Abstract
Corcyra cephalonica (S.) commonly called rice meal moth or rice moth is an important stored-product pest all over the world. The larvae feed on broken grains of cereal, pulses, oilseeds, dried fruits, nuts, and spices by constructing the silken webs. The excessive use of pesticides and chemical compounds used for the management of this pest led to the development of resistance and also harms the environment. To overcome these problems in the recent years, green nanotechnology has emerged as a promising tool for pest control. The present study of the experiment was conducted at the Centre for Nanotechnology Laboratory, UAS, Raichur. The green nanoparticles of zinc, copper, and silica were biosynthesized from Spinach leaves; tulasi leaves and paddy husk respectively, and these nanoparticles were characterized by Zetasizer, UV–Vis spectroscopy, X-ray diffraction (XRD), and Scanning electron microscope (SEM). The biophysical characterization revealed that the zinc, copper, and silica nanoparticle has Spindle, spherical, and agglomerated spindle-shaped with a mean particle size of 87.94, 84.15 and 23.65 d. nm respectively. The pesticidal effects of these green nanoparticles were used as stored product insect protectants compared to malathion as a standard reference. Data obtained from different concentrations (250, 500, 750, 1000, 1250, and 1500 ppm) of zinc, copper, and silica green nanoparticles indicated that the increase in concentration and exposure period resulted in increase in larval mortality, pupal mortality and adult deformity. Among the different concentrations, 1500 ppm of zinc, copper, and silica nanoparticles proved to be superior. Similarly, of the different nanoparticles, silica nanoparticles excelled followed by zinc and copper nanoparticles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sorghum (Sorghum bicolor (L.) Moench) is a premier crop of the semi-arid tropics which ranks fourth after rice, wheat and maize and is a major staple food in several parts of the world. It is a dry land crop grown in Kharif and Rabi seasons for the utility as food, feed, forage and industrial raw material. Besides production constraints, the insect pest problem, improper sanitation and storing methods result in both quantitative and qualitative storage losses in sorghum. In storage, the losses are mainly due to insects, mites and pathogens (Kumar et al. 2018). Every year, one-third of the world grain crop is lost during storage; much of this is particularly due to insect attack.
The rice moth, Corcyra cephalonica (S.) is an important stored-product pest all over the world. It is probably one of the most catholic feeders among the storage pests which feeds on a wide variety of materials viz., rice, corn, cocoa, dried fruits like almonds, date palm, nuts, chocolates, biscuits, oilcake, coffee and other seeds and also when they become fully grown, larvae contaminate the grain by producing dense webbing containing their fecal material and cast skins. In addition to consumption, they spin dense silken fibers which web their fecal material, exuviae together with stored grains, and generally cause qualitative and quantitative losses and reduce seed germination (Attia et al. 2020).
Fumigants and residual insecticides are commonly used to combat stored grain pests. In recent years, consumer awareness of the health hazard from residual toxicity and the growing problem of insect resistance to these conventional insecticides have led the researchers to look for alternative strategies for stored grains protection. Nanoparticles represent a new generation of environmental remediation technologies that could provide a cost-effective solution to some of the most challenging environmental clean-up problems (Chinnamuthu and Murugesa 2009).
Green nanotechnology has goals to produce nanomaterials and products without harming the environment or human health and producing nano products that provide solutions to environmental problems. It uses existing principles of green chemistry and green engineering to make nanomaterials and nano-products without toxic ingredients at low temperatures using less energy and renewable inputs (Gnanasangeetha and Thambavani 2014). ‘Green synthesis’ or ‘Biogenic synthesis’ of nanoparticles shows better advancement over chemical and physical methods as it is lesser toxic, cost-effective and environmentally friendly (Vidya et al. 2013).
Little research has been carried out to investigate the toxic effects of green nanoparticles on insects, especially on storage pests. The present-day practice of treating seeds with malathion and fumigation are indispensable but inevitable tools. In this regard, a novel approach like the use of nanoparticles synthesized from botanicals, against major stored grain pests is a new avenue.
