Abstract
Cabbage butterfly (Pieris brassicae) is major pest of cabbage. More than 50% yield losses have been attributed due to this pest annually. Seven insect parasitic nematodes i.e. Steinerma carpocapsae, S. feltiae, S. Pakitananese, S. asiaticum, S. glaseri, Heterorhabditis bacteriophora and H. indica were evaluated on 4th larval instar of P. brassicae at the different concentrations. Two best performing EPNs (S. glaseri and H. bacteriophora) were selected and evaluated against 2nd, 3rd and 4th larval instars of Pieris brassicae at 1500 infective juveniles/ml. H. bacteriophora spp. and S. glaseri spp. were sprayed at 1500 IJs/ml on the cabbage leaves alone or in combination (S. glaseri + H. bacteriophora) and 2nd, 3rd and 4th larval instars were fed on them. Among seven EPNs, hundred percent mortality of P. brassicae was recorded in the case of H. bacteriophora and S. glaseri at1500 IJs/ml concentration after 48 h. Both EPNs spp. were found highly effective against all larval instars of P. brassicae. After 48 h of exposure of both EPNs spp. on all larval instar, 100% mortality was recorded. The combined application of H. bacteriophora + S. glaseri on cabbage leaves resulted into 100% mortality of all larval instars of P. brassicae. From present study, it may be concluded that H. bacteriophora and S. glaseri at 1500 IJs/ml concentration found very effective against P. brassicae in laboratory conditions and may be used in the field conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cabbage (Brassica oleracea var. capitata Linn.) is one of the essential vegetable crop grown in Pakistan. It is used as a fresh salad, cooked or processed into preserved products such as sauerkraut. It is the cheapest source of useful minerals and food nutrients needed for the balanced human diet (Ashfaq et al. 2018). Successful production of cabbage is facing numerous constraints of fungal, bacterial, viral, insects and nematodes (Krauthausen et al. 2018; Sharma et al. 2018). Among these, infestations of defoliating caterpillars like cabbage butterfly (Pieris brassicae) are the major one (Saeed et al. 2017).
P. brassicae is a major pest of cabbage in Pakistan (Saeed et al. 2017). In Khyber Pakhthoon Khwa province, the insect severily damages the cruciferous vegetables throughout the year, single larva can consume 74–80 cm2 leaf area and cause economic losses. In India, the yield losses due to P. brassicae ranges from 30 to 40% annualy (Ali and Rizvi 2007; Kular and Kumar 2017). The losses may increase up to 75% percent if susceptible cultivar and favourable climatic conditions prevailes (Chaudhuri et al. 2001; Krishnamoorthy 2004) Larvae of P. brassicae feed in such a way leaving the only skeleton of the plant and consumes all the leaves of the plant (Mazurkiewicz et al. 2017). The severe attack resulting damage or deformed cabbage heads are develop which were 100% rejected by the end consumer (Uddin et al. 2007). The severity of the incidence of insect pests is greatly influenced by the prevailing climatic conditions (Meena et al. 2013).
Management of P. brassicae through the application of hazardous pesticides is an easy approach to minimize the pest population but it causes the development of insecticides resistance and presence of pesticide residues in cabbage heads. P. brassicae has established numerous resistance against a series of pesticides belongs to carbamates, synthetic pyrethroids and organophosphates (Ullah et al. 2016). It is an alarming threat to consumer health safety, soil and water pollution and biodegradation of pesticides etc. (Anjum 2018). Prevailing conditions stress to find out newly effective options to control P. brassicae. EPN enters into the hemolymph of insect pests through anus, mouth, spiracles or cuticle. After successful approaching in the body of insect, EPNs release bacteria already carrying in their intestine (Forst et al. 1997). The symbiotic bacteria quickly multiply and release toxins inside the hemolymph of insects, which primarily destroy the immune system of the host. Due to discharged toxins, the mortality of insects takes place between 24 to 48 h. On the other hand, bacteria convert the body parts of the host into the nutrient soup. EPNs consume this nutrient soup quickly, which ultimately supports the maximum multiplication of nematodes. When the nutrient soup is consumed, the EPNs breakdown the cadaver of insect pests and moves towards the other larvae (Forset and Clarke 2002; Poinar Jr 1990). In Pakistan, there is no systemic work has been done on the efficacy of EPNs against the management of P. brassicae. Therefore, keeping in view the efficient performance of EPNs as biocontrol agents, the present study was planned to evaluate environment-friendly nematode species against P. brassicae in lab conditions.
