne. Dorset, U.K
Jiang J, Liu X, Zhang Z, Liu F, Mu W (2019) Lethal and sublethal impact of sulfoxaflor on three species of Trichogramma parasitoid wasps (Hymenoptera: Trichogrammatidae). Biol Cont 134:32–37
Jiang J, Ma D, Zhang Z, Yu C, Liu F, Mu W (2018) Favorable compatibility of nitenpyram with the aphid predator, Coccinella septempunctata L. (Coleoptera: Coccinellidae). Envir Sci Pollu Res, 25(27):27393–27401
Kay M, Wobbrock JO (2015) ARTool: An R software package for the aligned rank transform for nonparametric factorial ANOVAs. https://cran.r-project.org/web/packages/ARTool Version 0.9.5
Khan MA (2017) Effect of selected baculoviruses on oviposition preference by Trichogramma chilonis (Trichogrammatidae: Hymenoptera). J King Saud Univ–Sci 29:214–220
Khan MA (2020) Lethal and parasitism effects of selected novel pesticides on adult Trichogramma chilonis (Hymenoptera: Trichogrammatidae). J Plant Dis Prot 127:81–90
Khan MA, Khan H, Farid A (2014) Assessment of the lethal and parasitism effects of Helicoverpa armigera Nucleopolyhedrovirus (HaNPV) on Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatidae). Sar J Agric 30(4):425–432
Khan MA, Khan H, Farid A, Ali A (2015a) Evaluation of Toxicity of some Novel Pesticides to Parasitism by Trichogramma chilonis (Hymenoptera: Trichogramm-atidae). J Agric Res 53(1):63–73
Khan MA, Khan H, Ruberson JR (2015b) Lethal and behavioral effects of selectednovel pesticides on adults of Trichogrammapretiosum (Hymenoptera: Trichogrammatidae). Pest Manag Sci 71:1640–1648
Khan MA, Ruberson JR (2017) Lethal effects of selected novel pesticides on immature stages of Trichogrammapretiosum (Hymenoptera:Trichogrammatidae). Pest Manag Sci 73(12):2465–2472
Landis DA, Wratten SD, Gurr GM (2000) Habitat management to conserve natural enemies of arthropod pests in agriculture. Ann Rev Ento 45:175–201
Leite GLD, de Paulo PD, Zanuncio JC, Tavares WD-S, Alvarenga AC, Dourado LR, Bispo EP, Soares MA (2017) Herbicide toxicity, selectivity and hormesis of nicosulfuron on 10 Trichogrammatidae (Hymenoptera) species parasitizing Anagasta ( = Ephestia) kuehniella(Lepidoptera: Pyralidae) eggs. J Environ Sci Health B 2:52(1):70–76
Lingathurai S PM, Raveen R, Priyatharsini PV, Sathikumaran R, Narayanan PCS (2015) Ecotoxicological performances and biochemical effect of selected pesticides on Trichogramma chilonis (Ishii) (Hymenoptera: Trichogrammatid- ae). J Entom Zool Stud 3(1):109–114
Lou YG, Zhang GR, Zhang WQ, Huc Y, Zhang J (2013) Biological control of rice insect pests in China. Biol Cont 67:8–12
Mahankuda B, Sawai HR, Gawande RW, Neharkar PS, Nagdeote VG (2019) Effect of insecticide residues on the adult survival rate of Trichogramma chilonis under laboratory condition. J Entomol Zool Stud 7(2):1349–1351
Manzoni CG, Grutzmacher AD, Giolo FP, Harter WDR, Castilhos RV, Paschoal MDF (2007) Side-effects of pesticides used in integrated production of apples to parasitoids of Trichogramma pretiosum Riley and Trichogramma atopovirilia Oatman & Platner (Hymenoptera: Trichogrammatidae). BioAssay 2:1–11
Martinson T, Williams L III, English-Loeb G (2001) Compatibility of chemical disease and insect management practices used in New York vineyards with biological control by Anagrus spp Mymaridae Hymenoptera parasitoids of Erthroneura leafhoppers. Biol Cont 22:227–234
Moens J, Tirry L, Clercq PD (2012) Susceptibility of cocooned pupae and adults of the parasitoid Microplitis mediator to selected insecticides. Phytopara 40:5–9
Moura AP, Carvalho GA, Pereira AE, Rocha LCD (2006) Selectivity evaluation of insecticides used to control tomato pests to Trichogramma pretiosum. BioCont 51:769–778
Nascimento PT, Fadini MAM, Valicente FH, Ribeiro PEA (2018) Does Bacillus thuringiensis have adverse effects on the host egg location by parasitoid wasps? Revis Brasil De Entomol 62:260–266
Parra JRP, Zucchi RA (1997) Trichogramma e o Controle Biol´ogico Aplicado. FEALQ, Piracicaba, Brazil, pp. 324
Parreira DS, Cruz RA, Zanuncio JC, Lemes PG, Rolim GS, Barbosa LR, Leite GLD, Serrão JE (2018) Essential oils cause detrimental effects on biological parameters of Trichogramma galloi immatures, J Pest Sci 91(2):887–895
Peterson LS (1993) Effects of 45 insecticides, acaricides and molluscicides on the rove beetle Aleochara bilineata (col.: staphylinidae) in the laboratory. Entomopha 38 (3):371–382
