Abstract
Screening the biological activities of plant secondary metabolites on economic pests can lead to discovery new ecofriendly biopesticides. The aim of this work was to evaluate the antifeedant, growth inhibitory and toxic activities of seven monoterpenes, two phenylpropenes and two sesquiterpenes on 2nd larval instar of Spodoptera littoralis. The tested compounds induced a significant antifeedant effect at various concentrations (500, 1000 and 2000 mg/kg), particularly after 6 and 9 days of exposure. Among the tested compounds, trans-cinnamaldehyde, α-terpinene, (−)-citronellal and 1,8-cineole were the most potent antifeedants after the three exposure periods. In general the tested compounds showed remarkable antifeedant activity after 9 days of exposure as their antifeedant indices ranged between 44.0 and 80.1%. On the other hand, the tested compounds drastically inhibited the growth of S. littoralis larvae at the tested concentrations. The larval growth inhibition ranged between 21.4 and 100% with cuminaldehyde, 1,8-cineole and eugenol being the most potent growth inhibitors. Some of the tested compounds caused significantly higher antifeedant and growth inhibitory effects than a reference insecticide, pyriproxifen. In general, the tested compounds showed higher growth inhibition than antifeedant effect. The tested compound also induced S. littoralis larval morality which improved with increasing exposure time and concentration. Cuminaldehyde, 1,8-cineole and (−)-carvone showed highest toxicity with 100.0, 97.0 and 77.0% mortality, respectively, at 2000 mg/kg after 9 days of exposure. Biochemical studies revealed that trans-cinnamaldehyde (IC50 = 0.03 mM), farnesol (IC50 = 0.04 mM) and eugenol (IC50 = 0.06 mM) are potent α-amylase inhibitors. These three compounds also caused significant inhibition of total proteases activity. This is the first report on antifeedant, growth inhibitory and insecticidal activities of the tested compounds on S. littoralis. Moreover, the strong bioactivity reported in this study indicated that these compounds have a potential to be used as bioinsecticides.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Developing new biopesticides from plant-derived products is an important issue in modern agricultural production systems. Recently, many countries around world have headed towards the use of integrated pest management (IPM) programs due to the several drawbacks associated with continuous use of synthetic pesticides (Czaja et al. 2015; Tonial et al. 2017). One of the main components of IPM is the application of plant extracts, oils and secondary metabolites in pest control as class of biopesticides. In this regard, plant products have been used as efficient toxicants, antifeedants, repellents and growth regulators against economic agricultural and public health insects (Miresmailli and Isman 2014; Pavela 2016; Szczepanik et al. 2016; Hernández-Carlos and Gamboa-Angulo 2019).
Certain classes of plant secondary metabolites, particularly monoterpenes, phenylpropenes and sesquiterpenes, are viewed as exceptionally promising natural pesticides. These three classes of compounds are usually present as major constituents in plant essential oils. These compounds have several ecological functions in plants, such as protection against insects, animals and pathogens, attraction of pollinators and allelopathy (Fischer et al. 1994; Langenheim 1994; Dudareva and Pichersky 2008). In addition, several studies have reported the wide spectrum of biological activities of monoterpenes, phenylpropenes and sesquiterpenes on economic insects as they can act as insecticides (Abdelgaleil et al. 2009; Wu et al. 2016; Saad et al. 2018), antifeedants (Gonzalez et al. 1997; Rajkumar et al. 2019), repellents (Watanabe et al. 2005; Peixoto et al. 2015), insect growth regulators (Céspedes et al. 2001; Zahran and Abdelgaleil 2011).
The cotton leafworm, Spodoptera littoralis (Boisduval), is among the most damaging lepidopterous insects to several important crops in subtropical and tropical zones. It attacks about 87 plant species belong to more than 40 families (Capinera 2008). Because of its highly polyphagous behavior, it is considered as a devastating insect. Therefore, some strategies have been developed to reduce the economic damage caused by S. littoralis, including the use of biorational chemical control and IPM.
Few reported studies were found in the literature on the antifeedant and growth inhibitory effects of monoterpenes, phenylpropenes and sesquiterpenes against S. littoralis (Gonzalez et al. 1997; Zapata et al. 2009; Ali et al. 2017). Therefore, the present study aimed to evaluate the antifeedant, growth inhibitory and toxic effects of seven monoterpenes [cuminaldehyde (major component of cumin oil), (−)-carvone (major component of caraway oil), (−)-citronellal (major component of citronella oil), 1,8-cineole (major component of eucalyptus oil), (−)α-pinene (major component of pine tree oil), α-terpinene (major component of citrus oils) and p-cymene (major component of cumin and thyme oils)], two phenylpropenes [trans-cinnamaldehyde (major component of cinnamon oil) and eugenol (major component of clove oil)] and two sesquiterpenes [(farnesol (component of lemon grass, citronella and other oils) and (Z,E)-nerolidol (major component of neroli and nerolina oils)] against the second larval instar of S. littoralis. Also, the inhibitory effects of selected compounds on the activity of two digestive enzymes, α-amylase and total proteases, were studied.