Materials and methods
Rearing of P. brassicae
For rearing of P. brassicae larvae, brassica plants were grown in small disposable cups. Plants were permitted to gain height of 6 to 7 cm which were used for the collection of eggs. 20–25 pupae were acquired from field and placed in cage for adults emergence. Swab of cotton was dipped in nectar and water solutions (1:9) and hanged in cage as nourishment to grow-up P. brassicae. When adults appeard, brassica plant were placed inside the cage. Female of P. brassicae laid eggs on brassica plants. Eggs hatched between 3 to 4 days. The 1st, 2nd, 3rd and 4th larval instars were allowed to consume the tender leaves. When 50 to 60% leaves were consumed, fresh leaves were provided. The larval stage completed within 12–14 days after this pupation period started which also completed in 6 days. With the help of camel hair brush, pupae were easily dislodged from the leaves. For rearing of P. brassicae, 12 h light and dark period with temperature 28 ± 4 °C were provided. In order to avoid any pathogenic contamination the rearing cages and culture room was cleaned with formalin. Furthermore, powdered based pesticide were used around the cages to save them from the attack of ants.
Multiplication of EPNs in G. mellonela
G. mellonela larvae were utilized as bait for duplication of EPNs. Seven species of EPNs i.e. S. carpocapsae, S. feltiae, S. Pakitananese, S. asiaticum, S. glaseri, H. bacteriophora, and H. indica were utilized during this study. The EPNs species were acquired from Nematological Laboratory, University of Agriculture, Faisalabad. Larvae were disinfected with 0.1% formaline solution to stay away any pathogenic infection after this kept in plastic petri plates already fixed with two whatman filter paper. Approximately, 1000 infective juveniles of each EPNs under study were inoculated to wax moth larvae kept in separate petri plates. EPNs were easily reconfirmed after the mortality of larvae with different colour shades. Larvae inoculated with Steinernema spp. confirmed with dark grey shadding and Heterorhabditis spp. express brik red shade (Wiesner 1993). Nematodes were harvested using White Trap method (White 1927). In vivo production of EPNs was conducted by the methods described by Poinar (1979). The plates were wrapped with para filim and record of each petri plate was maintained and kept in the incubator at 27 °C.
Counting and storage of EPNs
EPNs obtained through white trap procedure were collected in a different plastic cups and permitted to settle down for three to four hours. In the wake of settling down, the extra water was removed. The concentration of nematodes/ml of suspension was determined by counting the nematodes in a counting dish under stereomicroscope (Olympus 5240). The average of three counts was taken to estimate the final nematode population/ml. When larger nematodes were to be counted (>100/ ml), then dilution method was used for this purpose. The EPNs concentration in the original suspension was determined by using the following formula:
The nematode concentration was standardized between 1500 and 2000 infective juveniles/ ml. EPNs were stored by adjusting the incubator temperature at 10 °C in small plastic cups.
Pathogenicity of seven EPNs spp. against P. brassicae in laboratory
Seven different EPN spp. (S. carpocapsae, S. feltiae, S. Pakitananese, S. asiaticum, S. glaseri, H. bacteriophora and H. indica) were assessed against 4th larval instar of P. brassicae at different concentrations (250, 500, 1000 and 1500 Ijs/ml). In control treatment, no EPN was applied. Each treatment was five times replicated with five larvae of P. brassicae in separate petri plates (9 cm dia) respectively. Larval mortality of the insect pest was recorded at different time intervals.
Pathogenicity of two effective EPNs on different larval instars of P. brassicae in laboratory
Two EPN spp. (H. bacteriophora and S. glaseri) found to be more efficient with the highest mortality percentage of insect pest was utilized against various larval instars with effective concentration i.e. 1500 Ijs/ml. In control treatment, no EPN was applied. Each treatment was five times replicated with five larvae of P. brassicae in separate petri plates. Larval mortality was noted after 12, 24, 36 and 48 h time intervals.
