Abstract
Efficacy of Alstonia boonei powders and extracts against Dermestes maculatus (Coleoptera: Dermestidae) infesting stored smoked dried catfish (Claria gariepinus) were investigated in the laboratory. The products were tested at 2.5 g, 5.0 g, 7.5 g, 10 g, 12.5 g and 15 g and 1%, 2%, 5%, 10%, 15% and 20%/100 g of smoked catfish respectively. The ability of the plant to protect smoked catfish in terms of mortality rates, number of eggs laid and adult emergence were evaluated. All the tested plant parts caused significant mortality (P < 0.05) of adult D. maculatus. Alstonia boonei stem bark powder was the most potent, causing 100% mortality at 7.5 g of 8 days post exposure, followed by leaf powder which evoked 87.5% mortality after 10 days of exposure. For the extracts, adult mortality of D. maculatus was positively correlated with dose and exposure duration. Alstonia boonei product extracts significantly reduced the development of D. maculatus compared to the control. Stem bark extract significantly prevented oviposition. The use of A. boonei stem bark is advocated for the control of D. maculatus infestation. Further study is needed to characterize the chemical constituents and understand its mode of action with the likely formulation into herbal insecticide as alternative to synthetic insecticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fish is one of the cheapest source of animal protein used to reduce cholesterols that cause high blood pressure amongst elders (Ileke et al. 2020a). Fish consumption provides an important nutrient to a large number of people globally and thus makes a very substantial contribution to human nutrition (Fasakin and Aberejo 2002; Azam et al. 2004; Akinwumi 2011; Ajayi et al. 2019; Jangampalli 2019; Ileke et al. 2020a). Smoked fish is a highly preferred item of many traditional dishes in Nigeria and it is a condiment that greatly enriches the flavour of various dishes and a good alternative to fresh fish, which in many places is not readily available (Obiakor et al. 2013; Adesina et al. 2016; Ajayi et al. 2019). Smoke-dried catfish consumption have also becomes a better alternative in reducing cholesterols that cause high blood pressure amongst elders (Ileke et al. 2020a). In Nigeria and many other developing countries, fish constitutes 50% of total animal protein intake compared to other protein sources (Adesina et al. 2016). Owing to its nutritional and health benefits, fish is readily available on the menu table of most households irrespective of their socio-economic status, age and religious background (Adesina et al. 2014). However, the enormous economic, nutritive and health benefits of smoke-dried catfish (Clarias gariepinus) are threatened by insect infestation (Odeyemi et al. 2000; Adesina et al. 2014, 2016; Ajayi et al. 2019; Ileke et al. 2020a). Dermestes maculatus is considered to be one of the major and serious pests of smoked fish, hides and skins (Odeyemi and Daramola 2000; Ileke et al. 2020a). About 50% losses in smoked fish products during storage had been attributed to D. maculatus infestation which eat away the fish muscles and consequently reduce the nutritive quality and market price of fish (Osuji 1974; Owoade 1993; Lale and Sastawa 1996; Odeyemi et al. 2000) and as well making it unfit for consumption.
The need to protect smoked fish from insect infestation is imperative considering its crucial role in ensuring food security and income generation. However, in the developing countries fish handlers did little or nothing to safeguard infestation of smoked dried fish. The health risk linked with application of synthetic insecticides and the less susceptibility of D. maculatus larvae and adults to synthetic insecticides make their use an unpopular control agent (Amusan and Okorie 2002; Onu and Baba 2003).
In view of synthetic insecticide drawback, there has been renewed interest worldwide to screen aromatic and medicinal plant materials for its insecticidal properties as alternatives to overdependence on synthetic insecticide in the control of storage insect pests (Idoko and Ileke 2020; Ileke et al. 2020b). Over the past two decades, various researchers have documented the insecticidal potentials of various plant materials for the management of D. maculatus (Odeyemi et al. 2000; Akinwumi et al. 2006; Jose and Adesina 2014; Adesina et al. 2014, 2016; Nwosu et al. 2018; Ajayi et al. 2019; Ileke et al. 2020a).
Alstonia boonei De Wild (Apocyanaceae) is an African evergreen deciduous tree that shed leaves annually. Height of the plant is about 45 m, and its trunk is 1.2 m in diameter (Ileke 2019). The plant claimed to have medicinal properties in some cultures and climes (Moronkola and Kunle 2012). In traditional African medicine, A. boonei is used widely for the treatment of malaria, fever, intestinal helminths, rheumatism, hypertension (Asuzu and Anaga 1991; Terashima 2003; Betti 2004; Abel and Busia 2005; Majekodunmi and Odeku 2009); for the treatment of chronic diarrhea and dysentery, fever, pain, intestinal disorders and as an antidote for Strophanthus poison (Amole and Ilori 2010). The extracts of the stem bark is commonly used to treat malaria and is listed in the African pharmacopoeia as an anti-malaria drug (Olajide et al. 2000). It had been reported that a liquid made from the stem bark and fruit is drunk once daily to treat impotence (Majekodunmi et al. 2008); rheumatism, reversible antifertility (Raji et al. 2005), and hypertension (Olajide et al. 2000; Abel and Busia 2005; Betti 2004; Terashima 2003). It also an anti-venom against snake bite and antidote against arrow poisoning (Moronkola and Kunle 2012). The insecticidal activity of A. boonei has been reported recently against Sesamia calamistis Hampson (Lepidoptera: Noctuidae) (Oigiangbe et al. 2007a), Maruca vitrata (Lepidoptera: Pyralidae) (Oigiangbe et al. 2007b) and Anopheles mosquito larvae (Omoya et al. 2012). Ileke et al. (2012, 2013, 2014); Ileke and Arotolu (2018); Ileke 2019) reported the effectiveness of A. boonei in the management of cowpea bruchid (Callosobruchus maculatus). Ileke (2019) reported the efficacy of A. boonei stembark oil as a long-term storage protectant against cowpea bruchid (C. maculatus). This study was conceived to investigate the insecticidal potential of A. boonei leaf, Stembark, root powders and extracts on adult mortality and reproductive performance of D. maculatus (oviposition, larva and adult emergence) on smoked dried catfish (Claria gariepinus).
