Abstract
The recent isolation of an atoxigenic strain of Aspergillus flavus (Link) (Eurotiales: Trichocomaceae), which was virulent to all stages of Xylosandrus compactus (Eichhoff) (Coleoptera: Curculionidae) has generated interest to investigate virulence of the entomopathogenic fungus against other crop pests, and how it compares with established commercial biopesticides. In this study, we tested the pathogenicity and virulence of the atoxigenic A. flavus strain to Ootheca mutabilis (Sahlberg) (Coleoptera: Chrysomelidae) in the laboratory compared to the commercial Metarhizium anisopliae (Metschnikoff Sorokin) (Hypocreales: Clavicipitaceae; OD®, ICIPE 69). Serial dilutions of both entomopathogenic fungi (1 × 106–1 × 109 conidia/ml) were evaluated against adult O. mutabilis. Distilled water and cypermethrin 5% EC were included as controls. The treatments were replicated four times and the bioassay was repeated once. Both fungi at 1 × 109 conidia/ml killed 100% of tested O. mutabilis within seven days in the 1st and 2nd trials. The concentration of both fungi that killed 50% of O. mutabilis was the same in both trials (5.3 × 106 conidia/ml). The time taken by A. flavus and ICIPE 69 to kill 50% of O. mutabilis was not significantly different in the 1st trial (3.4 ± 0.1 days and 2.9 ± 0.4 days, respectively). However, ICIPE 69 took a significantly shorter time (2.1 ± 0.4 days) than A. flavus (3.6 ± 0.2 days) to kill 50% of O. mutabilis in the 2nd trial. We concluded that A. flavus is comparably as pathogenic and virulent (in terms of cumulative mortality and LC50) against O. mutabilis as ICIPE 69, and therefore both fungi may be viable biopesticides for commercialization against this pest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bean leaf beetles, Ootheca spp. (Coleoptera: Chrysomelidae) are important indigenous agricultural field pests of the common bean Phaseolus vulgaris (Linnaeus) (Fabales: Fabaceae) in Sub-Saharan Africa (SSA) (Paul et al. 2007; Bazie et al. 2019). Two species in the genus Ootheca, namely O. bennigseni (Weise) and O. mutabilis (Sahlberg) are common in eastern and southern Africa (Abate and Ampofo 1996; Kankwatsa 2018). These beetles attack the roots, leaves, flowers and bean pods, causing variable damage depending on the location; season and environmental conditions (Grobbelaar 2008; Mwanauta et al. 2015). Attack on roots by the early instar larvae may go unnoticed but the older larvae feed on lateral roots, causing wilting and premature senescence in bean plants. Emergence of adults usually coincides with germination of host plants at the onset of the rainy season. In case of a prolonged dry season, teneral adults undergo diapause until the onset of the next rainy season when they emerge and start feeding on leaves of the newly planted legumes. Adult damage to the growing shoot of the young seedling destroys the entire plant. These beetles continue to feed on the foliage between veins of growing legume plants, and cause extensive defoliation. Grain yield losses of the common bean attributed to Ootheca spp. range from 18% to 31% and complete crop loss can occur under severe infestation (Karel and Rweyemamu, 1984; Mwanauta et al. 2015; Obanyi et al. 2017).
Common bean farmers rely on cultural practices, botanicals and synthetic pesticides to control Ootheca spp. (Paul et al. 2007; Buruchara et al. 2010; Bazie et al. 2019). The cultural methods for controlling these pests include post-harvest tillage, crop rotation with non-host plants e.g. maize or sunflower, genetic mixtures and delayed sowing of common bean. These methods are cheap, dependable and specific, and do not possess detrimental side effects of synthetic pesticides. However, cultural methods do not provide complete economic control of the pests; their efficacy is difficult to assess; they require careful timing and long-term planning, and some are labor intensive (e.g. post-harvest tillage) to small-scale resource poor farmers who rely on the hand-hoe for land preparation. The botanical pesticides that deter Ootheca spp. and reduce damage to common beans include an aqueous extract from vernonia Vernonia lasiopus var. iodocalyx (Hoffmann) (Asterales: Asteraceae) leaves and Neem Azadirachta indica (De Jussieu) (Sapindales: Meliaceae) seed extracts. These botanical pesticides have low preparation and use costs, selective in action, and minimally toxic to humans, livestock and beneficial non-target organisms (Umoetok et al. 2009). However, botanical pesticides have short residual periods, slow effect on the pests, require frequent spraying and availability of their raw materials is seasonal. The active ingredients in botanical pesticides are liable to decomposition, their preparations have complex compositions and are not easy to standardize (El-Wakeil 2013).
Foliar application of synthetic pyrethroids for example, cypermethrin EC and lambda cyhalothrin EC are effective in controlling Ootheca spp. (Schwartz and Corrales, 1989; Paul et al. 2007; Tembo et al. 2018). Nevertheless, regular application of broad-spectrum pesticides are not recommended since their repeated use may result into resistance across a wider spectrum of common bean pests (Srinivasan 2014). Additionally, chemical pesticides can cause contamination of ground water and riparian habitats, outbreaks of secondary pests, decrease in biodiversity, and safety risks for humans, and wild and domestic animals (Dolinski and Lacey 2007; Mwanauta et al. 2015). Therefore, crop protection focus has shifted to integrated pest management (IPM), with emphasis on the use of biological control agents such as entomopathogens (Yubak Dhoj et al. 2008; Tesfaye and Seyoum 2010).