Pathogenicity of EPNs on P. brassicae feedings on cabbage leaves
Leaves of cabbage obtained from susceptible hybrid (CB-60) were kept in well-sterilized plastic boxes separately. Two best performing spp. of EPNs (H. bacteriophora spp. and S. glaseri spp.) were sprayed at high concentrations (1500 IJs) of infective juveniles over the leaves alone or in combination (S. glaseri + H. bacteriophora). Distilled water was sprayed on cabbage leaves, considered as control. Cabbage leaves on which EPNs sprayed allowed to dry for a few minutes and then nourished to testing instars for one day. Before offering, each larva was starved for 3 h and then sprayed on leaves. Each treatment was five times replicated with 10 larvae of P. brassicae and kept in separate boxes. Larval mortality was recorded at different time intervals i.e. after 12, 24, 36 and 48 h.
Statistical analysis
Recorded data was analyzed through Analysis of Variance (ANOVA) and treatments means were compared by Fisher’s Least Significant Difference (LSD) test. Data was processed statistically through SAS (9.3) software (Inc., 2011–2012) and was represented by Microsoft Excel (2019).
Results
Pathogenicity of seven EPNs spp. against P. brassicae in laboratory
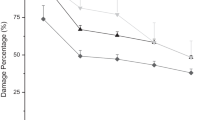
In this study seven different spp. of EPNs were evaluated for their pathogenic effects on 4th stage P. brassicae larvae in petri plate bioassay. After 12 h of EPNs treatment to 4th larval instar of P. brassicae in petri plate bioassays, 5.46, 8.46, 12.53 and 21.46% mortality observed with S. feltiae at 250, 500, 1000 and 1500 infective juveniles/ ml.
There was no mortality found with S. pakistanese and S. asiaticum at 250, 500, 1000 and 1500 infective juvenile concentration after 12 h. There was 20.53%, 48.53%, 68.50% and 88.43% mortality of P. brassicae larvae seen in H. bacteriophora at a different level of concentrations followed by S. glaseri with 80. 46% at 1500 IJs/ml after 24 h of treatment respectively. When time was increased to 36 h there were corresponding increase in mortality which was recorded 65.50, 70.40, 81.43 and 100% with S. glaseri followed by H. bacteriophora with 41.50, 76.60, 81.43 and 95.50% at 250, 500, 1000 and 1500 infective juveniles/ ml concentration respectively (Table 1). While after 36 h of treatment, there were 70.50, 61.50, 56.36 and 48.40% mortality found in 4th larval instar larvae of P. brassicae with S. feltiae at different concentrations (1500, 1000, 500 and 250 infective juveniles/ ml) followed by 54.43, 41.46, 31.50 and 21.46% mortality by S. carpocapsae with different infective nematodes concentrations respectively.
After 48 h, 77.40, 69.70, 60.50 and 54.36% mortality found in the case of where S. feltiae at different concentrations (1500, 1000, 500 and 250 infective juveniles/ ml) followed by 60.50, 57.43, 49.46 and 43.46% mortality by S. carpocapsae with different EPNs concentrations respectively.
H. bacteriophora and S. glaseri showed maximum mortality (100%) at higher concentrations of infective juveniles i.e. 1500 and 1000 IJs/ml. Results show that 9.46, 19.56, 27.43 and 38.36% mortality of 4th larval instar larvae of P. brassicae was found in S. carpocapsae treatment which was minimum as compared to the other treatments after 48 h (Table 1).
Pathogenicity of two effective EPNs on different larval instars P. brassicae in laboratory
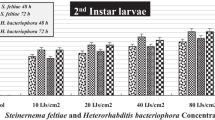
In the 2nd larval instar of P. brassicae, 60.50% mortality was recorded where H. bacteriophora was applied while 25.56% mortality of larvae was recorded due to S. glaseri after 12 h. Mortality percentage increased with the increase in the time interval. After 24 h, 100% mortality was seen where H. bacteriophora was applied and 60.53% larval mortality was observed in S. glaseri treatment. After 36 and 48 h of the interval, 100% mortality was recorded in both treatments. No mortality of larva was seen in the control treatment.