Materials and method
Studies on the bioefficacy of green synthesized zinc, copper, and silica nanoparticles on important storage pest Corcyra cephalonica were carried out during 2017–18 at Centre for Nanotechnology, UAS, Raichur, Karnataka. Raichur is situated in the northern dry region (Zone 2) of Karnataka located between 16° 15′ N latitude and 77° 20′ E longitude with 398.37 m above mean sea level. The spinach leaves and tulasi leaves were collected from University campus and rice husk was collected from rice mill and sorghum seeds (M35-1) were collected from seed unit, UAS, Raichur and Zinc nitrate hexahydrate [Zn(NO3)2.6H2O], Copper sulphate pentahydrate (Cu SO4 5H2O) and Silicon dioxide (SiO2) chemicals were procured from M/s. High Media, Bangalore.
Biosynthesis of zinc oxide nanoparticles from spinach leaves
The spinach leaves were washed thoroughly with distilled water and dried using a solar tunnel dryer. The dried leaves were ground using a pulverizer to make into a fine powder and passed through a 100 mesh sieve (150 µm). Five grams of dried powder was added to 100 ml of ethanol and kept for 24 h in a 250 ml conical flask and filtered through filter paper (Whatman No.1). The filtrate was stored at 4 ˚C for further experiments. The leaf extract of spinach (50 ml) was boiled at 60–80 ˚C using a magnetic stirrer. Zinc nitrate hexahydrate [Zn (NO3)2.6H2O] was used as a precursor. One mM zinc nitrate solution was prepared using distilled water. The solution was added to the leaf extract when the temperature reached 60 ˚C and boiled for 30 min or until color changed. A change in the color from dark green to pale yellow indicates the formation of zinc oxide nanoparticles (Fig. 1a) (Amrita et al. 2015).
Biosynthesis of copper oxide nanoparticles from tulasi leaves
Tulasi leaves were cleaned and washed thoroughly with distilled water and subsequently dried in solar tunnel dryer at 40 ˚C for 2 days to remove moisture completely. Dried leaves were ground using a mixer grinder to make into a fine powder. The obtained powder was passed through a 20 mesh sieve (840 µm) to get a uniform size. 10 g of uniformly sized powder was taken in a beaker along with 100 ml of deionized water and it was allowed to boil at 60˚C for 30 min. under reflux condition and cooled down to room temperature. The prepared solution was double filtered through Whatman No.1 filter paper thereby powdered leafy materials were filtered out and a clear solution was obtained. The filtrate was stored at 4˚C for further experiments. The plant extract of tulasi leaves (25 ml) was mixed with 100 ml of 1 mM aqueous copper sulphate pentahydrate (CuSO4.5H2O) solution under the continuous stirring. After complete mixing of leaf extract with a precursor, the mixture was kept for incubation at 31˚ C for 24 h. A change in the color from light green to dark green was observed and this indicated the formation of copper nanoparticles (Fig. 1b) (Mekal et al. 2016).
Biosynthesis of silicon oxide nanoparticles from rice husk
Rice husk was washed thoroughly with potable water to remove the soluble particles, dust and other contaminants. The cleaned rice husk was dried in a hot air oven at 110 °C for 24 h. The dried rice husk was refluxed with an acidic solution of 1 N HCl at 85 °C for 90 min. The sample was cooled at room temperature and kept intact for about 24 h. Then the sample was decanted and thoroughly washed with warm distilled water until the rinse became free from acid. The wet refluxed rice husk was subsequently dried in a hot air oven at 110 °C for 24 h. The dried rice husk was subjected to heat treatment in the muffle furnace at 700 °C for 2 h to obtain the ash.