Materials and methods
Dermestus maculatus culture
The initial source of D. maculatus culture used for this study was obtained from infested smoked C. gariepinus collected from smoked dried fish stand at Erekesan market, Akure, Ondo State, Nigeria. Adult and larvae D. maculatus were introduced into 2 L plane glass kilner jar containing dried fish. The jars were covered with muslin cloth and placed in an insect rearing cage at ambient temperature of 28 + 2 °C, 75 + 5% relative humidity and 12 h D:L. Newly emerged larvae (0–3 days) were removed from stock culture and placed on fresh uninfected smoked fish. The parent adult insects were removed after 2 - 3 weeks oviposition period. In order to promote oviposition, water was supplied by introducing cotton soaked into the breeding cage (Odeyemi and Daramola 2000; Philip-Attah 2019). Subsequent generation was used for further bioassays.
Collection and preparation of catfish, C. gariepinus
Catfish (C. gariepinus) weighing about 100 g after smoking, free of salt and seasoning were obtained from Hatchery Research Laboratory, Department of Animal and Environmental Biology, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria. The smoked dried fish were sterilized by re-heating at 40 °C for one hour in a hot air oven (Gallenkamp Oven) in the laboratory (Atijegbe 2004; Adesina et al. 2016), and allow to cool at room temperature in the laboratory in other to prevent mouldiness (Adedire et al. 2011).
Collection and preparation of Alstonia boonei powders
The leaf, stem bark and root of A. boonei were harvested from a farm at Aponmu, Akure, Ondo State, Nigeria. These plant parts were brought into the laboratory, washed thoroughly with clean running water and air-dried for four weeks. Thereafter, the plant parts were pulverized separately into fine powder using an electric blender (Supermaster ®, Model SMB 2977, Japan). Subsequently, the powders were sieved (perforation of 1 mm2), kept separately in sealed plastic containers and stored in a refrigerator at 4 °C until use.
Preparation of A. boonei extracts
Alstonia boonei leaf, stem bark and root powders were extracted using absolute n-hexane as solvent. The use of n-hexane to extract A. boonei for toxicity test against Callosobruchus maculatus have been established (Ileke et al. 2013; Ileke and Arotolu 2018; Ileke 2019). Four hundred grammes (500g) of A. boonei leaf, stem bark and root powders were soaked separately in an extraction bottle containing 800 ml of absolute n-hexane. The mixture was stirred at an interval of 6 h with a glass rod and extraction was terminated after 72 h (Ileke et al. 2016). Filtration was carried out using a double layer of Whatman No. 1 filter papers and solvent evaporated using a rotary evaporator at 30 to 40 °C with rotary speed of 3 to 6 rpm for 8 h (Udo 2011). The resulting extracts were air dried. From this stock solution, different extract concentrations of 1, 2, 5, 10, 15 and 20% were prepared separately as follows: 1% concentration was made by diluting 0.1 ml of extract in 9.9 ml of n-hexane; 2% concentration was made by diluting 0.2 ml of extract in 9.8 ml of n-hexane t; 5% concentration was made by diluting 0.5 ml of extract in 9.5 ml of n-hexane; 10% concentration was made by diluting 1 ml of extract in 9 ml of n-hexane; 15% concentration was made by diluting 1.5 ml of extract in 8.5 ml of n-hexane. Similarly, 20% concentration was made by diluting 1 ml of extract in 8 ml of n-hexane (Ashamo and Akinnawonu 2012).
Phytochemical screening of the plants
Qualitative analysis
Alkaloid determination
Alkaloid qualitative determination was carried out using the methods described by Trease and Evans (1985); Hikino et al. (1984). 0.5 g of A. boonei parts extract was added to 5 ml of 1% aqueous hydrochloric acid on a steam water bath. This was stirred regularly before filtration. Few drops of Dragendorf reagent was added to filtrate (1 ml). A blue black turbidity was formed which indicate the presence of alkaloid.
Saponin determination
Analytical qualitative determination of saponin was carried out using the methods reported by Ejikeme et al. (2014); Ezeonu and Ejikeme 2016). 50 cm3 of distilled water was added to 0.5 g each of A. boonei parts powders in a beaker placed on a steam water bath for 10 min and stirred occasionally before filtration. 10 cm3 of filtrate was added to 5 cm3 of a mixture of distilled water and agitated vigorously to form a stable persistent lather. Three drops of olive oil formed an emulsion which indicate the presence of saponin.
Phlobatannin determination
Phlobatannin qualitative determination methodology used in this research was carried out using the methods reported by Ezeonu and Ejikeme 2016). 0.5 g each of A. boonei leaf, stem bark and root powders were weighed into beaker and 50 cm3 of distilled water was added. The mixture was allowed to extract for 24 h. 10 cm3 of each extract was boiled with 1% aqueous hydrochloric acid (5 cm3). Deposit of red precipitate indicate the presence of Phlobatannin.
Anthraquinone determination
The method described by Amadi et al. (2004) was adopted for the analytical determination of Anthraquinone. 10 ml of benzene was added to 0.5 g each of A. boonei leaf, stem bark and root extracts and shake gently before filtration. 5 ml of 10% NH4 solution was added to the filtrate (10 cm3) and shake thoroughly. The formation of pink red or violet colour in the ammonia layer shows the presence of free anthraquinone.