Materials and methods
Test insect
A susceptible strain of cotton leafworm, Spodoptera littoralis Boisduval (Lepidoptera: Noctuidae) was kept under laboratory conditions at 26 ± 2 °C and 70 ± 5% RH. The larvae were fed on Ricinus communis L. (Euphorbiaceae) leaves as described by El-Defrawi et al. (1964). The second-larval instar of S. littoralis was chosen in this study because it is the first damaging larval stage. Also, this stage takes more time to reach the pupal stage than third and fourth stages that allow measuring antifeedant, growth inhibitory and toxic effects after 6 and 9 days of treatment.
Test compounds
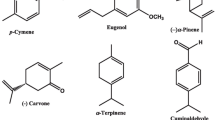
Seven monoterpenes, two phenylpropenes and two sesquiterpenes were purchased from Sigma–Aldrich Chemical Co., Steinheim, Germany, and used in this study. The compounds are cuminaldehyde (98%), (−)-carvone (98%), (−)-citronellal (95%), 1,8-cineole (99%), (−)α-pinene (98%), α-terpinene (85%), p-cymene (99%), trans-cinnamaldehyde (99%), eugenol (99%) farnesol (95%) and (Z,E)-nerolidol (98%). Chemical structures of these compounds are shown in Fig. 1. A reference insecticide, pyriproxyfen (98%), was obtained from Kafr El-Zayat Pesticides and Chemicals Co., Egypt. All solvents and reagents used in experiments were of high performance liquid chromatography (HPLC) grade.
Antifeedant, growth inhibition and toxicity assay
Monoterpenes, phenylpropenes and sesquiterpenes were evaluated for their antifeedant, growth inhibitory and toxic effects on the second-larval instar of S. littoralis by using diet-non-choice method (Abdelgaleil and El-Aswad 2005). The solutions of compounds were first prepared in acetone and incorporated with the artificial diet (Bakry et al. 1973) to give concentrations of 500, 1000 and 2000 mg/kg. Control treatment was diet mixed acetone at concentration of 0.5% (v/w). After complete evaporation of the solvent, 10 g of treated diet was placed in each Petri dish (9.0 cm diameter). Then 10 preweighed second larval instars were introduced to each Petri dish. Five replicates were carried out for each concentration. The eaten diet by each larva was determined after 3, 6 and 9 days of feeding by weighing the remaining diet in each Petri dish. Then, antifeedant index was calculated by the equation:
where C is the weight of diet consumed by each larva in control and T is the weight of diet consumed by each larva in the treatment (Abdelgaleil and El-Aswad 2005). The growth inhibition of larvae was assayed relative to control based on larval weight gain through 3, 6 and 9 days of feeding on the treated diet. The growth inhibition of larvae was calculated from the following equation:
where CL is the gain of larval weight in the control and TL is the gain of larval weight in the treatment (Abdelgaleil and El-Aswad 2005). The larval mortality was recorded after 3, 6 and 9 days of feeding on treated diet and the mortality percentages were calculated.
In vitro inhibition of α-amylase activity assay
Midguts of the 4th and 5th larval instars of S. littoralis were collected, excised, washed with ice-cold saline solution (0.9% NaCl) repeatedly to remove foodstuff. One gram of total larvae was homogenized in 5 ml glass distilled water using Polytron Kinemetica on ice. The homogenate was centrifuged at 15000 rpm for 15 min at 4 °C using IEC-CRU 5000 cooling centrifuge. The supernatant was used for α-amylase activity assay. The in vitro inhibition of total proteases activity was determined by incubating the enzyme for 30 min at 37 °C with different concentrations (0.005–1.0 mM) of tested compounds prepared in acetone. Emulsifying agent, Triton-X 100, was added at concentration of 0.01% to enzyme solution. The control treatments were prepared by adding 20 μl of acetone without tested compounds. Activity of α-amylase was assayed according to Kaufman and Tietz (1980). Fifty μl of enzyme source was added to an assay mixture in final volume 1 ml contains 2.3 mM 2-chloro 4-nitrophenyl-α-D-maltotrioside (CNPG3), 350 mM NaCl, 6 mM calcium acetate, 600 mM potassium thiocyanate and 100 mM Good’s buffer pH 6. An assay mixture without enzyme was used as the blank. The change in absorption at 405 nm was monitored on Sequoia-Turner Model 340 spectrophotometer for 4 min. Activity of α-amylase was calculated as ∆OD405/mg protein/min. The inhibition percentage of α-amylase activity was calculated. The concentrations of the tested compounds that inhibited 50% the enzyme activity (IC50) were determined from a linear regression analysis (Finney 1971).