A sample of 20 g ash was stirred in 160 ml of 2.5 M NaOH solution. The solution was heated in a covered beaker at 90 °C for 3 h by stirring constantly and filtered through filter paper (Whatman No. 4). The obtained viscous, transparent and colorless filtrate solution was allowed to cool at room temperature and 10 M H2SO4 was then added under constant stirring at controlled conditions until it reached to pH 2, then NH4OH was used to adjust pH level up to 8.5 and was allowed to stand at room temperature for 3 h. White silica precipitate was washed repeatedly with the distilled water until the filtrate was completely free from alkali. The silica was dried in a hot air oven at 105 °C temperature for 24 h (Fig. 1c) (Raffie et al. 2012).
Characterization of biosynthesized zinc, copper and silica nanoparticles
Biosynthesized green nanoparticles were subjected to characterization for the identification of its size, morphology and functional group. These nanoparticles were characterized by using Zetasizer (Malvern, ZETA Sizer, nano383 issue 5.0, England) was used (dynamic light scattering) to study the average particle diameter (nm), UV–Visible spectrophotometer measures the extinction (scatter + absorption) of light passing through a sample state and refractive index near the nanoparticles surface, which makes UV–Visible nanoparticles have unique optical properties that are sensitive to the size, shape, concentration, agglomeration for identifying, characterizing and studying the nanoparticles. X-ray diffraction (XRD) is a rapid analytical technique primarily used for phase identification of nanoparticles and the scanning electron microscope (SEM) image of the test sample surface is obtained by scanning it with a high energy beam of electrons in a vacuum chamber.
Maintenance of pure culture of rice moth on broken sorghum seeds
Stored grain insect pest rice moth was collected from the biological control laboratory of UAS, Raichur, and the culture was further maintained in plastic jars of two kg capacity containing broken sorghum seeds. The larvae that emerged from this culture were used throughout the period of experimentation.
Bioassay studies for rice moth on sorghum seeds
Effects of biosynthesized zinc, copper and silicon green nanoparticles on larvae of Corcyra cephalonica were determined by contact toxicity assay at seven doses of 125, 250, 500, 750, 1000, 1250 and 1500 ppm of nanoparticle kg-1 sorghum seeds. The experiments were carried out in Completely Randomized Design with three replications each consisted of 10 larvae of Corcyra cephalonica in small plastic screw-capped jars containing 100 g of broken sorghum seeds in each jar were treated individually with nanoparticles and the plastic box were closed with a muslin cloth and fastened tightly with the help of rubber band. Then, the jars were shaken manually for approximately 60 s to achieve equal distribution of nanoparticles on broken sorghum seeds. The pesticidal effects of these green nanoparticles were used as stored product insect protectants compared to malathion as a standard reference. In one additional set, nanoparticle was not mixed with broken sorghum seeds and this set served as control. After that 10 larvae of rice moth were introduced into each jar. This procedure was repeated until mortality data ranging from 20 to 80 percent was obtained. The treatment including control was replicated thrice and LC50 was calculated by probit analysis. All bioassays were performed at 30 ± 1 ˚C and 65 ± 5% RH. Insect mortality was checked after 1, 3, 5, 7, and 10 days after treatment.
Data collection and statistical analysis
For direct toxicity tests, insect mortalities were recorded at 1, 3, 5, 7, and 10 days interval. Data was corrected by Abbot's formula and analyzed statistically using ANOVA 2 and MSTAT-C. The mean values were adjusted using the Duncan Multiple Range Test. Median lethal doses were calculated using probit analysis with log10 transformation of concentration of green nanoparticles. Data for a lethal time were corrected using Abbott formula and analyzed using the method of Finney.
Result
The results obtained from the characterization of zinc, copper and silica nanoparticles for identification of their size and morphology are discussed below in terms of dynamic light scattering (Zetasizer) analysis, UV–visible spectrophotometer analysis and scanning electron microscopy (SEM).
Characterization of biosynthesized zinc, copper and silica nanoparticles
Dynamic light scattering (Zetasizer) for particle size analysis
The zinc oxide nanoparticles were characterized for average particle diameter from the intensity distribution analysis by using zetasizer. The results revealed that the average particle diameter of Zinc oxide nanoparticles was 20.67 nm as seen in Fig. 2a.