Tannin determination
The method described by Ezeonu and Ejikeme (2016) was adopted for the qualitative determination of tannin. 50 cm3 of distilled water was added to 0.5 g each of A. boonei leaf, stem bark and root powders in a beaker placed on a steam water bath for 10 min before filtration. Thereafter, 3 drops of 0.1% ferric chloride was added to the filtrate (5 cm3). Formation of a brownish green colouration confirmed the presence of tannin.
Flavonoid determination
Flavonoid qualitative determination using the method reported by Ejikeme et al. (2014); Ezeonu and Ejikeme (2016). 50 cm3 of distilled water was added to 0.5 g each of A. boonei leaf, stem bark and root powders in a beaker and allowed to extract for 2 h before filtration. 5 cm3 of 1.0 M dilute ammonia (NH4) solution was added to 10 cm3 each of the plant part filtrates before the addition of 5 cm3 of concentrated tetraoxosulphate (VI) acid (H2SO4) that formed a yellow colouration which disappeared on standing. This indicate the presence of flavonoid.
Insect bioassay
Toxicity of A. boonei leaf, stembark and root powders on D. maculatus adults
Powders of A. boonei leaf, stembark and root were thoroughly admixed separately at 2.5, 5.0, 7.5, 10, 12.5 and 15 g/100 g of C. gariepinus in plastic container (60 mm depth and 80 mm in diameter) in four replicates of the treated and controls laid out in Complete Randomised Block Design in insect cage. Ten pairs (10 males: 10 females) of newly emerged D. maculatus adult of <24 h were introduced. Insect mortality was assessed every 2 days for 10 days. Dead beetles were those that did not move or respond to pin probing (response to sharp pin). At the end of day 10, all insects, both dead and alive were removed from each container. Number of dead insects were counted and recorded. Percentage adult mortality was corrected (Abbott 1925).
Toxicity of A. boonei Leaf, Stembark and Root powders on Reproductive Performance of Dermestes maculatus
Powders of A. boonei leaf, stembark and root were thoroughly admixed separately at 2.5, 5.0, 7.5, 10, 12.5 and 15 g/100 g of C. gariepinus in plastic container (60 mm depth and 80 mm in diameter) in four replicates of the treated and controls laid out in Complete Randomised Block Design in insect cage. Ten pairs (10 males: 10 females) of newly emerged D. maculatus adult of <24 h were introduced. At the end of day 10, all insects, both dead and alive were removed from each container. Number of eggs laid by the adult female insects were counted and recorded every 24 h for 21 days until first filial adult emergence. The number reaching larva and adult stages were recorded and expressed as percentage hatching rate and adult emergence as described by Odeyemi and Daramola (2000).
Toxicity of A. boonei leaf, Stembark and root extracts on D. maculatus adults
Alstonia boonei leaf, stembark and root extracts were thoroughly rubbed separately using Camel brush on the sterilized smoked dried C. gariepinus at concentrations 1, 2, 5, 10, 15 and 20%/100 g in plastic container (60 mm depth and 80 mm in diameter). The treated fish samples were then wide-open to dryness for 5–10 min depending on concentration to eliminate traces of n-hexane. Ten pairs (10 males: 10 females) of newly emerged D. maculatus adult of <24 h were introduced. Four replicates of the treated and controls were laid out in Complete Randomised Block Design in insect cage. Insect mortality was assessed every 2 days for 10 days. Dead beetles were those that did not move or respond to pin probing (response to sharp pin). At the end of day 10, all insects, both dead and alive were removed from each container. Number of dead insects were counted and recorded. Percentage adult mortality was corrected (Abbot 1925).
Toxicity of A. boonei leaf, Stembark and root extracts on reproductive performance of Dermestes maculatus
Alstonia boonei leaf, stembark and root extracts were thoroughly rubbed separately at concentrations of 1, 2, 5, 10, 15 and 20%/100 g of smoked catfish, C. gariepinus in plastic container (80 mm depth and 100 mm in diameter). The treated fish samples were then wide-open to dryness for 5–10 min depending on concentration to eliminate traces of n-hexane. Ten pairs (10 males: 10 females) of newly emerged D. maculatus adult of <24 h were introduced. At the end of day 10, all insects, both dead and alive were removed from each container. Number of eggs laid by the adult female insects were counted and recorded every 24 h for 21 days until first filial adult emergence. The number reaching larva and adult stages were recorded and expressed as percentage hatching rate and adult emergence as described by Odeyemi and Daramola (2000).
Statistical analysis
All data were subjected to analysis of variance (ANOVA), and means were separated using the Tukey’s Honest Significant Difference Test at 5% probability level (SAS 2003). Prior to ANOVA, data observed in percentages and counts were subjected to arcsine and square root transformation to meet the conventions of normality and homogeneity of variance (Adesina and Ofuya 2011; Adesina and Mobolade-Adesina 2016). Log-Probit model analysis was carried out on percentage mortality of the hide beetle to evaluate the 50% lethal dose and concentration (LD50 and LC50) and 90% lethal dose and concentration (LD90 and LC90) (Finney 1971).
Results
Phytochemicals screening of Hexanoic extracts of Alstonia boonei
Tables 1 presented the result of the phytochemical screening of the hexanoic extracts of A. boonei leaf, stem bark and root. Alkaloids. saponins and tannins were present in all the plant parts tested. Flavonoid was present only in A. boonei stem bark extract while phlobatannins and anthraquinones were absent in the experimental plant parts.