In vitro inhibition of total proteases activity assay
Midguts of the 4th and 5th larval instars of S. littoralis were collected, excised, washed with ice-cold saline solution (0.9% NaCl) repeatedly to remove foodstuff. Midguts were then homogenized in (1: 10 w/v) 100 mM Tris-HCl buffer pH 7 using Polytron Kinemetica on ice. The homogenate was centrifuged at 4000 rpm for 15 min at 4 °C using IEC-CRU 5000 cooling centrifuge. The supernatant was used for total proteolytic activity estimation. The in vitro inhibition of total proteases activity was determined by incubating the enzyme for 30 min at 37 °C with different concentrations (0.02–20 mM) of tested compounds prepared in acetone. Emulsifying agent, Triton-X 100, was added at concentration of 0.01% to enzyme solution. The control treatments were prepared by adding 20 μl of acetone without tested compounds. Then, the total proteolytic activity was measured using asocasein as a substrate according to (Olga et al. 2002; Mohen and Gujar 2003). The homogenate was incubated in a total volume 60 μl of assay buffer (100 mM Tris-HCl pH 8) for 20 min at 37 °C before addition of 200 μl of 2% azocazein (w/v in assay buffer). The reaction was allowed to proceed for 180 min at 37 °C, and then stopped by addition of 300 μl cold 10% trichloroacetic acid (TCA). The reaction mixture was centrifuged at 3000 rpm for 10 min IEC-CRU 5000 cooling centrifuge. Excess acidity was neutralized by adding 10 μl NaOH (10 N) to the reaction mixture and absorbance was measured at 440 nm using Sequoia-Turner Model 340 spectrophotometer. An assay mixture without enzyme was used as the blank. The inhibition percentage of total proteases activity and IC50 of the tested compounds were calculated as previously described.
Statistical analysis
Significant differences among mean values of antifeedant indices, growth inhibitory and mortality percentages were determined (P = 0.05) by using one-way analysis of variance followed by Student–Newman–Keuls test (Cohort software Inc. 1985). The enzyme inhibition percentages were subjected to probit analysis (Finney 1971) to obtain IC50 values, using SPSS 21.0 (SPSS, Chicago, IL, USA).
Results
Antifeedant activity of monoterpenes, phenylpropenes and sesquiterpenes
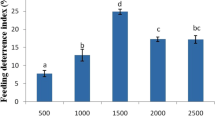
The antifeeding activity of the monoterpenes, phenylpropenes and sesquiterpenes were tested against the 2nd larval instar of S. littoralis on semi-artificial diet. The tested compounds showed different levels of feed-deterrence activities at various concentrations. The antifeedant indices of the tested compounds after 3, 6 and 9 days of feeding on treated diet at concentrations of 500, 1000 and 2000 mg/kg are shown in Table 1. The antifeedant activity of tested compounds enhanced significantly with increasing the treatment period. After 3 days of feeding on treated diet, trans-cinnamaldehyde revealed the strongest antifeedant activity at the three tested concentrations. The antifeeant indices of this compound were 33.3, 44.4 and 44.4% at 500, 1000 and 2000, respectively. The other compounds had significantly lower antifeeant indices ranged between 1.9 and 37.0%. After 6 days, α-terpinene caused the highest feed deterrence, followed by trans-cinnamaldehyde, eugenol, and (−)-carvone. Their antifeedant indices were 50.0, 44.4, 38.7, and 37.9 at concentration of 500 mg/kg, respectively. In addition, 1,8-cineole revealed the highest antifeedant activity at 1000 and 2000 mg/kg with antifeedant indices of 56.5 and 78.2%, respectively. The antifeedant indices of the tested compounds ranged between 44.0 and 80.1% after 9 days of treatment. Trans-Cinnamaldehyde caused the highest antifeedant activity at 500 and 2000 mg/kg, while citronellal was the most potent at 1000 mg/kg. (−)-Carvone, trans-cinnamaldehyde and p-cymene were more potent antifeedant than a reference insecticide, pyriproxifen, at the tested concentrations after 3 days of treatment.