Similarly, the average size of biosynthesized copper nanoparticles was found to be 82.41d.nm as seen in Fig. 2b.
The results of Zetasizer revealed that the average particle diameter of biosynthesized silica nanoparticles was 26.19 nm as seen in Fig. 2c.
UV–visible spectrophotometer for absorbance analysis
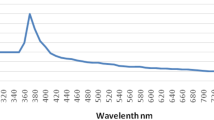
The reduction of zinc ions into zinc oxide nanoparticles was observed as the color changed from dark green to pale yellow. The reductions of Zinc oxide nanoparticles were characterized by UV–Visible spectrophotometer. This analysis showed an absorbance peak at 375.4 nm, which was specific for Zinc oxide nanoparticles.
Similarly, The UV- Visible spectrum of Copper nanoparticles recorded a maximum absorption band edge at 560 nm.
The UV–Visible spectrum of silica nanoparticles recorded a maximum absorption band edge of 310 nm in 1.95 absorbance.
X-ray Diffraction (XRD) analysis
The X-ray diffraction pattern of the zinc oxide nanoparticles showed broad hallow at 2θ = 30–40° which confirms the zinc nanoparticle (Fig. 3a).
Similarly, the copper nanoparticles showed the broad halo at about 2θ = 20–40° region which confirms the amorphous structure of copper nanoparticles as seen in Fig. 3b.
The X-ray diffraction pattern of silica nanoparticles is characterized by a broad halo band of absorbance at about 2θ = 15–25° region which confirms the amorphous structure of the biosynthesized silica nanoparticles as seen in Fig. 3c.
Scanning electron microscope (SEM) for surface morphology analysis
The morphological features of Zinc oxide nanoparticles were characterized by scanning electron microscope. The Zinc oxide nanoparticles formed were spindle in shape (Figure 4a).
Similarly, SEM analysis showed that the copper nanoparticles were spherical in shape (Fig. 4b). Silica nanoparticles were in the agglomerated form with spherical shape (Fig. 4c).
To standardize the dose of zinc, copper and silica green nanoparticles against rice moth on sorghum seeds
Effect of zinc green nanoparticle on larval mortality of rice moth in sorghum seeds
Among the different concentration of zinc oxide green nanoparticles evaluated against the rice moth, zinc oxide green nanoparticles @1500 ppm at one day after treatment showed 30 percent mortality of larva compared to 46.66 percent mortality in malathion 5D @ 1%, but more than 50 per cent of larval mortality was recorded in zinc oxide green nanoparticles @ 1250, 1500 ppm which showed 56.66 and 66.66 percent mortality at five days after treatment. However, significantly highest larval mortality of cent per cent was observed in zinc oxide green nanoparticles @ 1500 ppm which was on par with chemical check malathion 5D @ 1% at ten days after treatment. Whereas lowest larval mortality (33.33%) was noticed at zinc oxide green nanoparticles @ 125 ppm followed by 150 ppm (53.33%) at ten days after treatment as seen in Table 1.
Effect of copper green nanoparticle on larval mortality of rice moth in sorghum seeds
The results of the present study elucidates the variation in the larval mortality of C. cephalonica due to the different concentrations of copper nanoparticle and time of exposure. One day after treatment copper green nanoparticle @ 1500 ppm showed 33.33 percent mortality of larva compared to 50.00 percent larval mortality in malathion 5D @ 1%, but five days after treatment copper green nanoparticle @ 1500 ppm recorded 76.66 percent larval mortality which was followed by its next lowest dosage @ 1250 ppm (63.33%). Whereas significantly highest larval mortality (100%) was noticed at ten days after treatment which was statistically on par with chemical malathion 5D @ 1%. However significantly lowest larval mortality (46.66%) of C. cephalonica was observed at copper green nanoparticle @ 125 ppm followed by copper green nanoparticle @ 150 ppm (63.33%) at ten days after treatment. These treatments proved to be superior over the control (Table 2).