Toxicity of A. boonei leaf, Stembark and root powders on percentage mortality of D. maculatus
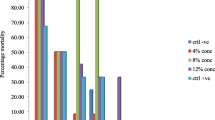
Results in Table 2 presented the toxicity of A. boonei leaf, stembark and root powders on adult mortality of D. maculatus. The result showed that A. boonei leaf, stembark and root powders were significantly (P < 0.05) toxic to the adult hide beetles at each tested concentrations compared to the untreated (control) in increasing order over the period of exposure. Alstonia boonei stembark powder was the most potent among the three powders triggering 100% adult mortality in dishes treated with 15 g/100 g and 7.5 g/100 g at 2nd and 8th days after exposure.
This was followed by the leaf powder of A. boonei which triggered 87.5% adult mortality at 4 and 10 days of treatment recorded in smoked fish treated with 15 and 7.5 g respectively. Also 15 g of the leave powder was able to produce 100% mortality at 10 days of exposure while the powder was the least toxic to adult hide beetle at all tested concentrations. The powder instigated highest mortality of 92.5% at 15 g dosage rate after 10 days of exposure.
Lethal dose (LD) of A. boonei leaf, Stembark and root powders on D. maculatus
The lethal doses of different parts of A. boonei powders against adult D. maculatus are given in Table 3. The required dosage calculated to cause 50% (LD50) and 90% (LD90) insect mortality after day 2 were 6.80 and 25.44 g; 4.27 and 14.33 g; and 8.99 and 42.03 g per 100 g of smoked fish for the leaf; stembark and root powders respectively. These values were observed to reduce as the period of exposure increased. From the calculations, the stembark was observed to have the lowest lethal dose across all period of exposure.
Delayed cumulative effects of A. boonei leaf, Stembark and root powders on reproductive performance of D. maculatus
Effects of A. boonei leaf, stembark and root powders on oviposition, larval development and adult emergence of D. maculatus are shown in Table 4. The effect of A. boonei leaf, stembark and root powders on D. maculatus progeny development revealed significant difference (P < 0.05) compared to the control in a dosage dependent manner. The various plant powders completely prevented the beetle from laying eggs except stembark, leaf and root powder that led to significant reduction in the number of eggs laid on those treated with 2.5, 5 and 7.5 g that recorded between 0.5 and 5.00 eggs respectively compared to control. Similarly, there were very low numbers of emerged larvae from the copulating adults that were exposed to powder treatments. Stembark powders completely prevented the emergence of larvae and adult insects from the few eggs laid. However, 17.5% and 20% larval and adult emergence were recorded on smoked fish treated with A. boonei leaf and root powders at rate 2.5 g/100 g of smoked fish.
Toxicity of A. boonei leaf, Stembark and root Hexanoic extracts on percentage mortality of D. maculatus
Result in Table 5 presented the toxicity of A. boonei leaf, stembark and root extracts on D. maculatus adult mortality. All the extracts were significantly (P < 0.05) toxic to the adult hide beetles at each of the concentrations tested compared to the control. Stembark extract was the most lethal causing 50, 87.50 and 100% mortality at 1, 2, 5, 10%, 15 and 20% concentration/100 g smoked catfish after 2 days of exposure. This was closely followed by A. boonei leaf instigating 32.50, 50, 67.50, 82.50, 97.50 and 100% adult mortality at rate 1, 2, 5, 10, 15 and 20% concentration/100 g smoked after 2 days of post treatment. At 8 days of exposure of D. maculatus to stembark extract at all the tested concentrations, 100% adult mortality was recorded. Alstonia boonei root extract was the least toxic to adult hide beetle at all tested concentrations. At the end of experiment, 100% mortality of hide beetles were recorded on smoked catfish treated with A. boonei leaf and stembark extracts at concentration 15% after 6 days of exposure. The experiment clearly indicated that D. maculatus adult mortality increased with increase in length of exposure and concentration of the extracts.
Lethal concentration of A. boonei leaf, Stembark and root extracts on D. maculatus
The lethal concentrations of different parts of A. boonei powders against adult D. maculatus are given in Table 6. The required concentrations calculated to cause 50% (LC50) and 90% (LC90) mortality in the insect after day 2 were 2.10 and 10.83%; 1.00 and 2.09%; and 3.39 and 31.76% per 100 g of smoked fish for the leaf; stembark and root powders respectively. These values were observed to reduce as the period of exposure increased. Some values were not calculated because most concentration caused maximum percentage mortality at such period of exposure.
Delayed cumulative effects of A. boonei leaf, Stembark and root Hexanoic extracts on reproductive performance of D. maculatus
Table 7 shown effects of A. boonei leaf, stembark and root powders on oviposition, larval development and adult emergence of D. maculatus. The effect of A. boonei leaf, stembark and root extracts on progeny development of D. maculatus revealed significant difference (P < 0.05) compared to the control. Alstonia boonei stembark extract significantly prevented the beetle from laying eggs. Similarly, there were very low mean numbers of emergent larvae of the insects from the new copulating adults that were exposed to extracts treatments. Stembark powders and leaf extract completely prevented the emergence of larvae and adult insects from the few eggs laid. However, 22.5% and 22.5% larva and adult emergence were recorded on smoked fish treated with A. boonei leaf and root extract at 1%/100 g concentration of the smoked catfish.
Discussion
The use of A. boonei in the management of insect pest of cereals, legumes and vectors of malaria as well as inhibition of bacteria and fungi growth have been reported (Oigiangbe et al. 2007a, b; Ileke and Oni 2011; Omoya et al. 2012; Ileke et al. 2012, 2013; Ojo and Ogunleye 2013; Ileke and Arotolu 2018; Ileke 2019). Although, this may be the first attempt to screen A. boonei for insecticidal potential against D. maculatus. The result obtained in this study is in agreement with the reports of many earlier researchers on the use of botanicals against suppression of D. maculatus infestation on smoked-dried fish (Onu and Baba 2003; Adebote et al. 2006; Akinwumi et al. 2007, 2011; Abdullahi et al. 2011; Mufutau 2012; Adesina et al. 2014, 2016; Ajayi et al. 2019; Philip-Attah 2019; AI Nta et al. 2019; Ileke et al. 2020a).