Growth inhibitory effect of monoterpenes, phenylpropenes and sesquiterpenes
The tested monoterpenes, phenylpropenes and sesquiterpenes caused a significant reduction on S. littoralis larval growth after 3, 6 and 9 days of feeding in treated diet with 500, 1000 and 2000 mg/kg (Table 2). Remarkable growth inhibition (GI) was observed after three days of treatment as the larval growth inhibition (GI) ranged between 45.4 and 100%. Cuminaldehyde (GI = 96.2%) and 1,8-cineole (GI = 95.9%) exhibited the strongest larval growth inhibition at 500 mg/kg. Both compounds caused higher growth inhibition than pyriproxyfen at this concentration. At 1000 mg/kg, 1,8-cineole (GI = 100.0%), eugenol (GI = 86.2%) and cuminaldehyde (GI = 86.0%) were the most potent growth inhibitors, while 1,8-cineole (GI = 100.0%) and cuminaldehyde (GI = 85.2%) had the highest growth inhibition at 2000 mg/kg. After 6 days of treatment, 1,8-cineole revealed the highest growth inhibition at the three tested concentrations with 82.8, 86.9 and 91.5% growth inhibition at 500, 1000 and 2000 mg/kg, respectively. After 9 days treatment, 1,8-cineole was the most potent growth inhibitor among the compounds at the three tested concentrations with growth inhibition percentages of 87.9, 73.5 and 87.4 at 500, 1000 and 2000 mg/kg, respectively. The other compounds showed growth inhibition ranged between 29.3 and 79.2%.
Insecticidal activity of monoterpenes, phenylpropenes and sesquiterpenes
The toxicity of monoterpenes, phenylpropenes and sesquiterpenes on the 2nd larval instar of S. littoralis after feeding on treated semi-artificial diet for 3, 6 and 9 days is presented in Table 3. The results showed that the mortality percentages enhanced by increasing the concentration and exposure period. Cuminaldehyde was the most potent compound at concentration of 500 mg/kg, while1,8-cineole and (−)-carvone revealed the highest insecticidal activity at concentrations of 1000 and 2000 mg/kg, respectively, after the three exposure times. At 2000 mg/kg, (−)-carvone induced complete larval mortality (100%) and 1,8-cineole caused 97.0% mortality after the three exposure times. Cuminaldehyde, 1,8-cineole and (−)-carvone showed higher toxicity than pyriproxifen. In contrary, farnesol, nerolidol, α-pinene and α-perpinene revealed weak insecticidal activity at the three tested concentrations.
Inhibitory effect of phenylpropenes and sesquiterpenes on α-amylase and total proteases
Among the tested compounds, trans-cinnamaldehyde, eugenol and farnesol caused high antifeedant and growth inhibition activities. Thus, these three compounds were tested for their inhibitory effects on α-amylase and total proteases isolated from S. littoralis larvae. The tested compounds revealed remarkable inhibitory effect on α-amylase activity (Table 4). trans-Cinnamaldehyde caused the highest inhibitory activity among the tested compound with IC50 value of 0.03 mM, followed by farnesol with IC50 values of 0.04 mM, then eugenol with IC50 value of 0.06 mM. On the other hand, eugenol (IC50 = of 0.24 mM) caused the greatest inhibition of the total proteases activity, followed by trans-cinnamaldehyde (IC50 = 1.12 mM) and farnesol (IC50 = 2.33 mM).
Discussion
Plants have numerous secondary metabolites that possess plant protection properties against different pests and pathogens. Application of these natural products in insect pest management programs has received much attention in recent years due to drawbacks associated with unwise use of synthetic insecticides. Particularly, the plant compounds with feeding deterrent and growth inhibitory properties gained more attention in IPM programs because these compounds are important mediators of plant–insect interactions and insect behavior manipulators. The feeding deterrent and growth regulatory properties of plant extracts and secondary metabolites have been studied against lepidopteran larvae and other insects (Ballesta-Acosta et al. 2008; Rani and Murty 2009; Zapata et al. 2009; Pavela 2010).