Peterson LS (1995) Effects of 37 fungicides on the rove beetle Aleochara bilineata (coleop: staphylinidae) in the laboratory. Entomophaga 40(2):145–152
Pinto JD (2006) A review of the New World genera of Trichogrammatidae (Hymenoptera). J Hymen 15:38–163
Pinto JD, Stouthamer R (1994) Systematics of the Trichogrammatidae with emphasis on Trichogramma. In: Wajnberg E, Hassan SA (eds) Biological control with egg parasitoids. CAB International, Wallingford, pp. 1–36
Polaszek A (2010) Species diversity and host associations of Trichogramma in Eurasia. In: Cônsoli FL, Parra JR, Zucchi RA (eds) Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma. Springer, Dordrecht, pp. 237–266
Pontes JP, Leite GLD, Bispo EPR, Tavares W de S, Menezes CWG de,Wilcken CF, Zanuncio JC (2020) A glyphosate‑based herbicide in a free-choice test on parasitism, emergence, and female‑biased sex ratio of 10 Trichogrammatidae. J Plant Dis Prot 127:73–79
R Core Team (2013) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Ruberson JR, Nemoto H, Hirose Y (1998) Pesticides and conservation of natural enemies in pest management. In: Barbosa P (ed) Conservation biological control. Academic Press, New York, pp 207–220
Sagheer M, Ashfaq M, M-ul H, Rana SA (2008) Integration of Some Biopesticides and Trichogramma chilonis for the Sustainable Management of Rice Leaf Folder Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae). Pak J Agri Sci 45(1):69–74
Sattar S, Ullah F, Saljoqi AUR, Arif M, Qazi JI (2011) Toxicity of some new insecticides against Trichogramma chilonis (Hymenoptera: Trichogrammatidae) under laboratory and extended laboratory conditions. Pak J Zool 43:1117–1125
Schuld M, Schmuck UR (2000) Effects of Thiacloprid, a New Chloronicotinyl Insecticide, on the Egg Parasitoid Trichogramma cacoeciaev. Ecotoxicolo 9:197–205
Sheng S, Wang J, Zhang X-rui, Liu Z-xiang, Yan M-wen, Shao Y, Zhou J-cheng, Wu Fu-an, Wang J (2021) Evaluation of Sensitivity to Phoxim and Cypermethrin in an Endoparasitoid, Meteorus pulchricornis (Wesmael) (Hymenoptera: Braconidae), and Its Parasitization Efficiency Under Insecticide Stress. J Insec Sci 21(1):10; 1–8
Singh PP, Varma GC (1986) Comparative toxicities of some pesticides to Chrysoperla carnea (Chrysopidae: Neuroptera) and Trichogrammabrasiliensis (Hymenoptera: Trichogrammatidae), two arthropod natural enemies of cotton pests. Agric Ecosyst Environ 15:23–30
Smith SM (1996) Biological control with Trichogramma: advances, successes, and potential of their use. Annu Rev Entomol 41:375–406
Snyder WE (2019) Give predators a complement: conserving natural enemy biodiversity to improve biocontrol. Biol Cont 135:73–82
Stark JD, Banken JO (1999) Importance of population structure at the time of toxicant exposure. Ecotox Environ Safety 42:282–287
Stark JD, Vargas R, Banks JE (2007) Incorporating ecologically relevant measures of pesticides effect for estimating the compatibility of pesticides and biocontrol agents. J Econ Entomol 100:1027–1032
Steiner H (1977) Standardized field test to measure side-effects of pesticides in the tree level. J Plant Dise Prot 84(3):164–166
Sterk G, Hassan SA, Baillod M, Bakker F, Bigler F, Blümel S, Bogenschütz H, Boller E, Bromand, B, Brun J, Calis JNM, Coremans-Pelseneer J, Duso C, Garrido A, Grove A, Heimbach U, Hokkanen H, Jacas J, Lewis G, Moreth L, Polgar L, Rovesti L, Samsoe-Peterson L, Sauphanor B, Schaub L, Stäubli A, Tuset JJ, Vainio A, de Veire M.V, Viggiani G, Viñuela E, Vogt H (1999) Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS-Working Group ‘‘Pesticides and Beneficial Organisms’’. BioControl 44:99–117
Suinaga FA, Picanc¸o M, Zanuncio JC, Bastos CS (1996) Seletividade fisiólogica de inseticidas a Podisus nigrispinus (Dallas, 1851) (Heteroptera: Pentatomidae) predador de lagartas desfolhadoras de eucalipto. Rev Árvore 20:407–414
Tabebordbara F, Shishehbora P, Ziaeea M, Sohrabi F (2020) Lethal and sublethal effects of two new insecticides spirotetramat and flupyradifurone in comparison to conventional insecticide deltamethrin on Trichogramma evanescens (Hymenoptera: Trichogrammatidae). J Asia-Pacif Entom 23:1114–1119
Thomson LJ, Hoffmann AA (2010) Natural enemies responses and pest control importance of local vegetation. Biol Cont 52:160–166