Alstonia boonei plant parts exhibited dosage dependent and period of exposure activities, the higher the concentration and period of exposure the more the mortality. The results are similar to the findings of Oigiangbe et al. (2007a), (b); Ileke and Oni (2011); Ileke et al. (2012); Omoya et al. (2012) who reported the susceptibility of Maruca vitrata, Sesamia calamists, Sitophilus zeamais, Callosobruchus maculatus and vector of malaria, Anopheles gambiae to Alstonia boonei leaf, stembark and root. This is also in agreement with Okorie et al. (1990) who reported 93% mortality of D. maculatus larvae and 100% mortality of all adults, when treated with neem seed powder at the rate of 2 g/25 g of the smoked Tilapia fish. Akinwumi (2011) reported 100% mortality of adult D. maculatus on exposure of Pipper guineense powder at the rate of 10 g/100 g of smoked fish after 7 days of experimental period. The study evidently shown that the both plant powders were most effective at higher concentration compared to the low concentration.
The mortality caused by the powders and extracts of A. boonei might be through desiccation of insects or through occlusion of their spiracles, thereby preventing respiration via tracheal system. (Adedire et al. 2011). Medicinal and aromatic plant products have been linked with paralysis and blockage of electron transportation in respiratory processes of insects, immobilization and toxicity and ultimately resulting in mortality (Singh et al. 2001; Moreira et al. 2007). Plant products had been reported to consist of complex mixtures of monoterpenes, sesquiterpenes and biogenetically related phenols (Trivedi et al. 2018; Ileke et al. 2020b). Their mode of actions against insect pests is through neurotoxic mode of action (Trivedi et al. 2018). The complex mixtures in plant products inhibited acetyl cholinesterase enzyme (AChE) action (Houghton et al. 2006). The inhibited acetyl cholinesterase enzyme (AChE) activity interferes with the neuromodulator octopamine (Kostyukovsky et al. 2002; Trivedi et al. 2018) which block GABA-gated chloride channels of the insect pest resulting to their death (Priestley et al. 2003). This supports the findings of other researchers who had earlier reported on the efficacy of insecticidal activities of various plant products as surface protectant against D. maculatus infestation (Okorie et al. 1990; Akinwumi et al. 2007; Akinwumi 2011).
In addition to adult mortalities, A. boonei stem bark powder and extract prevented egg laying as fewer eggs were laid on smoked fish treated with A. boonei stem bark powder and extract compared with control. Complete oviposition suppression was observed with stembark power at 5-15 g, leaf and root powder at 10-15 g; while for extracts treated fish samples, zero oviposition was recorded from 1% stembark, 2–20% leaf and 5–20% root extract. The significant reduction in the number of egg laid, could be due to toxicity of the plant products. Plant products were known to interfere with oviposition, egg hatching and also impede the production of important enzymes such as those responsible for moulting thus inhibiting growth and general development of the insect pests (Moreira et al. 2007; Ileke and Oni 2011). The observed significantly low oviposition and larvae emergence from this study collaborates with the findings of Kosini and Nukenine (2017) who stated that, apart from adult toxicity, extracts of Gnidia kaussiana significantly inhibited C. maculatus oviposition and/or exterminated the larvae at developmental stages after eggs laid on Vigna subterranea. Jamil et al. (1984) opined that crude extracts retard development and caused mortality of larvae, cuticle melanisation resulting in the disruption of the endocrine system controlling the growth and moulting of larvae.
The insecticidal activity of A. boonei may be attributed to its active chemical components. Botanicals are known to possess bioactive chemical substances like terpenes, saponins, tannins, flavonoids and alkaloids among others which have been found to possess reasonable antifungal, antibacterial, antioxidant or insecticidal efficacy (Fernando et al. 2005). The presence of flavonoids in A. boonei stem bark could be responsible for higher mortality of D. maculatus.
Conclusion
The results obtained from the study showed that A. boonei stem bark powder and extract were the most potent in the management of D. maculatus, causing significant adult mortality and suppressed larva and adult emergence. Thus, it use should be promoted among fishermen and fish traders for the control of D. maculatus infestation. Further study is needed to characterize and isolate the plant bioactive chemical constituents and understand the plant products mode of action with the likely formulation into herbal insecticide as alternative to synthetic insecticides.