Several commercial products based on different species in genera of entomopathogenic fungi (EPF) such as, Metarhizium, Beauveria, Verticillium, Nomuraea, Aspergillus, Entomophthora, Paecilomyces and Neozygites have been formulated; targeting important pests and vectors (Sileshi et al. 2013; Bhan et al. 2015; Erler and Ates 2015; Bohara et al. 2018). Entomopathogenic fungi are eco-friendly alternatives for managing insect pests of public health and agricultural importance (Cravanzola et al., 1997; Charnley and Collins, 2007; Teshome and Tefera 2009; Farooq and Freed 2016). In the genus Metarhizium, M. anisopliae based biopesticides are frequently used to manage many insect pests including: Maladera affinis (Blanchard) (Coleoptera: Scarabaeidae); Alaus oculatus (Linnaeus) (Coleoptera: Elateridae) (Krishnamurthy et al. 1989); Holotrichia serrata (Fabricius) (Coleoptera: Scarabaeidae) (Cassell and Kuhar 2009) and some orthopterans (locusts and grasshoppers) (Magalhaes et al. 2000). In the genus Aspergillus, A. flavus has S- and L-strains or morphotypes, depending on sclerotia morphology (Dorner 2004; Donner et al. 2010; Ohkura et al. 2018). The L-strain produces fewer large sclerotia and abundant conidia, with varying aflatoxin-producing capabilities and most of them are atoxigenic (i.e., do not produce aflatoxins). The S-strain of A. flavus produces larger quantities of small sclerotia and more aflatoxins than the L-strain, restraining the former from application in vector and insect pest control (Powell et al. 2013). Most L-strain isolates of A. flavus are basic active agents in biocontrol products that are used to manage aflatoxin contamination (Probst et al. 2010). The atoxigenic L-strain of A. flavus is used as a biopesticide against Anopheles stephensi (Liston) (Diptera: Culicidae) and Culex quinquefasciatus (Say) (Diptera: Culicidae) (Bhan et al. 2015). Aspergillus flavus was reported effective against the red spider mite Oligonychus coffeae (Nietner) (Acari: Tetranychidae), a serious pest of tea in India (Mazid et al. 2015). Mukasa et al. (2018) isolated an atoxigenic strain of A. flavus in Uganda which was highly lethal to all development stages of Xylosandrus compactus (Eichhoff) (Coleoptera: Curculionidae: Scolytinae), causing mortalities of between 70% and 100% in the laboratory and 71% and 79% in the field; and suggested testing the entomopathogen against other species of insect pests. This isolate was, therefore, worthy of evaluation for control of important pests such as O. mutabilis, in comparison to known commercial biological control agents.
In this study, we investigated the comparative pathogenicity and virulence of the atoxigenic A. flavus and the commercial entomopathogenic biopesticide ICIPE 69, M. anisopliae, against O. mutabilis in the laboratory with a view of evaluating the potential of commercializing the isolate of A. flavus against this pest. We chose O. mutabilis because our recent studies (unpublished) showed this species of bean leaf beetles to be more widely spread and damaging to the common bean in Uganda than its sister species O. bennigseni.
Materials and methods
Insects
The 1st batch of O. mutabilis was collected during March to May 2017 planting season from Manibe sub-county in Arua district (03°05′20″N, 30°57′24″E and 1181 m above sea level (masl)); whereas the second batch was collected during March to May 2018 planting season from Agweng sub-county in Lira district (02°29′08″N, 32°54′35″E and 1084 masl). Both sub-counties were selected in consultation with agricultural extension officers and local farmers, based on heavy infestation of common bean gardens by the beetles. In the two sub-counties, O. mutabilis were collected from common bean gardens where pesticides had not been used during the season. Adult beetles were collected by handpicking and placed in translucent plastic containers (150 mm height × 60 mm top internal diameter × 40 mm bottom internal diameter; Crown Industries Ltd., Nairobi, Kenya). The containers were ventilated by creating three circular windows measuring 30 mm in diameter on opposite sides and on the lid of the container. The windows were fitted with muslin cloth to prevent beetles from escaping. Fresh tender common bean leaves from the gardens where beetles were collected were added to the containers as food. The top windows of the containers were covered with moistened wads of cotton (Carex, Inik Industries Ltd., Wakiso, Uganda) to maintain RH at between 40% and 60% (Beck and Blumer 2011). The translucent plastic containers (with beetles inside) were transported to the Entomology and Plant Pathology Laboratory at the National Crops Resources Research Institute (NaCRRI), Namulonge, Wakiso, Uganda (00o 31′ 30″N, 32o 36′ 54″E and 1127 masl) from where they were sorted and identified according to Kortenhaus and Wagner (2010). Sorted beetles were placed into clean translucent plastic containers described above and provided with fresh tender common bean leaves (NABE 16, raised in the legume sunscreen at NaCRRI). All adult insects were eligible for the bioassay, regardless of sex.
Entomopathogenic fungi
A commercial oil-formulated biopesticide M. anisopliae, (Real M. anisopliae 69 OD® - ICIPE 69, Real IPM Company, Kenya Limited) manufactured to control whiteflies, mealybugs, thrips and snout beetles; and a soil-borne isolate of atoxigenic A. flavus (L- strain) from Mubende district in Uganda (GPS coordinates kept private), reported to be highly lethal to all growth stages of X. compactus (Mukasa et al. 2018) were used in this study. The two entomopathogenic fungi were passaged through adult O. mutabilis to prevent attenuation (Butt and Goettel 2000; Tumuhaise et al. 2015) and loss of virulence, which would result from maintaining the fungi on artificial substrate (Safavi 2012). The spores from mycosed cadavers of O. mutabilis were propagated on Potato Dextrose Agar (PDA) (Oxoid, CMO 139, England) medium and incubated at room temperature for 20 days to obtain fungal cultures for subsequent bioassays.
Preparation of conidial suspensions and assessment of their viability
For both A. flavus and ICIPE 69, conidia from a 20-day old culture (described above) were harvested with a sterile loop needle and suspended in an aqueous solution of 0.05% Tween 80 (Himedia Laboratories Pvt. Ltd., 23 Vadhan Industries, Mumbai, India) as a wetting agent and sterile distilled water in 10 ml glass bottles with 10 glass beads (3 mm). The fungal suspensions in glass bottles were vortexed on a mechanical shaker (5 min at 700 oscillations/min) to break-up the conidial clumps, and filtered through a double layer of sterile muslin cloth to remove coagulated media (Slade et al. 1987; Sileshi et al. 2013).