Effect of silica green nanoparticle on larval mortality of rice moth in sorghum seeds
At one day after treatment Silica green nanoparticle @1500 ppm exhibited 36.66 percent mortality followed by its next lowest dosage @1250 ppm (33.33%). Whereas seven days of treatment no mortality was recorded in untreated check, while significantly highest mortality (100.00%) was recorded in Malathion 5D @ 1% dust followed by silica nanoparticle @1500 ppm and 1250 ppm with 93.33% and 86.66% larval mortality respectively. The larval mortality of the rice moth significantly increased as concentrations and exposure periods of silica green nanoparticles increased. Cent percent mortality of larva was recorded from malathion 5D @ 1% and silica green nanoparticle @1500 ppm and 1250 ppm. There was no mortality recorded in untreated check as seen in Table 3.
Response–mortality relationship concerning different green nanoparticles against C. cephalonica
It was found that when the larvae of C. cephaolnica were exposed to green nanoparticles for different concentrations and different time periods, the LC50 values for C. cephalonica larva was 1666, 1449 and 1160 ppm for an exposure period of 48 h in zinc, copper and silica nanoparticles. For an exposure period of 72 h, the LC50 values for larva were 1060, 1204, and 1996 ppm while at 120 h, the LC50 values for an adult were 685, 566, and 412 ppm in zinc, copper, and silica green nanoparticles (Table 4).
Discussion
Characterization of biosynthesized zinc, copper and silica nanoparticles
The results obtained from the characterization of zinc oxide nanoparticles for identification of their size and morphology are discussed below in terms of dynamic light scattering (Zetasizer) analysis, UV–visible spectrophotometer analysis, and scanning electron microscopy (SEM).
The zinc oxide nanoparticles were characterized for average particle diameter from the intensity distribution analysis using zetasizer. The results revealed that the average particle diameter of Zinc oxide nanoparticles was 20.67 nm. In accordance with the present result Supraja et al. (2015) obtained 20.3 d.nm. The variation in particle size was probably due to change in climatic conditions during biosynthesis (Zainala et al. 2013). Similarly, the average size of biosynthesized copper nanoparticles was found to be 82.41d.nm. This is in agreement with previous findings which suggested that, as the reaction temperature increases, both synthesis rate and conversion of copper nanoparticles increased. The average particle size decreased from 110 nm at 25 0c to 45 nm at 95 0c (Lee et al. 2011). The results of Zetasizer revealed that the average particle diameter of biosynthesized SNPs was 26.19 d. nm. The results are in line with the findings of Das et al. (2014).
The reductions of Zinc oxide nanoparticles were characterized by UV–Visible spectrophotometer. This analysis showed an absorbance peak at 375.4 nm, which was specific for Zinc oxide nanoparticles. The result is in agreement with the findings of Awwad et al. (2014) with 374 nm. Similarly, The UV- Visible spectrum of Copper nanoparticles recorded a maximum absorption band edge at 560 nm. Sarnyadevi et al. (2014) found that the copper nanoparticles formation was confirmed from the peak at 531 nm with the UV-range of 560–640 nm, and hence it is evident with the current result. The UV–Visible spectrum of silica nanoparticles recorded a maximum absorption band edge of 310 nm in 1.95 absorbances. These optical features are similar to those obtained in previous reports and attributed to the Si–O-Si bond confirming the presence of silica nanoparticles (Djangang et al. 2015).
The X-ray diffraction pattern of the zinc oxide nanoparticles showed broad hallow at 2θ = 30–40° which confirms the zinc nanoparticle. Similarly, the copper nanoparticles showed the broad halo at about 2θ = 20–40° region which confirms the amorphous structure of copper nanoparticles. The results are in line with Fatma et al. (2017) who observed peaks at 2θ values of 42.47 0. The X-ray diffraction pattern of silica nanoparticles is characterized by a broad halo band of absorbance at about 2θ = 15–25° region which confirms the amorphous structure of the biosynthesized silica nanoparticles. Similar results were recorded by (Djangang et al. 2015) who reported the amorphous structure of the silica nanoparticles from rice husk.