References
Abbot W (1925) A method of computing the effectiveness of an insecticide. J Econ Entomol 18:265–267
Abdullahi N, Auwal F, Bello B, Yushau M (2011) Laboratory efficacy of nutmeg (Monodora myristica) powder against the beetle Dermestid maculatus (Coleoptera: Dermestidae) on treated smoked catfish (Clarias gariepinus) in Kano state of Nigeria. Intl J Pharma Appl Sci 2(1):52–55
Abel C, Busia K (2005) An exploratory Ethnobotanical study of the practice of herbal medicine by the Akan peoples of Ghana (Ethnobotanical study: herbal medicine in Ghana) Alt Med Rev 42
Adebote DA, Abolude DS, Oniye SJ, Oladodo SS, Hassan MM (2006) Larvicidal and repellant action of Detarium microcarpum seed oil against the larvae of Dermestes lardarius (coleopteran: Dermestidae) in dried Clarias gariepinus fish. J Entomol 38(3):245–253
Adedire CO, Obembe OO, Akinkurolere RO, Oduleye O (2011) Response of Callosobruchus maculatus (Coleoptera: Chysomelidae) to extracts of cashew kernels. J Plant Dis Prot 118(2):75–79
Adesina JM, Mobolade-Adesina TE (2016) Tolerance activities of Callasobruchus maculatus (F.) (Coleoptera: chrysomelidae) against Secamone afzelii (Schult) K. Schum leaf extracts. Jordan J Agric Sci 12(4):1141–1154
Adesina JM, Ofuya TI (2011) Evaluation of leaf and vine powders of Secamone afzelii (Schult) K. Schum for control of Callosobruchus maculatus (fab.) (Coleoptera: bruchidae) in stored cowpea vigna unguiculata (L.) Walp. South Asian J Exp Biol 1(3):158–163
Adesina JM, Jose AR, Adetuyi OO, Olorunfemi DA (2014) Larvicidal activity of Phyllanthus fraternus powder in suppressing Dermestes maculatus Degeer (Coleoptera: Dermestidae) infestation on smoked African catfish (Clarias gariepinus). Intl J Aquacul 4(11):67–72
Adesina JM, Jose AR, Rajashaker Y (2016) Bio-efficacy of Clerodendrum capitatum against Dermestes maculatus larvae infestation on smoked catfish (Clarias gariepinus). Brazilian J Biol Sci 3(5):37–44
Ahmad W, Shilpa S, Sanjay K (2017) Phytochemical screening and antimicrobial study of Euphorbia hirta extracts. J Med Plant Stud 2:183–186
Ajayi OE, Oladele OO, Fabunmi GO (2019) Differential inhibitory potentials of three plant extracts against Dermestes maculatus Degeer and microorganisms in smoked catfish, Clarias gariepinus Burchell. Food Qual Saf 3(3):201–208. https://doi.org/10.1093/fqsafe/fyz017
Akinwumi FO (2011) Evaluation of some plant materials for the control of smoked fish Pest, Dermestes maculatus Degeer (Coleoptera: Dermestidae) in Clarias pariepinus Burchell (Pisces: Clariidae). ARPN J Agric Biol Sci 6(7):65–69
Akinwumi FO, Fasakin EA, Adedire CO (2006) Progeny inhibiting effect of four plant product against the leather beetle and copra beetles on smoked African mud cat fish Clarias gariepinus Burchell. J Biol Sci 6(6):1023–1028
Akinwumi FO, Fasakin EA, Adedire CO (2007) Toxic and repellence activities of four plant extracts against Dermestes maculatus on smoked African mud catfish, Clarias gariepinus, Burchell. J Entomol 4(2):149–154
Amadi BA, Agomuo EN, Ibegbulem CO (2004) Research methods in biochemistry. Supreme Publishers, Owerri
Amole OO, Ilori OO (2010) Antimicrobial activity of the aqueous and ethanolic extracts of the stem bark of Alstonia boonei. Intern J Phytopharmacol 1(2):119–123
Amusan AAS, Okorie TG (2002) The use of Piper guineense, fruit oil as protectant of dried fish against Dermestes maculatus (De Geer) infestation. Global J Pure Appl Sci 8(2):197–201
Ashamo MO, Akinnawonu O (2012) Insecticidal efficacy of some plant powders and extracts against the Angoumois moth, Sitotroga cerealella (Olivier) [Lepidoptera: Gelechiidae]. Arch Phytopath Crop Prot 45(9):1051–1058
Asuzu U, Anaga AO (1991) Pharmacological screening of the aqueous extract of Alstonia boonei stem barck. Filoterapia 63:411–417.3
Atijegbe SR (2004) Infestation of smoked fish in Ghana. M.Phil. Thesis in Entomology University of Ghana, pp 103
Azam K, Ali MY, Asaduzzaman M, Basher MZ, Hossain MM (2004) Biochemical assessment of selected fresh fish. J Biol Sci 4:9–10
Betti JL (2004) An ethnobotanical study of medicinal plants among the Baka pygmies in the dja biosphere reserve, Cameroon. Afri Study Mono 25(1):1–27
Ejikeme CM, Ezeonu CS, Eboatu AN (2014) Determination of physical and phytochemical constituents of some tropical timbers indigenous to Niger Delta area of Nigeria. Europ Scien J 10(18):247–270
Ezeonu CS, Ejikeme CM (2016) Qualitative and quantitative determination of phytochemical contents of indigenous Nigerian softwoods. New J Sci Article ID 5601327, 9 pages:1–9. https://doi.org/10.1155/2016/5601327
Fasakin EA, Aberejo BA (2002) Effect of some pulverized plant materials on the developmental stages of fish beetle, Dermestes maculatus Degeer in smoked cat fish, Clarias gariepinus during storage. Bioresour Technol 85(2):173–177
Fernando AC, Silva KFSD, Santos KKD, Junior KALR, Ana AEGS (2005) Activities of some Brazilian plant against larvae of the mosquito Aedes aegypti. Fitoterapiai 76:234–239
Finney DJ (1971) Probit Analysis, 3rd edn. Cambridge University Press, Cambridge
Hikino H, Kiso Y, Wagner H, Fiebi M (1984) Antihepatotoxic actions of flavonolignans from Silybum marianum fruits. Planta Med 50(3):248–250