The concentration of conidia in the filtrate was estimated using a heamocytometer (CAT. No. 1103, China) under a light microscope (Axioplan, Carl Zeiss Microscopy, D07740-Jena, Germany) (Goettel and Inglis 1997; Inglis et al. 2012). Briefly, the conidial concentration was quantified by loading a uniform suspension of conidia into the coverslip-affixed chambers of the haemocytometer. The haemocytometer was placed under the microscope at ×10 objective to facilitate localization of the grid, and counting started two minutes after the conidia had settled. The focus was on four large squares (consisting of 16 small squares) at each corner. Conidia in each of the four squares and at the right hand and bottom boundary lines were counted while those on the left border and upper boundary lines were skipped as per the classical procedure (Araujo et al. 2004; Pasco et al. 2016; Rishi et al. 2016). The average number of conidia for the four squares was calculated and used to estimate the conidia/ml of suspension (average conidia count per large square × 104).
Conidial viabilities of A. flavus and ICIPE 69 were tested according to Mohammed (2013). A standard conidial suspension of 3 × 106 conidia/ml of each entomopathogenic fungus was prepared. For each conidial suspension, an aliquot of 0.1 ml of the 3 × 106 conidia/ml suspension was placed on PDA plate with a sterile glass rod; sealed with parafilm (Penchiney, Menasha W154952, India) and incubated in complete darkness at 27 °C and 75% RH for 24 h (Teshome and Tefera 2009; Huang et al. 2012; Mazid et al. 2015). Germination was terminated by adding a drop of 0.5% formaldehyde (Loba Chemie Pvt. Ltd., Mumbai, India) onto the plate. Two sterile microscope cover slips (Chance Propper Ltd., West Mids, England) were placed on the plate, and germination percentage was determined by counting 100 spores under the cover slip on the plate under a light microscope at ×400 magnifications. Only conidia with a germ tube at least twice the size of the spore were considered to have germinated (Inglis et al. 2012). The procedure was replicated four times for each entomopathogenic fungus and the mean spore germinations were computed as 92.4 ± 3% and 94.6 ± 2% for A. flavus and ICIPE 69, respectively. These levels of viability were all way higher than 80%, which is sufficient to cause substantial mortality on target pests (Ekesi et al. 1998; Teshome and Tefera 2009).
Preparation of conidial dilutions and experimental controls
The surfaces of 20-day old cultures of A. flavus and ICIPE 69 were harvested using a sterile loop needle and separately suspended in 10 ml glass bottles containing 10 glass beads and aqueous solution of 0.05% Tween 80. The suspensions were vortexed and filtered through double layers of sterile muslin cloth. A stock suspension of 1 × 109 conidia/ml was estimated for each entomopathogenic fungus using a haemocytometer. Serial dilutions of 1 × 108, 1 × 107, and 1 × 106 conidia/ml of distilled water were made from the stock suspensions of each test entomopathogenic fungus. A standard chemical pesticide, CyperLacer® (cypermethrin 5% EC; Agrochemicals Pvt., Ltd. Gujarat, India) and sterile distilled water (containing 0.05% Tween 80 only) were prepared as controls. Cypermethrin 5% EC was selected because it’s commonly used for controlling field insect pests of the common bean in Uganda. The pesticide - cypermethrin 5% EC- was prepared by mixing 75 ml in five liters of water as per the manufacturer’s recommendation.
Dose-response bioassay
The 10 treatments (four from each entomopathogenic fungus, one for distilled water and one for the chemical pesticide) were replicated four times. Each treatment was applied on 10 adult O. mutabilis (irrespective of sex) in a translucent circular plastic container (Everfresh No. 4 (530), Kenpoly, Kenya) measuring 110 mm diameter × 65 mm height. The containers were lined at the bottom with a layer of moist cotton wool beneath a circular filter paper (Whatman No. 1, 1001–090) to absorb excess suspension, and covered with muslin cloth on top. Aliquots (2.5 ml) of each conidial suspension were directly sprayed on the beetles in the containers, wetting them completely, using handheld plastic trigger sprayers (Yuyao Lucky Commodity Co., Ltd., China) of 1.5 l capacity (Butt and Goettel 2000; Sanjaya et al. 2013; Mukasa et al. 2018). Similarly, beetles in each of the control containers were separately sprayed with 2.5 ml of (a) sterile distilled water and (b) CyperLacer®. Separate sprayers were used for each treatment to prevent cross-contamination. For each fungal suspension, lower concentrations were consecutively sprayed first to minimize leftover effects on concentrations. The beetles were provided with tender common bean leaves from the sunscreen at NaCRRI daily throughout the experiments. The experiments were conducted at 25 ± 4 °C and 60 ± 5% RH in the Entomology and Plant Pathology Laboratory at NaCRRI. The number of dead beetles in each treatment was recorded daily for seven days, starting 24 h after spraying (Burges, 1981; Tumuhaise et al. 2015; Rishi et al. 2016).
To confirm fungal infection in O. mutabilis, cadavers from A. flavus and ICIPE 69 treated insects were separately surface sterilized with 0.2% sodium hypochlorite solution (Orbit Chemical Industries Ltd. Nairobi, Kenya) for three seconds; washed twice in sterile distilled water and placed individually on moist filter paper in fresh sterile Petri dishes (100 mm × 17 mm). They were incubated at 27 °C for seven days. After three to five days, mycelia were observed growing out of the cadavers. The retrieved mycelia were cultured on PDA medium for 20 days, and compared with those obtained from passaging of the two EPF through the beetles (i.e. colony growth and appearance, pigmentation at the reverse of PDA plate and diffusion of pigmented metabolites into the Potato Dextrose Broth medium). The bioassay was repeated once.