The morphological features of Zinc oxide nanoparticles were characterized by scanning electron microscope. The Zinc oxide nanoparticles formed were spindle in shape. Similar results reported by Noorjahan et al. (2015) for zinc nanoparticles. Similarly, SEM analysis showed that the copper nanoparticles were spherical in shape. The results are in line with Hariprasad et al. (2016). Silica nanoparticles were in the agglomerated form with a spherical shape supported by findings of Djangang et al. (2015).
To standardize the dose of zinc, copper and silica green nanoparticles against rice moth on sorghum seeds
Among the different concentration of zinc oxide nanoparticle tested against rice moth, the dosage @1500 ppm proved to be superior wherein larval mortality varied in the range of 30 to 100 percent from one to ten days after treatment, whereas highest mortality occurred in malathion 5D @ 1% which was superior overall treatments and there was no larval mortality noticed in control.
The results of the present study showed the variation in the larval mortality of C. cephalonica due to the different concentrations of copper nanoparticle and time of exposure. Treatment with higher concentration i.e., copper nanoparticle at 1250 ppm and @ 1500 ppm recorded highest larval mortality which ranged from 30.00 to 93.33 and 33.33 to 100 percent from 1st to 10th day after treatment respectively and 100 percent mortality was noticed in malathion 5D @ 1% on 10th after treatment and there was no larval mortality recorded in control.
Since there are no reviews on the effect of zinc and copper nanoparticle against the larval mortality of rice moth, related reviews of different nanoparticles tested on other lepidopterans are discussed.
Comparable to present work Wahab and Anwar (2015) reported that nanoparticles at 0.01 g quantity, caused mortality and malformation of H. armigera larvae. Mortality recorded was 100% and 73.33% in the case of ZnO and CuO nanoparticles respectively. While that ratio decreased to 86.67% in the case of ZnO + Vertimec and increased to 100% with Vertimec + CuO, however Vertimec itself caused 33.33%. Siva and Santosh Kumar (2015) reported the Larvicidal activity of aqueous extract of A. indica and synthesized Ag NPs showed 54.82, 68.16, 87. 69% and 64.04, 86.38, and 100% mortality against H. armigera at 30, 40, and 50 mg/mL, respectively, and the activity was statistically significant compared with control.
The results showed the variation in the larval mortality of C. cephlaonica due to the different concentrations of silica green nanoparticles and time of exposure. Treatment with higher concentration i.e., silica nanoparticle at 1250 ppm and silica nanoparticle at 1500 ppm recorded highest larval mortality which ranged from 33.33 to 100.00% and 36.66 to 100% from 1st to 10th day after treatment and 100 percent mortality was noticed in Malathion 5D @ 1% on 10th day after treatment and there was no larval mortality occur in control.
The present findings are similar to the results of Vani and Brindha (2013) who reported that the mortality rate increased with an increase in exposure time and concentration of silica nanoparticle when grains (Khumbu) admixed with 10 mg and 80 mg of silica nanoparticle achieved better control in C. cephalonica. Eight days of exposure to silica nanoparticle IV caused 100 percent mortality than the other treatments which was significantly superior over the untreated control. In support of present finding the effect of nanoparticles on other lepidopteran pests, Debnath et al. (2011) found that SiNPs was much more effective on the second instar larvae of Spodoptera litura F. (Lepidoptera: Noctuidae) especially, hydrophilic SiNPs at 1.25 g/kg which killed all the larvae within 24 h after treatment. Chakravarthy et al. (2012) examined the potential adverse effects of Ag and TiO2 nanoparticles on S. litura in the laboratory. Their data of second instar S. litura larvae indicated that TiO2NPs showed a maximum of 73.79% larval mortality (Fig. 5a) at 2400 ppm and the least was 18.50 percent at 150 ppm.
Silica nanoparticles do not affect the looseness and bulk density of grain mass like DE even with the highest dose used in our bioassay. This was also evident from our experiment, where nano-silica was effective because of their enormously increased exposed surfaces which could interact with the insect cuticle. Damage occurs to the insects’ protective wax coat on the cuticle, both by sorption and abrasion. Biosynthesized silica nanoparticle act with the same efficacy on Sitophilus oryzae.