Houghton PJ, Ren Y, Howes MJ (2006) Acetylcholinesterase inhibitors from plants and fungi. Nat Prod Rep 23(2):181–199
Idoko JE, Ileke KD (2020) Comparative evaluation of insecticidal properties of essential oils of some selected botanicals as bio-pesticides against cowpea bruchid, Callosobruchus maculatus (Fabricius) of stored cowpea. Bullentin Nat Res Cent 44(119):1–7
Ileke KD (2019) The efficacy of Alstonia boonei Stembark oil as a long-term storage protectant against cowpea Bruchid, Callosobruchus maculatus (fab.) (Coleoptera: Chrysomelidae). Jordan J Biol Sci 12(3):329–337
Ileke KD, Adesina JM, Obajulaye EO (2016) Synergetic effects of two botanicals entomocides as pest-protectants in maize grains. J Biol Res 89(2):33–39
Ileke KD, Arotolu TE (2018) Bioefficacy of Alstonia boonei Leaf Extract against Cowpea beetle Callosobrochus maculatus infesting stored cowpea seeds in storage. Brizillian J Biol Sci 5(11):673–681. https://doi.org/10.21472/bjbs.051106
Ileke KD, Oni MO (2011) Toxicity of some plant powders to maize weevil, Sitophilus zeamais (Coleoptera: Curculionidae) on stored wheat grains. Afri J Agric Res 6(13):3023–3048
Ileke KD, Odeyemi OO, Ashamo MO (2012) Insecticidal activity of Alstonia boonei De wild powder against cowpea bruchid, Callosobruchus maculatus (fab.) [Coleoptera: Chrysomelidae] in stored cowpea seeds. Intl J Biol 4(2):125–131
Ileke KD, Odeyemi OO, Ashamo MO (2013) Response of cowpea Bruchid, Callosobruchus maculatus (Fabr.) [Coleoptera: Chrysomelidae] to cheese wood, Alstonia boonei De wild stem bark oil extracted with different solvents. Arch Phytopath Crop Prot 46(11):1357–1370
Ileke KD, Odeyemi OO, Ashamo MO (2014) Phytochemical screening and effectiveness of Alstonia boonei De wild oils as an entomocides in the management of cowpea bruchid, Callosobruchus maculatus (fab.) (Coleoptera: Chrysomelidae). International J Hort 4(6):24–23
Ileke KD, Ojo DO, Obembe OM, Ogunbiyi YK (2020a) Susceptibility of Hide beetle, Dermestes maculatus (De Geer) [Coleoptera: Dermestidae] to powder and extract of two species of Capsicum fruits on smoke-dried catfish, Clarias gariepinus (Burchell) [Pisces: Clariidae]. Egyptian Acad J Biol Sci A Entomol 13(3):97–111
Ileke KD, Idoko JE, Ojo DO, Adesina BC (2020b) Evaluation of botanical powders and extracts from Nigerian plants as protectants of maize grains against maize weevil, Sitophilus zeamais (Motschulsky) [Coleoptera: Curculionidae]. Biocat Agricul Biotech 27:101702. https://doi.org/10.1016/j.bcab.2020.101702
Jamil K, Rani U, Thyagarajan G (1984) Water hyacinth–a potential new juvenile hormone mimic. Int Pest Control 26:106–108
Jangampalli AP (2019) Aquaculture role in global food security with nutritional value: a review. Trans Animal Sci 3(2):903–910. https://doi.org/10.1093/tas/txz012
Jose AR, Adesina JM (2014) Larval susceptibility of Dermestes maculatus (De geer) (Cleoptera: Derestidae) to Secamone afzelli K. Schum leaf on smoked dried fish. Intl J Aquac 4(17):102–107
Kosini D, Nukenine EN (2017) Bioactivity of novel botanical insecticide from Gnidia kaussiana (Thymeleaceae) against Callosobruchus maculatus (Coleoptera: Chrysomelidae) in Stored Vigna subterranea (Fabaceae) Grains. J Insect Sci 17(1): 31; 1–7
Kostyukovsky M, Rafaeli A, Gileadi C, Demchenko N, Shaaya E (2002) Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: possible mode of action against insect pests. Pest Manag Sci 58:1101–1106
Lale NES, Sastawa BM (1996) The effects of sun drying on the infestation of the African catfish (Clarias gariepinus) by post-harvest insects in the Lake Chad District of Nigeria. Intl J Pest Manag 42:281–283
Majekodunmi SO, Odeku OA (2009) Effects of interacting variables on the formulation of Alstonia boonei De wild (Apocynaceae) tablets. Acta Pharma Sci 51:141–148
Majekodunmi SO, Adegoke OA, Odeku OA (2008) Formulation of the extract of the stem bark of Alstonia boonei as tablet dosage form. Tropical J Pharma Res 7(2):987–994
Moreira MD, Picanço MC, Cláudio de Almeida LB, Guedes RNC, Ribeiro de Campos M, Silva GA, Martins JC (2007) Plant compounds insecticide activity against Coleoptera pests of stored products. Pesq Agrop Brasileira 7:909–915
Moronkola DO, Kunle OF (2012) Essential oil compositions of leaf, Stem Bark and Root of Alstonia Boonei De Wild (Apocyanaceae). Intl J Biol Pharma Res 3(1):51–60
Mufutau AA (2012) Evaluation of the efficacy of neem seed oil (NSO) extract for the control of Dermestes maculatus degeer, 1774 (Coleoptera: Dermestidae) in Clarias gariepinus (Burchell, 1822) (Pisces: Claridae). Munis Entomol Zool 7(2):1188–1194
Nta AI, Andem AB, Okererke CL, Odey CO (2019) Bio-efficacy of some plant oil extracts as surface protectant against leather beetle, Dermestes maculatus (De geer) on smoked fish of Clarias gariepinus. J Entomol Zool Stud 7(3):51–56
Nwosu LC, Obi OA, Azoro VA, Dialoke SA, Onah AE, Zakka U, Azeez OM, Eluwa AN, Azuine UA, Ukpai KU, Arotolu TE, Lawal IA, Ugagu GM, Uwalaka OA, Petgave GM, Obilo RU, Aguwa UO (2018) The efficacy of the plant extracts of Afrostyrax kamerunensis, Monodora myristica, Moringa oleifera and Azadirachta indica against the infestation of the leather beetle, Dermestes maculatus De Geer in smoked African mud catfish, Clarias gariepinus Burchell. Jordan J Biol Sci 11(5):511–515