Statistical analysis
Since mortality in the chemical pesticide treatment was 100% within the first 24 h, and no mortality was recorded in the control treated with sterile distilled water in the 1st and 2nd trials; data from the two controls were not included in the statistical analyses (Tesfaye and Seyoum 2010; Pasco et al. 2016). For each trial, cumulative mortalities of O. mutabilis from replicates of A. flavus and ICIPE 69 at the highest concentration (1 × 109 conidia/ml) by day seven were both 100%; therefore, these data did not require statistical comparison. To generate the LC50 (conidial concentrations that were lethal to 50% of O. mutabilis) on the last day and LT50 (lethal time at which 50% of O. mutabilis were killed) at the highest conidial concentration for each entomopathogenic fungus, data on dead (0) and alive (1) adults of O. mutabilis for each EPF in the two trials were subjected to Generalized Linear Model (GLM) procedure with binomial distribution error and logit link (Warton and Hui 2011). The ratios of residual deviances to degrees of freedom from the GLM outputs approximated to 1 in all cases, indicating appropriateness of the models. The function ‘dose.p’ from the ‘MASS’ library was used to produce LC50 and LT50 estimates, which were compared between the two EPF by a Two Sample t-test at α = 0.05. All the analyses were carried out in R-statistical computer software version 3.4.3 (R Development Core Team 2017).
Results
Mortality of Ootheca mutabilis in the control treatments
The mortalities of O. mutabilis in the control treatments of the standard chemical pesticide (CyperLacer®) were 100% in all replicates in both trials after 24 h. However, no mortalities were recorded in control treatments of sterile distilled water (containing 0.05% Tween 80) in all treatments in both trials after seven days.
Pathogenicity of Aspergillus flavus and ICIPE 69 against Ootheca mutabilis
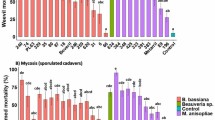
The cumulative mortalities of O. mutabilis treated with A. flavus and ICIPE 69 at 1× 109 conidia/ml on the 7th day in the 1st and 2nd trials were 100%.
Median lethal concentrations of Aspergillus flavus and ICIPE 69 against Ootheca mutabilis
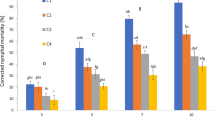
The median lethal concentrations of A. flavus and ICIPE 69 on the 7th day (approximately 5.3 × 106 conidia/ml for both entomopathogenic fungi) were not significantly different in both trials (t = 0.596, df = 6, P = 0.573 and t = 0.289, df = 6, P = 0.782 for trials 1 and 2, respectively) (Fig. 1).
Median lethal time of Aspergillus flavus and ICIPE 69 against Ootheca mutabilis
Whereas the LT50 for A. flavus and ICIPE 69 against O. mutabilis at the highest entomopathogenic fungal conidial concentrations were not significantly different in the 1st trial (t = 1.103, df = 6, P = 0.313), the LT50 of A. flavus (3.6 ± 0.2 days) was significantly longer than that of ICIPE 69 (2.1 ± 0.4 days) in the 2nd trial (t = 3.331, df = 6, P = 0.016) (Fig. 2).
Discussion
The standard pesticide (cypermethrin EC 5%) caused maximum mortalities (100%) of O. mutabilis in both trials after 24 h of application. This is an indication of the quick knock down and kill properties of broad-spectrum synthetic insecticides like cypermethrin. This finding concurs with that of Reynolds et al. (2017) in which topical application of cypermethrin EC on the Queensland fruit fly Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) caused maximum mortality (100%) in the first 24 h. Cypermethrin EC is used in the management of Ootheca spp. in leguminous crops in Tanzania (Tembo et al. 2018). As is common with other insect pest species, cypermethrin EC is an important option in reducing larval populations of the fruit borer Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) and the pea pod borer Etiella zinckenella (Treitschke) (Lepidoptera: Pyraloidea) in cowpeas in Egypt (Mahmoud 2011). In India, aphids Myzus persicae (Sulzer) (Hemiptera: Aphididae), whiteflies Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) and thrips Thrips tabaci (Lindeman) (Thysanoptera: Thripidae) that attack tomatoes Lycopersicon esculentum Miller (Solanales: Solanaceae) are managed by applying cypermethrin EC (Wagh et al. 2017). In California, United States of America (USA), a number of agricultural crop pests including the western flower thrips Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae); pea leafminer Liriomyza langei (Frick) (Diptera: Agromyzidae); American serpentine leafminer Liriomyza trifolii (Burgess) (Diptera: Agromyzidae) and vegetable leafminer Liriomyza sativae (Blanchard) (Diptera: Agromyzidae) are managed with cypermethrin EC (Shimat et al. 2017). In Cameroon, cowpea pests O. mutabilis; flower thrips, Megalurothrips sjostedti (Trybom) (Thysanoptera: Thripidae); Aphids, Aphis craccivora (Koch) (Hemiptera: Aphididae); legume pod borer, Maruca vitrata (Fabricius) (Lepidoptera: Crambidae) and pod sucking bugs - Clavigralla tomentosicolis(Stal) (Hemiptera: Coreidae) and Anoplocnemis curvipes (Fabricius) (Hemiptera: Coreidae)- are managed with cypermethrin EC (Djidjonri et al. 2019).
However, Thatheyus and Selvam (2013) and Srinivasan (2014) caution against reliance on synthetic pyrethroids in the control of insect pests because they are non-selective and, therefore, their repeated use may cause resistance across a range of common bean pests. Cypermethrin EC is detrimental to the ladybird beetle Coccinella magnifica(Latreille) (Coleoptera: Coccinellidae), which is a natural enemy of bean aphids Aphis spp. (Hemiptera: Aphididae) and bean flies Ophiomyia spp. (Diptera: Agromyzidae). Cypermethrin is equally harmful to predatory spiders Deinopis spp. (Araneae: Deinopidae) of Ophiomyia spp. and parasitoid flies Istocheta spp. (Diptera: Tachinidae) of coleopterans, including Ootheca spp. (Mohan et al. 2013; Wagh et al. 2017). This highlights the need to develop an IPM strategy for O. mutabilis control without or with limited use of synthetic pesticides.