At the lower concentration of these nanoparticles treated with the broken sorghum seeds, the behavior of the rice moth larvae was normal on the first day. With increase in concentration and number of days there was reduction in feeding of rice moth larvae, larvae had sluggish movements and turned to black color three days after treatment. On the seventh day after treatment in the higher concentration, the larvae became stiff and brittle. Whereas in the lower concentration more than 30 percent of larvae went for pupation. The pupal mortality (Fig. 5b) and malformed pupa were also noticed as the concentration of the nanoparticles increased and pupa also turned to black color and there was no adult emergence of rice moth in the higher concentration, similarly in the lower concentration malformed and deformed emergence of the adult moths were noticed. There was no larval mortality, pupal deformity and adult deformity noticed in the untreated control.
Response–mortality relationship concerning different green nanoparticles against C. cephlaonica
It was found that when the larvae of C. cephaolnica were exposed to green nanoparticles for different concentrations and different periods, the LC50 values for C. cephalonica larva were 1666, 1449, and 1160 ppm for an exposure period of 48 h in zinc, copper and silica nanoparticles. For an exposure period of 72 h, the LC50 values for larva were 1060, 1204, and 1996 ppm while at 120 h, the LC50 values for an adult were 685, 566, and 412 ppm in zinc, copper and silica green nanoparticles. The present findings are similar to Vani and Brindha, (2013) who reported the LC50 value of silica nanoparticles on the larva of rice moth after 48 h was 200 ppm on bajra seeds.
Conclusion
The biosynthesis of Zinc, copper and silica green nanoparticles from spinach leaves, tulasi, and rice husk is an environmentally friendly, simple and efficient route for the synthesis of nanoparticles which could be an alternative to chemical and physical methods, different analytical characterization techniques such as Zetasizer, UV–Visible spectrophotometer, XRD and SEM are used for the confirmation of particle size, the surface morphology of these green nanoparticles. Zinc, copper, and silica green nanoparticles showed potential entomotoxicity against rice moth. Among different concentrations of green nanoparticles, 1500 ppm proved to be effective by recording cent percent mortality of larvae, pupae, and no emergence of adults and without any seed damage and weight loss during the storage condition. This study could lead to open up newer pathways of using biosynthesized green nanoparticles for the control of stored grain insect pests, which could be an alternative for insecticidal agents.
References
Amrita R, Reena SL, Mohammad J, Kapil L (2015) Antibacterial activity of Zinc oxide nanoparticles prepared from Brassica oleraceae leaves extract. Int J Adv Res 3:322–328
Attia RG, Rizk SA, Hussein M, Fattah HMA, Ma’moun KMM, SA (2020) Effect of cinnamon oil encapsulated with silica nanoparticles on some biological and biochemical aspects of the rice moth, Corcyra cephalonica (Staint.)(Lepidoptera: Pyralidae). Annal Agri Scie 65:1–5
Awwad AM, Albiss B, Ahmad AL (2014) Green synthesis, characterization and optical properties of Zinc oxide nanosheets using Olea europea leaf extract. Adv Mat Lett 5:520–524
Chakravarthy AK, Chandrashekharaiah SB, Kandakoor A, Bhattacharya K, Hanabala KG, Ramesh P (2012) Bio efficacy of inorganic nanoparticles CdS, nano-Ag and nano-TiO2 against Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae). Current Biotica 6:271–281