Obiakor MO, Ezeonyejiaku CD, Ezenwelu CO (2013) Biotoxicity and eco-friendly characterization of plant materials as green pesticides for the control of fish beetles (Demestes maculatus). J Life Sci Biomedi 3(4):300–307
Odeyemi OO, Daramola AM (2000) Storage practices in the tropics: food storage and pest problems. First edition, vol 1. Dave Collins Publication, Nigeria, 235pp
Odeyemi OO, Owoade RA, Akinkurolere O (2000) Toxicity and population suppression effects of Parkia clappatoniana on dried fish pests (Dermestes maculatus and Necrobia rufipes). Global J Pure Appl Sci 6(2):191–195
Oigiangbe ON, Igbinosa IB, Tamo M (2007a) Insecticidal activity of the medicinal plant, Alstonia boonei De wild, against Sesamia calamistis Hampson. J Zhejiang Univ Sci B 8(10):752–755
Oigiangbe ON, Igbinosa IB, Tamo M (2007b) Bioactivity of extracts of Alstonia boonei De wild stem bark against Maruca vitrata (Lepidoptera: Pyralidae). Adv Sci Tech 1(1):67–70
Ojo DO, Ogunleye RF (2013) Comparative effectiveness of the powders of some underutilized botanicals for the control of Sitophilus zeamais (Motschulsky) (Coleoptera: Curculionidae). Intl J Pure Appl Sci Tech 16(2):55–62
Okorie TG, Siyanbola OO, Ebochuo VO (1990) Neem seed powder as a protectant for dried Tilapia fish against Dermestes maculatus Degeer infestation. Insect Sci Appl 11(2):153–157
Olajide AO, Awe SO, Makinde JM, Ekhelar AI, Olusola A, Morebise O, Okpako DT (2000) Studies on the anti-inflammatory, antipyretic and analgesic properties of Alstonia boonei stem bark. Journal of Ethnopharmacology 71(1, 2):179–186
Omoya F, Oladipupo K, Abe A, Udensi O (2012) Bioactivity, qualitative and quantitative components of Alstonia boonei leaf extracts on Anopheles mosquito larvae in Nigeria. J Med Bioeng 1(1):39–41
Onu I, Baba GO (2003) Evaluation of neem products (Azadirachta indica a. Juss: Meliaceae) for the control of Dermestid beetle (Dermestes maculatus De Geer) (Coleoptera: Dermestidae) on dried fish. Nig J Entomol 20:105–115
Osuji FNC (1974) Beetle infestation of dried fish purchased from a Nigerian market, with special reference to Dermestes maculatus Degeer. Nig J Entomol 1(1):69–79
Owoade RA (1993) Storage of dried fish, Clarias lazera (curvier and Valencienres) in different packaging materials and control of the major pest, Dermestes maculates (De Geer) M. Tech. Thesis. Federal University of Technology, Akure, p 54
Philip-Attah TM (2019) Evaluation of ethanolic extracts of Piper guineense, Dennettia tripetala and Capsicum frutescens as protectant of smoked fish, Clarias gariepinus (Pisces: Clariidea) against Dermestes maculatus (Coleoptera: Dermestidae). The J Basic Appl Zool 80(7):1–7. https://doi.org/10.1186/s41936-019-0079-1
Priestley CM, Williamson EM, Wafford KA, Sattelle D, Thymol B (2003) A constituent of thyme essential oil, is a positive allosteric modulator of human GABAA receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br J Pharmacol 140:1363–1372
Raji YI, Salmon TM, Akinsomsoye OS (2005) Reproductive functions in male rats treated with methanol extract of Alstonia boonei stem bark. Afr J Biomedical Res 8(2):105–111
SAS Institute. (2003). The SAS system version 9.1 for windows. SAS Institute, Cary
Singh UP, Prithiviraj B, Sarma BK, Singh M, Ray AB (2001) Role of garlic (Allium sativum L.) in human and plant diseases. Indian J Exp Biol 39:310–322
Terashima H (2003) Names, use and attributes of plants and animals among the Ituri forest foragers: a comparative ethnobotanical and ethnozoological study. Afri study mono 28(Suppl):7–24
Trease GE, Evans WC (1985) Pharmacognosy, 14th edn. London, W.B, Sanders Company
Trivedi A, Nayak N, Kumar J (2018) Recent advances and review on use of botanicals from medicinal and aromatic plants in stored grain pest management. J Entomol Zool Stud 6(3):295–300
Udo IO (2011) Potentials of Zanthoxylum xanthoxyloides (LAM.) for the control of stored product insect pests. J Stored Prod Postharvest Res 2(3):40–44
Funding
The research work was funded by the authors.
Author information
Authors and Affiliations
Contributions
KDI, conceived and design the study, source for and prepared the plant materials, carried out data analysis and proofread the manuscript. JMA, carried out the phytochemical screening, source for relevant literatures and prepared the manuscript. AOA, source for and prepared the fish samples KDI and MSA carried out the insect bioassay and collect all data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and /or animals
The research work did not involve animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ileke, K.D., Adesina, J.M., Abidemi-Iromini, A.O. et al. Entomocide effect of Alstonia boonei De wild on reproductive performance of Dermestes maculatus (Coleoptera: Dermestidae) infestation on smoked catfish Claria gariepinus (Pisces: Clariidea). Int J Trop Insect Sci 41, 1293–1304 (2021). https://doi.org/10.1007/s42690-020-00321-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-020-00321-6