There were no mortalities of O. mutabilis in the second control of sterile distilled water containing 0.05% tween 80 during the first seven days of the experiment in both trials. Absence of mortality in the water control has been reported in several researches, for example, no mortalities were reported in the water control within the first 10 days of treating the maize weevil Sitophilus zeamais (Mostch) (Coleoptera: Curculionidae) with B. bassiana and M. anisopliae (Teshome and Tefera 2009). Additionally, Pasco et al. (2016) reported no mortalities in the water control in 14 days during the assessment of the efficacy of topical, leaf residue, and soil drench applications with Isaria fumosorosea Wise (Hypocreales: Cordycipitaceae) blastospores (Ifr strain 3581) for management of citrus root weevils Diaprepes abbreviatus (Linnaeus) (Coleoptera: Curculionidae). Absence of mortalities in the control without a killing agent indicates that the beetles are hardy and, therefore, may perpetuate in the field unless controlled. This finding may explain the high abundance of Ootheca spp. during rainy periods, in coincidence with germinating legumes (Buruchara et al. 2010). The teneral adults of O. mutabilis can also withstand harsh field conditions of prolonged droughts by undergoing an obligatory diapause until onset of the next rainy season when they emerge and start feeding on leaves of newly planted legumes. However, cases of mortalities in experiments involving water controls are also commonly reported in literature, for example: Sileshi et al. (2013) recorded 6.67% mortality in termites Macrotermes (Isoptera: Termitidae) during an evaluation of M. anisopliae and B. bassiana against these pest in six days; Kirubakaran et al. (2015) recorded about 5% mortality in C. quinquefasciatus in seven days of the experiment; and Bayissa et al. (2017) recorded between 15% and 20% mortality in the water control treatments in the bioassay involving the three aphids Aphis gossypii (Glover), Brevicoryne brassicae (Linnaeus) and Lipaphis pseudobrassicae (Davis) (Hemiptera: Aphididae).
In both trials, the cumulative mortalities of O. mutabilis treated with 1 × 109 conidia/ml of both A. flavus and ICIPE 69 on the 7th day were 100%. This implies that both EPF were equally pathogenic and can hence be potential biopesticides in managing O. mutabilis. This is consistent with Fernandes et al. (2012) that arachnids and insect pests including coleopterans are susceptible to fungal infections, for example, M. anisopliae infects a wide range of beetles, including the white grub of Hoplia philanthus (Füessly) (Coleoptera: Scarabaeidae) (Ansari et al. 2006) and the rhinoceros beetle Oryctes rhinoceros (Linnaeus) (Coleoptera: Scarabaeidae) (Bedford 2014). Other agricultural pests that are susceptible to M. anisopliae infections include B. brassicae and L. pseudobrassicae (Bayissa et al. 2017); M. vitrata (Tumuhaise et al. 2015) and polyphagous thrips (Wu et al. 2018). The atoxigenic strains of A. flavus are commercially registered products in USA for managing aflatoxins in pre- and post-harvested cotton, peanut and corn through competing and displacing toxigenic strains (Dorner 2004; Abbas et al. 2011). However, the same strains are reportedly pathogenic to coleopterans, such as the shot-hole borer Scolytus nitidus (Schedl) (Coleoptera: Curculionidae: Scolytinae) (Buhroo et al. 2002); pupae of the small hive beetle Aethina tumida (Murray) (Coleoptera: Nitidulidae) (Rong et al. 2004); the almond bark beetles Scolytus amygdali (Guerin-Meneville) (Coleoptera: Curculionidae: Scolytinae) (Asma et al. 2017) and the coffee twig borer X. compactus (Mukasa et al. 2018). Larvae of the southern house mosquito ( C. quinquefasciatus) are also susceptible to A. flavus (Bhan et al. 2015). The current study broadens the spectrum of insect pests that are susceptible to A. flavus. It also provides potential for commercial production of atoxigenic A. flavus for O. mutabilis control. However, further research is required to determine the pathogenicity of the entomopathogenic fungus to all developmental stages of O. mutabilis as well as its efficacy against the pest in the field.
Based on dose-mortality relationships, the LC50 estimates for A. flavus and ICIPE 69 were comparable indicating that both EPF are equally virulent to O. mutabilis. Besides probable innate similarities in the virulence of the two EPF, similar laboratory conditions (such as relative humidity, temperature and light) under which they were tested may also explain this finding. These conditions are vital determinants of the rates and quantities of fungi that germinate, sporulate and infect the pests (Goettel and Inglis 1997; Sanjaya et al. 2013). The LC50 estimates depend on the number of infectious propagules in contact with the host cuticle. Propagules have to reach susceptible sites on the host cuticle, for example under the elytra and the intersegmental membranes (Bedford 2014). The viability of infectious propagules on the host cuticle is interrupted by preening, ecdysis and basking in the sun. Insect vigor, handling and rearing conditions affect the susceptibility of the insect to viable propagules (Butt and Goettel 2000; Ibrahim et al. 2015). To our knowledge, this is the first report of the virulence of A. flavus and ICIPE 69 against O. mutabilis; which broadens the spectrum of control options against this important common bean pest. However, as noted above, further research is required to determine the virulence of these entomopathogens on other developmental stages of O. mutabilis, and under field conditions prior to their endorsement for use in the management of the pest.