In the current study, monoterpenes, phenylpropenes and sesquiterpenes revealed pronounced antifeeding activity on the second larval instar of S. littoralis. Among the tested compounds, trans-cinnamaldehyde, 1,8-cineole, (−)-citronellal and farnesol were the most effective antifeedant, particularly after 9 days of treatment. The results indicated that the antifeedant activity of tested compounds was time dependent. For example, trans-cinnamaldehyde and p-cymene were relatively the most active antifeedants after 3 days of treatment, while 1,8-cineole and α-terpinene revealed the highest antifeedant activity after 6 days of treatment. Additionally, trans-cinnamaldehyde and (−)-citronellal were the most effective antifeedants after 9 days of treatment. The quick antifeedant activity (after 3 days) of compounds may be attributed to the repellent effect of these compounds or to their unacceptable taste for the larvae. So the larvae did not eat at the beginning but after starvation the larvae started feeding. The late antifeedant activity (after 6 and 9 days) of compounds may be due to the inhibitory effect of compounds on digestive enzymes which allowed larvae to eat at the beginning and stop eating later on. The enhancing of antifeedant activity of all compounds with increasing exposure time could be attributed to the combine effect of repellent, unacceptable taste and inhibition of digestive enzymes.
In the literature, there are no recorded studies on the antifeeding activity of tested compounds on S. littoralis larvae. However, essential oils whose major constituents are monoterpenes and sesquiterpenes have been shown to have antifeedant activity against the fourth larval instar of S. littoralis (Ali et al. 2017). Similarly, Gonzalez et al. (1997) explained the antifeedant activity of sesquiterpenes isolated from seven Celastraceae species against fifth larval instar of S. littoralis. Other phytochemicals, such as diterpenes (eriocephalin, salviacoccin, aethiopinone and oxocandesalvone), coumarins (oxypeucedanin, xanthotoxin, isoimperatorin and prangol), drimanes (drimendiol, isodrimeninol, isotadeonal and polygodial) and limonoids (khayalactol, khayanolide D, 2-hydroxyseneganolide, 1-O-acetylkhayanolide A, khayanolide A and methyl angolensate) have been described to show significant antifeedant activity against larval instars of this insect (Ballesta-Acosta et al. 2008; Zapata et al. 2009). On the other hand, some of the tested compounds have been shown to possess antifeedant activity against other insects. For example, eugenol and 1,8-cineole revealed high feeding-deterrence effect on Rhyzopertha dominica and Tribolium castaneum (Ukeh and Umoetok 2011). Other monoterpenes, such as thymol, (+)-limonene and (±)-camphene had antifeedant activity against T. castaneum, R. dominica and Solanum tuberosum (Szczepanik et al. 2009; Kanda et al. 2016).
The results indicated that the tested compounds are promising growth inhibitors of second larval instar of S. littoralis for the first time. These results are supported by earlier studies in which some sesquiterpenes, such as drimendiol, isodrimeninol were reported to cause growth inhibition of S. littoralis larvae (Zapata et al. 2009). It is also documented that eudesmane sesquiterpenes inhibited the growth of S. frugiperda, (Sosa et al. 2017). Moreover, some triterpenes caused growth inhibition of S. littoralis larvae, such as limonoids from Khaya senegalensis, Chukrasia tabularis and Swietenia mahogani (El-Aswad et al. 2003; Abdelgaleil and El-Aswad 2005). The potent growth inhibitory effects observed here indicated that the tested monoterpens, phenylpropenes and sesquiterpenes could strongly delay the development of the insect, produce smaller pupa, reduce adult emergence and also decrease the fecundity and fertility of the emerged females which led to drastic reduction in insect population as well as make insects more susceptible to diseases and other control methods. Therefore, these compounds could be used as promising candidates in the plant protection programs of this insect.
Besides their effects on feeding-deterrence and growth inhibition, the tested compounds also showed insecticidal activity against of S. littoralis larvae. However, the majority of compounds showed less toxic effect than growth inhibition and antifeedant effects. The results also showed that (−)-carvone and 1,8-cineole are the most active toxicants against the 2nd larval instar of S. littoralis. This finding is supported by earlier studies in which (−)-carvone and 1,8-cineole showed strong fumigant and contact toxicities against the third larval instar of this insect (Abdelgaleil 2010). The toxicity of phenylpropenes and monoterpenes observed in the present study is also supported by earlier studies in which trans-ethyl cinnamate, thymol, carvacrol, trans-anethole and piperitone revealed contact toxicity against the third larval instar (Abdelgaleil et al. 2008; Pavela 2014), and γ-terpinene and terpinen-4-ol caused contact toxicity against the fourth larval instar of S. littoralis (Abdelgaleil et al. 2008; Abbassy et al. 2009). Moreover, few sesquiterpenes have been reported to cause residual toxicity against S. littoralis larvae (Srivastav et al. 1990; Gonzalez et al. 1997).