In the 3rd larval instar, S. glaseri and H. bacteriophora exhibited 10.50 and 30.50% mortality respectively after 12 h of application. After 24 h interval, 80.53 and 50.46% mortality recorded in case H. bacteriophora and S. glaseri respectively. Hundred percent mortality was recorded in the case of H. bacteriophora while 90.46% due to S.
glaseri respectively after 36 h. After 48 h, 100% mortality of P. brassicae larvae recorded in both treatments after 48 h. No mortality was seen in control treatment at all time intervals.
In the 4th larval instar, S. glaseri and H. bacteriophora showed 10.66 and 18.50% mortality respectively of P. brassicae larvae after 12 h of application. After 24 h, 35.53% mortality was seen due to S. glaseri application and 75.33% moratlity was observed where H. bacteriophora was applied. Hundred percent mortality was recorded in the case of H. bacteriophora, while 81.50% by S. glaseri after 36 h. After 48 h of application, 100% mortality was noted in the 4th larval instar of P. brassicae in both treatments. In control treatment, 0% mortality was recorded irrespective to the time intervals (Table 2).
Pathogenicity of EPNs on P. brassicae feedings on cabbage leaves
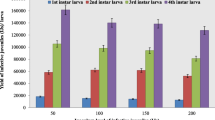
In 2nd larval instar, maximum mortality was seen when EPNs were applied in combination (H. bacteriophora + S. glaseri) as compared to alone (S. glaseri and H. bacteriophora) after 12 h. After 24 h, 100% mortality was seen when EPNs were appllied in combination (H. bacteriophora + S. glaseri), S. glaseri and H. bacteriophora exhibited 95.46 and 65.50% mortality of P. brassicae larvae respectively when apllied separately. After 36 h of interval, 100% mortality of P. brassicae larvae was recorded in case of H. bacteriophora + S. glaseri and S. glaseri treatments except H. bacteriophora where 80.53% mortality was recored. After 48 h inoculation, 100% mortality of P. brassicae was recorded in all EPNs treatments.
In 3rd larval instar, 35.53% mortality of P. brassicae larvae noted due to the application of H. bacteriophora after 12 h. After 24 h of inoculation, 70.46% mortality recorded with S. glaseri. Similarly, 36 h after pathogenic EPNs spray, 90.06% mortality was seen in case of combine application of EPNs (H. bacteriophora + S. glaseri). After 48 h of exposure, 90.04% and 80.46% mortality assessed in the case of S. glaseri and H. bacteriophora respectively while 100% mortality was seen because of the combine application of EPNs treatments (H. bacteriophora + S. glaseri). In control, no mortality was seen.
In 4th larval instar, 49.46% mortality was recorded where EPNs were applied in combination (H. bacteriophora + S. glaseri) while 25.50% mortality was recorded with S. glaseri after 12 h of application. After 24 h of exposure, 55.46 and 45.43% mortality was assessed in the case of S. glaseri and H. bacteriophora respectively. After 36 h of nematodes spray, 64.43% was seen in case of H. bacteriophora and 80.46% mortality of P. brassicae larvae observed where EPNs (H. bacteriophora + S. glaseri) @ 1500 infective juvenile/ml. After 48 h of exposure, 90.02 and 80.46% mortality was recorded with S. glaseri and H. bacteriophora treatments while 100% mortality recorded by the application EPNs (H. bacteriophora + S. glaseri). No mortality was seen in control treatment at all the time intervals (Table 3).
Discussion
EPNs associated with symbiotic bacteria (Xenorhabdus spp. and Photorhabdus spp.) converts the body parts of the host into a nutrient soup on which nematodes feed. In the first part of the present study, minimum mortality of P. brassicae with exposure of S. pakistanese, S. asiaticum spp. and H. indica spp. maybe due to their weak association or slow releasing of mutualistic bacteria into the haemocoel of the insect. On the other hand, highly virulent EPNs like H. bacteriophora spp. and S. glaseri spp. after entering into the host immediately released bacteria. Bacteria release broad-spectrum antibiotics responsible for the mortality of the insect pest in minimum time by breaking down of the tissues of insect and rapidly converts the body parts of host into the nutrient soup and provide food for the development and multiplication of EPN. H. bacteriophora spp. and S. glaseri spp. consume the nutrient soup quickly which ultimately supports the maximum production of nematodes due to competition of food and space H. bacteriophora spp. and S. glaseri spp. breakdown of the dead body of P. brassicae and move towards the other larvae is one of major the reasons regarding higher mortality percentage.