The LT50 of ICIPE 69 was significantly shorter than that of A. flavus in the 2nd trial. Intra- and inter-specific differences in virulence is common among EPF (Ekesi et al. 1998; Wu et al. 2018). The high virulence of ICIPE 69 among other isolates of M. anisopliae and those of Beauveria bassiana was reported for M. vitrata (Tumuhaise et al. 2015). It is probable that ICIPE 69 produces superior toxins that are responsible for faster death of O. mutabilis than those produced by A. flavus. Nonetheless, the delay to kill O. mutabilis by A. flavus may be of less significance if the incapacitating effect of infection can reduce the beetle’s capacity to damage the common bean. The ability of pests to damage crops reduces drastically after treatment with infectious propagules of EPF (Inglis et al. 2012; Safavi 2012; Bedford 2014). The various researchers attribute anti-feedant effects of EPF to the production of toxins and mechanical disruption of insect tissues by hyphal growth.
In conclusion, the current study demonstrates that A. flavus and ICIPE 69 are equally pathogenic and virulent (in terms of cumulative mortalities and concentrations required to kill 50% of the pests) to O. mutabilis. However, the two EPF require different times to kill 50% of this pest. Both EPF are potential alternatives to the use of synthetic pesticides in the management of O. mutabilis. This provides a leeway to develop atoxigenic A. flavus into a commercial biopesticide for O. mutabilis control. We recommend further studies on the pathogenicity and virulence of A. flavus and ICIPE 69 involving other developmental stages of O. mutabilis, and under field conditions.
References
Abate T, Ampofo JKO (1996) Insect pests of beans in Africa: their ecology and management. Annu Rev Entomol 41:45–73
Abbas HK, Weaver MA, Horn BW, Carbone I, Monacell JT, Shier WT (2011) Selection of Aspergillus flavus isolates for biological control of aflatoxins in corn. Toxin 30:59–70
Ansari M, Shah F, Tirry L, Moens M (2006) Field trials against Hoplia philanthus (Coleoptera: Scarabaeidae) with a combination of an entomopathogenic nematode and the fungus Metarhizium anisopliae CLO 53. Biol Control 39:453–459
Araujo R, Rodrigues AG, Pina-Vaz C (2004) A fast, practical and reproducible procedure for the standardization of the cell density of an Aspergillus suspension. J Med Microbiol 53:783–786
Asma, Z., Ahmed, M., Ayberk, H., Qiu, B., Cuthbertson, A., Varlese, R., Lombardi, N., Mannion, C., Daami-Remadi, M. and Braham, M., 2017. Occurrence, characterization and pathogenicity test of the fungi, Aspergillus flavus and Fusarium oxysporum isolated from cadavers of Scolytus amygdale Guerin-Meneville (Coleoptera: Curculionidae: Scolytinae). Egypt J biol Pest control. 27
Bayissa W, Ekesi S, Mohamed S, Kaaya G, Wagacha J, Hanna R, Maniania N (2017) Selection of fungal isolates for virulence against three aphid pest species of crucifers and okra. J Pest Sci 90:355–368
Bazie S, Ebabuye Y, Kim SW, Lee YS (2019) Integrated management of haricot bean foliage beetle in northeastern Ethiopia. J Entomol 37:1–5
Beck, C.W. and Blumer, L.S., 2011. A handbook on bean beetles, Callosobruchus maculatus, Emory University, Atlanta
Bedford GO (2014) Advances in the control of rhinoceros beetle. Oryctes rhinoceros in oil palm J Oil Palm Res 26:183–194
Bhan S, Mohan L, Srivastava C (2015) Synergistic larvicidal potential of temephos and entomopathogenic fungus, Aspergillus flavus against filarial vector, Culex quinquefaciatus (say). Int J Mosq Res 2:33–37
Bohara, J.R., Maharjan, S., Poudel, A., Karki, K., Bist, V., Regmi, R., Marahatta, S. and Kafle, L., 2018. Efficacy different concentration of Metarhizium anisopliae (Metsch.) Sorokin against white grub atlaboratory condition in Chitwan, Nepal. Assessment. 102, 99
Buhroo AA, Chishti M, Masoodi MA (2002) Biocontrol agents of shot-hole borer, Scolytus nitidus Schedl.(Coleoptera: Scolytidae) infesting apple orchards. India J Plant Pro 30:71–73
Buruchara, R., Mukaruziga, C. and Ampofo, K.O., 2010. Bean disease and pest identification and management. International Center for Tropical Agriculture (CIAT), Kampala
Butt, T. and Goettel, M., 2000. Bioassays of entomogenous fungi, in: Ascher, a.N.a.K.R.S. (Ed.), bioassays of Entomopathogenic microbes and nematodes. CABI publishing, London
Cassell, M.E. and Kuhar, T.P., 2009. Bean leaf beetle biology and management in snap beans. Online at: https://www.cabdirect.org/cabdirect/abstract/20127800801( accessed 12 APRIL 2018)
Djidjonri FP, Nukenine EN, Hartmut K (2019) Abundance and diversity of insect pests on maize, cowpea and okra in a comparative experiment testing effects of intercropping and insecticide in the Cameroonian Guinean-Savannah and Sudano-Sahelian agro-ecological zones. J Exp Agric Int 29:1–20
Dolinski C, Lacey LA (2007) Microbial control of arthropod pests of tropical tree fruits. Neotrop Entomol 36:161–179
Donner M, Atehnkeng J, Sikora RA, Bandyopadhyay R, Cotty PJ (2010) Molecular characterization of atoxigenic strains for biological control of aflatoxins in Nigeria. Food addit contam A 27:575–590
Dorner JW (2004) Biological control of aflatoxin contamination of crops: toxin review. J Toxicol 23:425–450
Ekesi S, Maniania N, Onu I, Löhr B (1998) Pathogenicity of entomopathogenic fungi (Hyphomycetes) to the legume flower thrips, Megalurothrips sjostedti (Trybom)(Thysan., Thripidae). J Appl Entomol 122:629–634
El-Wakeil NE (2013) Botanical pesticides and their mode of action. Gesunde Pflanzen 65:125–149