It has been suggested that the antifeedant compounds deter insect feeding via sensory perception, such as having an unpalatable taste to insects and/or via postingestive effects (Abdelgaleil and El-Sabrout 2018). Essential oils and their major constituents (monoterpenes, phenylpropenes and sesquiterpenes) possess aromatic properties and cause insects disgusted by food and reduce or stop feeding (Arasu et al. 2013). Some essential oils and monoterpenes have been reported to inhibit α-amylase and other digestive enzymes (Basak and Candan 2010; Sudha et al. 2011; Kohl et al. 2015). The results of this study supported the later hypothesis as the tested compounds induced inhibitory effects on α-amylase and total proteases activities. In fact, other plant secondary metabolites, such as terpenes (squalene, lupeol, oleanoic acid, ursolic acid and betulinic acid) have been shown to inhibit α-amylase activity (de Sales et al. 2012).
Although the effect of monoterpenes, phenylpropenes and sesquiterpenes on total proteases were not previously reported some terpenoids, such as diterpenes and triterpenes and have been shown to inhibit digestive proteases of Colorado potato beetle, Leptinotarsa decemlineata (Ortego et al. 1999). Furthermore, azadirachtin, tetranortriterpene, has been reported to inhibit the activity of digestive proteases in larvae of S. litura which indicated its disruption effects on the digestive process in insects (Koul et al. 1996), Manduca sexta (Timmins and Reynolds 1992) and S. littoralis (Abou-Taleb 2016).
In conclusion, the tested monoterpenes, phenylpropenes and sesquiterpenes caused interesting antifeedant and growth inhibitory effects as well as induced toxicity of S. littoralis larvae. Therefore, these compounds, particularly, trans-cinnamaldehyde, cuminaldehyde, 1,8-cineole, (−)-carvone and eugenol, could be serve as effective bio-insecticides for managing this polyphagous insect. However, studies on their binary mixtures and their mixtures with synergists are recommended to enhance their efficacy against target insect. Further studies are also needed on formulations and stability of these compounds under field condition. Also, the efficacy of the formulations on target and non-target organisms should be addressed before commercial use.
References
Abbassy MA, Abdelgaleil SAM, Rabie RYA (2009) Insecticidal and synergistic effects of Majorana hortensis essential oil and some of its major constituents. Entomol Exp Appl 131:225–232
Abdelgaleil SAM (2010) Molluscicidal and insecticidal potential of monoterpenes on the white garden snail, Theba pisana (Muller) and the cotton leafworm, Spodoptera littoralis (Boisduval). Appl Entomol Zool 45:425–433
Abdelgaleil SAM, El-Aswad AF (2005) Antifeedant and growth inhibitory effects of tetranortriterpenoids isolated from three meliaceous species on the cotton leafworm, Spodoptera littoralis (Boisd.). Res J Appl Sci 1:234–241
Abdelgaleil SAM, El-Sabrout AM (2018) Anti-nutritional, antifeedant, growth-disrupting and insecticidal effects of four plant essential oils on Spodoptera littoralis (Lepidoptera: Noctuidae). J Crop Prot 7:135–150
Abdelgaleil SAM, Abbassy MA, Belal AH, Abdel-Rasoul MAA (2008) Bioactivity of two monoterpenoids isolated from Artemisia judaica L. Bioresouce Technol 99:5947–5950
Abdelgaleil SAM, Mohamed MIE, Badawy MEI, El-Arami SAA (2009) Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J Chem Ecol 35:518–525
Abou-Taleb HK (2016) Effects of azadirachtin and methoxyfenozide on some biological and biochemical parameters of cotton leafworm, Spodoptera littoralis (Lepidoptera: Noctuidae). Egypt Sci J Pestic 2:17–26
Ali AM, Mohamed DS, Shaurub EH, Elsayed AM (2017) Antifeedant activity and some biochemical effects of garlic and lemon essential oils on Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). J Entomol Zool Stud 5:1476–1482
Arasu MV, Al-Dhabi NA, Saritha V, Duraipandiyan V, Muthukumar C, Kim SJ (2013) Antifeedant, larvicidal and growth inhibitory bioactivities of novel polyketide metabolite isolated from Streptomyces sp. AP-123 against Helicoverpa armigera and Spodoptera litura. BMC Microbiol 13:105