Chinnamuthu CR, Murugesa BP (2009) Nanotechnology and Agroecosystem. Madras Agric J 96:17–31
Das D, Yang Y, Brien JSO, Breznan D, Nimesh S, Bernatchez S, Hill M, Sayari A, Vincent R, Kumarathasan P (2014) Synthesis and physicochemical characterization of mesoporous SiO2 nanoparticles. J Nanomater 62:11–12
Debnath N, Das S, Seth D, Chandra R, Bhattacharya SC Goswami A (2011) Entomotoxic effect of silica nanoparticles against Sitophilus oryzae (L.). J Pest Sci 84:99–105
Djangang CN, Mlowe S, Njopwouo D, Revaprasadu N (2015) One-step synthesis of silica nanoparticles by thermolysis of rice husk ash using nontoxic chemicals ethanol and polyethylene glycol. J Applicable Chem 4:1218–1226
Fatma S, Kalainila P, Ravindran E, Renganathan S (2017) Green synthesis of copper nanoparticle from Passiflora foetida leaf extract and its antibacterial activity. Asian J Pharm Clin Res 10:79–83
Gnanasangeetha D, Thambavani SD (2014) Facile and eco-friendly method for the synthesis of Zinc oxide nanoparticles using Azadirachta and Emblica. Int J Pharm Sci Res 5:2866–2873
Hariprasad S, Bai GS, Santhoshkumar J, Madhu CH, Sravani D (2016) Green synthesis of copper nanoparticles by Arevalanata leaves extract and their antimicrobial activities. Int J Chem Tech Res 9:98–105
Kumar KM, Tambe VJ, Rehaman SK, Choudhuri BN, Thakur KD (2018) Effect of different diets on the biology of rice moth, Corcyra cephalonica (Stainton). J Entomol Zool Stud 6:251–254
Lee HJ, Lee G, Jang NR, Yun JH, Song JY, Soo B (2011) Biological synthesis of copper nanoparticles using plant extract. Bioprocess Bio Syst Eng 1:371–374
Mekal J, Rajan MR, Ramesh R (2016) Green synthesis and characterization of copper nanoparticles using tulsi (Ocimum sanctum) leaf extract. Indian J Res 5:14–16
Noorjahan CM, Shahina SKJ, Deepika T, Rafiq S (2015) Green synthesis and characterization of Zinc oxide nanoparticles from Neem (Azadirachta indicia). Int J Sci Eng Technol Res 4:5751–5753
Rafiee E, Shahebrahimi S, Feyzi M, Shaterzadeh M (2012) Optimization and characterization of nanosilica produced from rice husk (common waste material). Int Nano Lett 2:1–8
Saranyaadevi K, Subha V, Ernest RS, Renganathan S (2014) Synthesis and characterization of copper nanoparticle using Capparis zeylanica leaf extract. Int J Chem Tech Res 8:4533–4541
Siva C, Santoshkumar M (2015) Pesticidal activity of eco-friendly synthesized silver nanoparticles using Aristolochia indica extract against Helicoverpa armigera (Lepidoptera: Noctuidae). Int J Adv Scie Res 5:2249–9954
Supraja N, Prasad NV, Krishna TG, David E (2015) Synthesis, characterization and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated Zinc oxide nanoparticles. Appl Nanosci 6:581–590
Vani C, Brindhaa U (2013) Silica nanoparticles as nanocides against Corcyracephalonica(s.), the stored grain pest. Int J Pharm Bio Sci 4:1108–1118
Vidya C, Hiremath S, Chandraprabha MN, Antonyraj LMA, Gopal IV, Jain A, Bansal K (2013) Green synthesis of ZnO nanoparticles by Calotropis gigantean. Int J Curr Eng Technol 1:118–120
Wahab RA, Anwar EM (2015) The effect of direct and indirect use of nanoparticles on cotton leafworm, Spodoptera littoralis. Int J Chemi Bio Sci 1:17–24
Zainala NA, Shukor SRA, Wabb HAA, Razak KA (2013) Study on the effect of synthesis parameters of silica nanoparticles entrapped with rifampicin. Chem Eng 32:432–440
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Biradar, W., Nadagouda, S., Aralimarad, P. et al. Entomotoxic effect of green nanoparticle an alternate strategy for stored grain pest management. Int J Trop Insect Sci 41, 2829–2840 (2021). https://doi.org/10.1007/s42690-021-00465-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00465-z