Erler, F. and Ates, A.O., 2015. Potential of two entomopathogenic fungi, Beauveria bassiana and Metarhizium anisopliae (Coleoptera: Scarabaeidae), as biological control agents against the June beetle. J Insect Sci 15
Farooq M, Freed M (2016) Infectivity of housefly, Musca domestica (Diptera:Muscidae) to different entomopathogenic fungi. Brazirian J Microbiol 110:1–10
Fernandes EKK, Bittencourt VREP, Roberts DW (2012) Perspectives on the potential of entomopathogenic fungi in biological control of ticks. Exp Parasitol 130:300–305
Goettel MS, Inglis GD (1997) Fungi: hyphomycetes. In: Lacey LA (ed) Manual of techniques in insect pathology. Academic Press, San Diego
Grobbelaar E (2008) On the identity of Ootheca bennigseni Weise, O. mutabilis (Schönherr) and O. meridiana sp. n.(Chrysomelidae: Galerucinae), bean and cowpea pests in the afrotropical region. Afr Entomol. 16:7–22
Huang Z, Shaukat A, Shunxiang R, Jianghu W, Zhang Y (2012) Influence of the entomopathogenic fungus Beauveria bassiana on Prynocaria Congener (Billberg) ( Coleoptera: Coccinellidae) under laboratory conditions. Pakistan J Zool 44:209–216
Ibrahim L, Laham L, Touma A, Ibrahim S (2015) Mass production, yield, quality, formulation and efficacy of entomopathogenic Metarhizium anisopliae conidia. British J Appl Sci Technol 9:427–440
Inglis GD, Enkerli J, Goettel MS (2012) Laboratory techniques used for entomopathogenic fungi: Hypocreales. In: Lacey LA (ed) Manual of techniques in invertebrate pathology. Academic Press, San Diego, pp 189–253
Kankwatsa P (2018) Efficacy and cost-benefit analysis of indigenous technical knowledge versus recommended integrated pest and disease management technologies on common beans in South Western Uganda. OALib J 5:1
Kirubakaran S, Haripriya R, Naveenraj D, Thirumalaivasan P (2015) Virulence of Metarhizium anisopliae soil and commercial isolates against Culex quinquefasciatus say, a vector of Bancroftian filariasis, and Aedes aegypti L., a vector of dengue fever. Eur J Biotechnol Biosci 3:39–47
Kortenhaus S, Wagner T (2010) Revision of Ootheca Chevrolat, 1837 from tropical Africa–redescriptions, descriptions of new species and identification key (Coleoptera: Chrysomelidae: Galerucinae). Zootaxa. 2659:1–52
Krishnamurthy K, Khan M, Avadhani K, Venkatesh J, Siddaramaiah A, Chakravarthy A, Gurumurthy B (1989) Three decades of cardamom research at regional research station. Technic Bulletin 97
Magalhaes BP, Lecoq M, Faria Md, Schmidt F, Guerra W (2000) Field trial with the entomopathogenic fungus Metarhizium anisopliae var. acridum against bands of the grasshopper Rhammatocerus schistocercoides in Brazil. Biocontr Sci Technol 10:427–441
Mahmoud MMS (2011) Persistence of new insecticides and their efficacy against insect pests of cowpea. Aust J Basic Appl Sci 5:82–89
Mazid S, Rajkhowa RC, Kalita JC (2015) Pathogenicity of Aspergillus Niger and Aspergillus flavus on red spider mite (Oligonychus coffeae Nietner), a serious pest of tea. J Entomol Zool Studies 3:11–13
Mohammed A (2013) An overview of distribution, biology and the management of common bean anthracnose. J Plant Pathol Microbiol 4:1–6
Mohan S, Nahar N, Ahmed KS (2013) Effects of Cyperin 10 EC and Neem extract on pollinator abundance, fruit setting and quality of mango. J Bangladesh Agric Univ 11:189–192
Mukasa Y, Kyamanywa S, Sserumaga JP, Otim M, Tumuhaise V, Erbaugh M, Egonyu JP (2018) An Atoxigenic L-strain of Aspergillus flavus (Eurotiales: Trichocomaceae) is pathogenic to the coffee twig borer, Xylosandrus compactus (Coleoptera: Curculionidea: Scolytinae). Environ Microbiol Rep. https://doi.org/10.1111/1758-2229.12705
Mwanauta, R.W., Mtei, K.M. and Ndakidemi, P.A., 2015. Potential of controlling common bean insect pests (bean stem maggot (Ophiomyia phaseoli), Ootheca (Ootheca bennigseni) and aphids (Aphis fabae) using agronomic, biological and botanical practices in field. Agric Sci 6, 489
Obanyi JN, Kamau AW, Ogecha JO (2017) Effects of common bean (Phaseolus vulgaris L.) cultivars and their mixtures with other legume species on bean foliage beetle (Ootheca spp) incidence, severity and grain yield in Western Kenya. World J Agric Res 5:156–161
Ohkura, M., Cotty, P. and Orbach, M., 2018. Comparative genomics of Aspergillus flavus S and L morphotypes yield insights into niche adaptation. G3. 3, 3915-3930
Pasco BA, Wayne BH, David GH, Mark AJ, Charles AP (2016) Efficacy of topical application, leaf residue or soil drench of blastospores of Isaria fumosorosea for citrus root weevil management: laboratory and greenhouse investigations. Insects. 7:66
Paul U, Ampofo J, Hilbeck A, Edwards P (2007) Evaluation of organic control methods of the bean beetle, Ootheca bennigseni, in East Africa. N Z Plant Prot 60:189
Powell KA, Renwick A, Peberdy JF (2013) The genus Aspergillus from taxonmy and genetics to industrial application. Plenum press, New York
Probst C, Schulthess F, Cotty PJ (2010) Impact of Aspergillus section Flavi community structure on the development of lethal levels of aflatoxins in Kenyan maize (Zea mays). J Appl Microbiol 108:600–610
Reynolds OL, Osborne TJ, Barchia I (2017) Efficacy of chemicals for the potential management of the Queensland fruit fly Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Insects. 8:49
R Development Core Team (2017) R: A language and Environment for Statistical Computing. Vienna, Austria R Foundation for Statistical Computing, 3.4.3, http://www.R-project.org.