Bakry N, Taman F, Zeid M (1973) Effect of nutrition, age and temperature on toxicity of insecticides to Spodoptera littoralis (Boisd). I Egypt Pest Cont Cong, Assiut, pp 105–115
Ballesta-Acosta MC, Pascual-Villalobos MJ, Rodriguez B (2008) The antifeedant activity of natural plant products towards the larva of Spodoptera littoralis. Span J Agric Res 6:85–91
Basak SS, Candan F (2010) Chemical composition and in vitro antioxidant and antidiabetic activities of Eucalyptus camaldulensis. Essent oil J Iran Chem Soc 7:216–226
Capinera JL (2008) Cotton Leafworm, Spodoptera littoralis (Boisduval). In: Capinera JL (ed) Encyclopedia of entomology. Springer, Dordrecht
Céspedes CL, Alarcón J, Aranda E, Becerra J, Silva M (2001) Insect growth regulator and insecticidal activity of ß-dihydroagarofurans from Maytenus spp. (Celastraceae). Z Naturforsch 56c:603–613
Cohort Software Inc (1985) Costat User’s Manual. Version 3. Tucson, AZ: Cohort
Czaja K, Góralczyk K, Struci’nski P, Hernik A, Korczn W, Minorczyk M, Lyczewska M, Ludwicki JK (2015) Biopesticides-towards increased consumer safety in the European Union. Pest Manag Sci 71:3–6
De Sales PM, De Souza PM, Simeoni LA, Magalhães PO, Silveira D (2012) α-Amylase inhibitors: a review of raw material and isolated compounds from plant source. J Pharm Pharmaceut Sci 15:141–183
Dudareva N, Pichersky E (2008) Metabolic engineering of plant volatiles. Curr Opin Biotechnol 19:181–189
El-Aswad AF, Abdelgaleil SAM, Nakatani M (2003) Feeding deterrent and growth inhibitory properties of limonoids from Khaya senegalensis (Desr.) against the cotton leafworm, Spodoptera littoralis (Boisd.). Pest Manag Sci 60:199–203
El-Defrawi ME, Tappozada A, Mansour N, Zeid M (1964) Toxicological studies on the Egyptian cotton leafworm Prodenia litura L.: susceptibility of different larval instars of Prodenia to insecticides. J Econ Entomol 57:591–593
Finney DJ (1971) Probit Analysis, 3rd edn. Cambridge University Press, London
Fischer NH, Williamson GB, Weidenhamer JD, Richardson DR (1994) In search of allelopathy in the Florida scrub: the role of terpenoids. J Chem Ecol 20:1355–1379
Gonzalez AG, Jimenez IA, Ravelo AG, Coll J, Gonzalez JA, Lloria J (1997) Antifeedant activity of sesquiterpene from celastraceae. Biochem Syst Ecol 25:513–519
Hernández-Carlos B, Gamboa-Angulo M (2019) Insecticidal and nematicidal contributions of Mexican flora in the search for safer biopesticides. Molecules 24:897–940
Kanda D, Kaur S, Koul O (2016) A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: acute toxins or feeding deterrents. J Pest Sci 90:531–545
Kaufman RA, Tietz NW (1980) Recent advances in measurements of amylase activity – a comparative study. Clin Chem 26:846–853
Kohl KD, Pitman BC, Robb JW, Connelly MD, Dearing FJS (2015) Monoterpenes as inhibitors of digestive enzymes and counter-adaptations in a specialist avian herbivore. J Comp Physiol B 185:425–434
Koul O, Shankar JS, Kapil RS (1996) The effect of neem allelochemicals on nutritional physiology of larval Spodoptera litura. Entomol Exp Appl 79:43–50
Langenheim JH (1994) Higher plant terpenoids: a phytocentric overview of their ecological roles. J Chem Ecol 20:1223–1280
Miresmailli S, Isman MB (2014) Botanical insecticides inspired by plant-herbivore chemical interactions. Trends Plant Sci 19:29–35
Mohen M, Gujar T (2003) Characterization and comparison of midgut proteases of Bacillus thuringiensis susceptible and resistant diamondback moth (Lepidoptera: Plutellidae). J Invertebr Pathol 82:1–11
Olga L, Ibrahim MM, Candas NC, Koller NC, Bauer LS, Bulla LA (2002) Changes in proteases activity and cry 3Aa toxin binding in the Colorado potato beetle: implications for insect resistance to Bacillus thuringiensis toxins. Insect Biochem Mol Biol 32:567–577
Ortego F, Lopez-Olguın J, Ruız M, Castanera P (1999) Effects of toxic and deterrent terpenoids on digestive protease and detoxication enzyme activities of Colorado potato beetle larvae. Pestic Biochem Physiol 63:76–84
Pavela R (2010) Antifeedant activity of plant extracts on Leptinotarsa decemlineata say. And Spodoptera littoralis bois. Larvae. Ind Crop Prod 32:213–219