H. bacteriophora spp. and S. glaseri spp. during 2nd and 3rd part of the present study were found to be very virulent with maximum mortality of various larval instar after 48 h. The duration of nematode emergence was shorter in 2nd larval stage as compared to other larval instars. The sound reason regarding the increase in mortality percentage with an increase in time is due to the presence of the defensive system in P. brassicae. Insect pest defense himself against EPN with two progressions i.e. haemocytic and humoral response. Haemocytic process comprising of celluar encapsulation, nodule formation and phagocytosis while the humoral response including melanotic encapsulation and production of antimicrobial peptides (Khush and Lemaitre 2000). EPNs release bacteria inside the host within minutes to hours, depending upon the strain. In any case, haemolymph of host detects EPNs as well as bacteria quickly and tries to restrict their pathogenic activities. The bacteria multiply inside the nodule and again enter into the haemolymph but this time suppress the insect immune system with releasing of lethal toxin after that mortality of insect takes place (Dunphy and Bourchier 1992). This may be the reason behind this increase in mortality % with the increase in time. After penetration of EPNs into the haemocoel, the insect immune system alarms immediately. Insect pest instantly produced phenoloxidase that deposit a layer of melanin around the EPNs as well as lysozyme and antibacterial peptides against bacterial infection (Khush and Lemaitre 2000).
The second reason might be that in small-sized larvae of P. brassicae the food for EPNs depleted quickly and nematodes were compelled to exit from the dead body of insects earlier. This situation does not exist in 4th larval stage because larger sized larvae food did not deplete quickly and multiplication of EPNs inside the dead body continues until the nutrient soup is totally exhausted. These outcomes of the present study are supported by the work of Pal and Prasad (2012) who find maximum mortality of fourth instars of P. brassicae larvae with higher infective juvenile concentration. Abdolmaleki et al. (2017a); Abdolmaleki et al. (2017b); Gorgadze et al. (2018); Zolfagharian et al. (2016) concluded that with increased nematode concentration and time, mortality percentage of P. brassicae larvae was also increased.
References
Abdolmaleki A, Maafi Z, Dastjerdi H, Naseri B, Ghasemi A (2017a) Immune defense of Pieris brassicae larvae in challenged with Heterorhabditis bacteriophora, its symbiotic bacteria and metabolites. Invert Surviv J 14:73–84. https://doi.org/10.25431/1824-307X/isj.v14i1.73-84
Abdolmaleki A, Rafiee Dastjerdi H, Tanha Maafi Z, Naseri B (2017b) Virulence of two entomopathogenic nematodes through their interaction with Beauveria bassiana and Bacillus thuringiensis against Pieris brassicae (Lepidoptera: Pieridae). J Crop Prot 6:287–299
Ali A, Rizvi PQ (2007) Developmental response of cabbage butterfly, Pieris brassicae L.(Lepidoptera: Pieridae) on different cole crops under laboratory and field condition. Asian J Plant Sci 6:1241–1245
Anjum MA (2018). The pesticides registered with recommendations for safe handling and use in Pakistan. Pakistan Agricultural Research Council, Ministry of National Food Security and Research, Islamabad
Ashfaq F, Butt MS, Nazir A, Jamil A (2018). Compositional analysis of Pakistani green and red cabbage Pak J Agric Sci 55
Chaudhuri N, Ghosh S, Ghosh J, Senapati S (2001) Incidence of insect pests of cabbage in relation to prevailing climatic conditions of Terai region. Ind J Entomol 63:421–428
Dunphy GB, Bourchier RS (1992) Responses of nonimmune larvae of the gypsy moth, Lymantria dispar, to bacteria and the influence of tannic acid. J Invertebr Pathol 60:26–32. https://doi.org/10.1016/0022-2011(92)90149-X