Rishi R, Pandey S, Borah RK, Kumar R, Borthakur N (2016) Efficacy of entomopathogenic fungi on Craspedonta leayana, a serious insect pest of Gmelina arborea. Current Life Sci 2:15–19
Rong I, Hill M, Hepburn R, Elzen P (2004) The susceptibility of small hive beetle (Aethina tumida Murray) pupae to fungal pathogens. Am Bee J 144:486–488
Safavi SA (2012) Attenuation of the entomopathogenic fungus Beauveria bassiana following serial in vitro transfers. Biologia. 67:1062–1068
Sanjaya Y, Ocampo VR, Caoili BL (2013) Infection process of entomopathogenic fungi Metarhizium anisopliae in the Tetrancyhus kanzawai (Kishida) (Tetranychidae: Acarina). J Agric Entomol 35:64–72
Shimat VJ, Tunyalee M, Kimberly S, Petr K (2017) Outlook of pyrethroid insecticides for pest management in the Salinas valley of California. J Integrated Pest Manag 8:1–11
Sileshi A, Sori W, Dawd M (2013) Laboratory evaluation of entomopathogenic fungi Metarhizium anisophilae and Beauveria bassiana against termite, Macrotermes (Isoptera: Termitidae). Asian J Plant Sci 12:1–10
Slade S, Harris R, Smith C, Andrews J, Nordheim E (1987) Microplate assay for Colletotrichum spore production. Appl Environ Microbiol 53:627–632
Srinivasan R (2014) Insect and mite pests on vegetable legumes: a field guide for identification and management. AVRDC - The World Vegetable Center, Shanhua
Tembo Y, Mkindi GA, Mkenda PA, Mpumi N, Mwanauta R, Stevenson PC, Ndakidemi PA, Belmain SR (2018) Pesticidal plant extracts improve yield and reduce insect pests on legume crops without harming beneficial arthropods. Front Plant Sci 9:1–10
Tesfaye D, Seyoum E (2010) Studies on the pathogenicity of native entomopathogenic fungal isolates on the cotton/melon aphid, Aphis gossypii (Homoptera: Aphididae) glover under different temperature regimes. Afr Entomol 18:302–312
Teshome A, Tefera T (2009) Susceptibility of Sitophilus zeamais (mostch.)(Coleoptera: curculionidae) to Beauveria bassiana and Metarhizium anisopliae. Ethiop J Sci 32:21–28
Thatheyus AJ, Selvam ADG (2013) Synthetic pyrethroids: toxicity and biodegradation. Appl Ecol Environ Sci 1:33–36
Tumuhaise V, Ekesi S, Mohamed S, Ndegwa P, Irungu L, Srinivasan R, Maniania N (2015) Pathogenicity and performance of two candidate isolates of Metarhizium anisopliae and Beauveria bassiana (Hypocreales: Clavicipitaceae) in four liquid culture media for the management of the legume pod borer Maruca vitrata (Lepidoptera: Crambidae). Int J Trop Insect Sci 35:34–47
Umoetok S, Osuagwu A, Udo I, Idiongette M, Ukeh D (2009) Effects of Azadirachta indica products on the management of Ootheca mutabilis on Telfairia occidentalis in Calabar. Southeast Nigeria Crop Prot 28:583–587
Wagh BM, Pagire KS, Thakare DP, Birangal AB (2017) Management of sucking pests by using newer insecticides and their effect on natural enemies in tomato (Lycopersicon esculentum mill.). Int J Curr Microbiol App Sci 6:615–622
Warton DI, Hui FK (2011) The arcsine is asinine: the analysis of proportions in ecology. Ecology. 92:3–10
Wu S, Tang L, Fang F, Li D, Yuan X, Lei Z, Gao Y (2018) Screening, efficacy and mechanisms of microbial control agents against sucking pest insects as thrips. Advances Insect Physiol 55:210–219
Yubak Dhoj G, Keller S, Nagel P, Kafle L (2008) Virulence of Metarhizium anisopliae and Beauveria bassiana against common white grubs in Nepal. J Formosan Entomol 28:11–20
Acknowledgments
We greatly acknowledge the Real IPM Company, Kenya and Mukasa et al. (2018) for providing the entomopathogenic fungi (ICIPE 69 and A. flavus, respectively) for this study. This study was funded by the Bill & Melinda Gates Foundation’s Programme for Emerging Agricultural Research Leaders (OPP1131470_2015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This study did not involve use of human and/or vertebrate animals.
Sources of funding
This study was funded by the Bill & Melinda Gates Foundation’s Programme for Emerging Agricultural Research Leaders (OPP1131470_2015); Grant recipient: Michael H. Otim). The Real IPM Company, Kenya donated the commercial OD® ICIPE 69 Metarhizium anisopliae used in this study, did not participate in any aspect of the study and approved the publication of the findings.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mugonza, J., Otim, M.H. & Egonyu, J.P. The comparative virulence of an atoxigenic strain of Aspergillus flavus (Eurotiales: Trichocomaceae) and the commercial ICIPE 69 Metarhizium anisopliae (Hypocreales: Clavicipitaceae) to the bean leaf beetle Ootheca mutabilis (Coleoptera: Chrysomelidae). Int J Trop Insect Sci 40, 403–411 (2020). https://doi.org/10.1007/s42690-019-00091-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-019-00091-w