Torres JB, Bueno A de F (2018) Conservation biological control using selective insecticides – A valuable tool for IPM. Bio Cont 126:53–64
You Y, Lin T, Wei H, Zeng Z, Fu J, Liu X, Lin R, Zhang Y (2016) Laboratory evaluation of the sublethal effects of four selective pesticides on the predatory mite Neoseiulus cucumeris (Oudemans). Syst Appl Acarol 21(11):1506–1514
Yu SJ (1998) Selectivity of insecticides to the spined soldier bug (Heteroptera: Pentatomidae) and its lepidopterous prey. J Econ Entomol 81:119–122
van den Bosch R, Stern VM (1962) The integration of chemical and biological control of arthropods pests. Annu Rev Ent 7:367–386
Varma GC, Singh PP (1987) Effect of insecticides on the emergence of Trichogramma brasiliensis (Hymenoptera: Trichogrammatidae) from parasitized host eggs. Entomoph 32:443–448
Vieira A, Oliveira L, Garcia P (2001) Effects of conventional pesticides on the pre-imaginal developmental stages and on adults of Trichogramma cordubensis (Hymenoptera: Trichogrammatidae). Biocont Sci Technol 11:527–534
Wahengbam J, Raut AM, Mandal SK, Banu AN (2018) Efficacy of new generation insecticides against Trichogramma chilonis Ishii and Trichogramma pretiosum Riley. J Ent Zool Stud 6(1):1361–1365
Wajnberg E, Hassan SA (1994) Biological Control with Egg Parasitoids.CAB International, Wallingford, Oxon, UK, pp. 286
Way MJ (1986) The role of biological control in integrated plant protection. In: Franz JM (ed) Biological Plant and Health Protection. Gustav Fischer Verlag, Stuttgart, pp 289–303
Wang DS, He YR, Guo XL, Luo YL (2012) Acute toxicities and sublethal effects of some conventional insecticides on Trichogramma chilonis (Hymenoptera: Trichogrammatidae). J Econ Entomol 105(4):1157–1163
Wang D, Lü L, He Y, Shi Q, Wang G (2016) Effects of Insecticides on Oviposition and Host Discrimination Behavior in Trichogramma chilonis (Hymenoptera: Trichogrammatidae). J Econ Entomol 109(6):2380–2387
Wang D, Lü L, He Y (2017) Effects of insecticides on sex pheromone communication and mating behavior in Trichogramma chilonis. J Pest Sci 91(1):65–78
Willow J, Silva A, Veromann E, Smagghe G (2019) Acute effect of low-dose thiacloprid exposure synergised by tebuconazole in a parasitoid wasp. PlosOne 14(2):e0212456. https://doi.org/10.1371/journal.pone.0212456
Wobbrock JO, Findlater L, Gergle D, Higgins J (2011) The aligned rank transform for nonparametric factorial analyses using only ANOVA procedures. In Proceedings of the ACM Conference on Human Factors in Computing Systems (CHI '11), 143–146. URL: https://depts.washington.edu/aimgroup/proj/art/
Zucchi RA, Querino RB, Monteiro RC (2010) Diversity and hosts of Trichogramma in the New World, with emphasis in South America. In: Cônsoli FL, Parra JR, Zucchi RA (eds) Egg Parasitoids in Agroecosystems with Emphasis on Trichogramma. Springer, Dordrecht, pp 219–236
Acknowledgements
I gratefully acknowledged the Higher Education Commission (HEC) of Pakistan financial support under indigenous PhD fellowship. I also wish to offer special thanks to the administration of the Nuclear Institute of Food and Agriculture (NIFA), Tarnab, Peshawar (Pakistan) for permission and provision of laboratory facilities to conduct experiments. I am also very grateful to Dr John R Ruberson, Chairman Department of Entomology, University of Nebraska, Lincoln. Nebraska, USA for English editing of the manuscript. I am very thankful to Jos Feys, senior research fellow at the KU Leuven University (Catholic University of Leuven, Belgium) for statistical analysis of the data.
Funding
This study was funded by Higher Education Commission of Pakistan under the indigenous 5000 PhD fellowship program, Batch IV with research grant number (PIN) 074–3591-BM4-011.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I am the sole author of the manuscript and declares no conflict of interest with any person, institution or organization.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, M.A. Lethal and parasitism effects of selected novel pesticides on the immature stages of Trichogramma chilonis (Trichogrammatidae: Hymenoptera). Int J Trop Insect Sci 42, 1077–1093 (2022). https://doi.org/10.1007/s42690-021-00580-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00580-x