Forset S, Clarke D (2002). Bacteria-nematode symbiosis. Entomopathogenic nematology. CABI Publishing, United Kingdom
Forst S, Dowds B, Boemare N, Stackebrandt E (1997) Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu Rev Microbiol 51:47–72. https://doi.org/10.1146/annurev.micro.51.1.47
Gorgadze O, Bakhtadze G, Nebieridze D (2018) Efficacy of local entomopathogenic nematodes against Pieris brassicae (lepidoptera: pieridae). Int J Dev Res 8:23900–23903
Khush RS, Lemaitre B (2000) Genes that fight infection: what the Drosophila genome says about animal immunity. Trends Genet 16:442–449. https://doi.org/10.1016/S0168-9525(00)02095-3
Krauthausen H-J, Hörner G, Zimmermann S, Voegele R, Brändle F (2018) Competence of Xanthomonas campestris from cruciferous weeds and wallflower (Erysimum cheiri) to induce black rot in cabbage. Eur J Plant Pathol 151:275–289. https://doi.org/10.1007/s10658-017-1371-x
Krishnamoorthy A (2004). Biological control of diamondback moth Plutella xylostella (L.), an Indian scenario with reference to past and future strategies. In: Proceedings of the International Symposium (Kirk, A. A and Bordat, D. eds.), Montpellier, France, CIRAD. pp. 204–211
Kular JS, Kumar S (2017) Quantification of avoidable yield losses in oilseed Brassica caused by insect pests. J Plant Prot Res 51:38–43
Mazurkiewicz A, Tumialis D, Pezowicz E, Skrzecz I, Błażejczyk G (2017) Sensitivity of Pieris brassicae, P. napi and P. rapae (Lepidoptera: Pieridae) larvae to native strains of Steinernema feltiae (Filipjev, 1934). Journal of Plant Diseases and Protection 124:521–524. https://doi.org/10.1007/s41348-017-0118-4
Meena R, Ameta O, Meena B (2013) Population dynamics of sucking pests and their correlation with weather parameters in chilli, Capsicum annum L. crop. The Bioscan 8:177–180
Pal R, Prasad C (2012) Efficacy of Entomopathogenic nematode, Heterorhabditis indica (Meerut strain) against Lepidopteran insect Pest of agriculture importance. Biosciences 5:321–325
Poinar GO (1979) Nematodes for biological control of insects. CRC press, Boca Raton
Poinar Jr GO (1990). Taxonomy and biology of Steinernematidae and Heterorhabditidae. Entomopathogenic nematodes in biological control 54
Saeed M, Shoukat RF, Zafar J (2017) Population dynamics of natural enemies and insect pest in different Brassica oleracea (cabbage) growing seasons with different production systems. J Entomol Zool Stud 5:1669–1674
Sharma R, Singha B, Choudhury SR, Sharma G (2018) Isolation and microscopic investigation of Entomopathogenic nematodes (EPNs) occurring in Barak Valley, Assam, India. Int J Curr Microbiol App Sci 7:1835–1839
Uddin M et al (2007) IPM approach for controlling two lepidopteran pests of cabbage in Bangladesh. Bangl J Entomol 17:19–29
Ullah M, Arshad M, Ali S, Iftikhar Y, Mahmood S (2016) Effect of thiamethoxam and some botanical extracts on Cotesia glomerata L.(Braconidae: hymenoptera): an endoparasitoid of Pieris brassicae (L.)(Pieridae: Lepidoptera). Egypt J biol. Pest Control 26:545
White G (1927) A method for obtaining infective nematode larvae from cultures. Science (Washington) 66:302–303
Wiesner A (1993). Die Induktion der Immunabwehr eines Insekts (Galleria mellonella, Lepidoptera) durch synthetische Materialien und arteigene haemolymphfaktoren. Verlag nicht ermittelbar
Zolfagharian M, Saeedizadeh A, Abbasipour H (2016) Efficacy of two entomopathogenic nematode species as potential biocontrol agents against the diamondback moth, Plutella xylostella (L.). Biol Control 30:78–83. https://doi.org/10.1016/j.tifs.2007.01.004
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abbas, W., Javed, N., Haq, I.U. et al. Pathogenicity of ENTOMOPATHOGENIC nematodes against cabbage butterfly (PIERIS BRASSICAE) LINNAEUS (LEPIDOPTERA: PIERIDAE) in laboratory conditions. Int J Trop Insect Sci 41, 525–531 (2021). https://doi.org/10.1007/s42690-020-00236-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-020-00236-2