Pavela R (2014) Acute, synergistic and antagonistic effects of some aromatic compounds on the Spodoptera littoralis Boisd. (Lep., Noctuidae) larvae. Ind Crop Prod 60:247–258
Pavela R (2016) History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects – a review. Plant Prot Sci 52:229–241
Peixoto MG, Bacci L, Blank AF, Araújo APA, Alves PB, Silva JHS, Santos AA, Oliveira AP, da Costa AS, Arrigoni-Blank MF (2015) Toxicity and repellency of essential oils of Lippia alba chemotypes and their major monoterpenes against stored grain insects. Ind Crop Prod 71:31–36
Rajkumar V, Gunasekaran C, Christy IK, Dharmaraj J, Chinnaraj P, Paul CA (2019) Toxicity, antifeedant and biochemical efficacy of Mentha piperita L. essential oil and their major constituents against stored grain pest. Pestic Biochem Physiol 156:138–144
Rani AS, Murty US (2009) Antifeedant activity of Spilanthes acmella flower head extract against Spodoptera litura (Fabricius). J Entomol Res 33:55–57
Saad MG, Abou-Taleb HK, Abdelgaleil SAM (2018) Insecticidal activity of monoterpenes and phenylpropenes against Sitophilus oryzae L. and their acetylcholinesterase and adenosine triphosphatases inhibitory effects. Appl Entomol Zool 53:173–181
Sosa A, Costa M, Salvatore A, Bardon A, Borkosky S, Vera N (2017) Insecticidal effects of eudesmanes from Pluchea sagittalis (Asteraceae) on Spodoptera frugiperda and Ceratitis capitata. Int J Eenviron Agric Biotechnol 2:2456–1878
Srivastav RP, Prokscht P, Wray V (1990) Toxicity and antifeedant activity of a sesquiterpene lactone from Encelia against Spodoptera littoralis. Phytochemistry 29:3445–3344
Sudha P, Zinjarde SS, Bhargava SY, Kumar AR (2011) Potent α-amylase inhibitory activity of Indian Ayurvedic medicinal plants. BMC Complement Altern Med 11:5
Szczepanik M, Szumny A, Wawrzeńczyk C (2009) The effect of α-methylenelactone group on the feeding deterrent activity of natural and synthetic alkenes against Colorado potato beetle, Leptinotarsa decemlineata say. Pol J Environ Stud 18:1107–1112
Szczepanik M, Gliszczynska A, Hnatejko M, Zawitowska B (2016) Effects of halolactones with strong feeding-deterrent activity on the growth and development of larvae of the lesser mealworm, Alphitobius diaperinus (Coleoptera: Tenebrionidae). Appl Entomol Zool 51:393–401
Timmins WA, Reynolds SE (1992) Azadirachtin inhibits secretion of trypsin in midgut of Manduca sexta caterpillars, reduced growth due to impaired protein digestion. Entomol Exp Appl 63:47–54
Tonial F, Maia BHLNS, Savi DC, vicente VA, Gomes RR (2017) Biological activity of Diaporthe terebinthifolii extracts against Phyllosticta citricarpa. FEMS Microbiol Lett 364:1–7
Ukeh DA, Umoetok SBA (2011) Repellent effects of five monoterpenoid odours against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.) in Calabar, Nigeria. Crop Prot 30:1351–1355
Watanabe Y, Mihara R, Mitsunaga T, Yoshimura T (2005) Termite repellent sesquiterpenoids from Callitris glaucophylla heartwood. Forest Ecol Manag 258:1918–1923
Wu H, Wu H, Wang W, Liu T, Qia M, Feng J, Li X, Liu Y (2016) Insecticidal activity of sesquiterpene lactones and monoterpenoid from the fruits of Carpesium abrotanoides. Ind Crop Prod 92:77–83
Zahran HA, Abdelgaleil SAM (2011) Insecticidal and developmental inhibitory properties of monoterpenes on Culex pipiens L. (Diptera: Culicidae). J Asia Pac Entomol 14:46–51
Zapata N, Budia F, Vinuela E, Medina P (2009) Antifeedant and growth inhibitory effects of extracts and drimanes of Drimys winteri stem bark against Spodoptera littoralis (Lep., Noctuidae). Ind Crop Prod 30:119–125
Acknowledgments
This research was partially funded by the Alexandria University Research Fund (ALEX-REP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Abdelgaleil, S.A.M., Abou-Taleb, H.K., Al-Nagar, N.M.A. et al. Antifeedant, growth regulatory and biochemical effects of terpenes and phenylpropenes on Spodoptera littoralis Boisduval. Int J Trop Insect Sci 40, 423–433 (2020). https://doi.org/10.1007/s42690-019-00093-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-019-00093-8