Abstract
The influence of intraguild predation between Orius albidipennis Reuter and two parasitoids, Eretmocerus mundus Mercet or Eretmocerus eremicus Rose and Zolnerowich, on the suppression of Bemisia tabaci Gennadius was investigated under laboratory conditions. Through a non-choice test, the 2nd and 3rd instar nymphs of B. tabaci and larval and pupal stages of both parasitoids were offered separately to both 5th instar nymphs and adults of O. albidipennis. In the choice test, various combinations of parasitized and unparasitized preys were provided for two stages of O. albidipennis, and their preference was recorded. Both predator stages readily preyed upon unparasitized and parasitized nymphs of B. tabaci. The most predation of O. albidipennis adults occurred on E. eremicus larvae, while for the 5th instar nymphs, it was recorded on E. eremicus larvae and pupae. The least predation of both stages was recorded on unparasitized nymphs of B. tabaci and E. mundus pupae. None of the prey combinations showed any obvious preference of adults and the 5th instars of O. albidipennis toward parasitized or unparasitized prey.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intraguild predation (IGP) is one of the possible interspecific interactions that occur when different natural enemies are released to control the same pest in a system (Polis et al. 1989). IGP commonly occurs in food webs, both in natural and managed systems, where species compete for a common pest prey/host. IGP may occur between predators, parasitoids, and predators and parasitoids (Polis et al. 1989; Rosenheim et al. 1995; Rosenheim 1998). Predator–parasitoid interaction occurs in two different modes. In addition, predators may prey directly on parasitoids or indirectly by consuming the parasitized host and the associated immature parasitoid (Rosenheim et al. 1995).
Insects represent different interactions with IGP. IGP may disrupt biological control and thus lead to an increase in the pest population (Erbilgin et al. 2004; Rosenheim et al. 1993; Sohrabi et al. 2013). However, the effect of IGP on biological control is not always negative (Herrick et al. 2008). Positive effects have also been shown when an increase occurs in the efficacy of natural enemies (Gardiner and Landis 2007; Schausberger and Walzer 2001; Snyder et al. 2004).
Bemisia tabaci Gennadius is a very harmful pest worldwide for many crops, especially greenhouse crops such as ornamentals, tomato, pepper, bean, eggplant, and cucumber. More than 600 species of different plants are the hosts of B. tabaci (Oliveira et al. 2001). Biological control of B. tabaci mostly relies on parasitoids from family Aphelinidae such as the genus Eretmocerus and Encarsia (Hymenoptera: Aphelinidae) (Gerling et al. 2001; Naranjo and Ellsworth 2005; Zandi-Sohani et al. 2009). Eretmocerus mundus Mercet is a parasitoid that is able to efficiently control B. tabaci (Gabarra et al. 2006; Stansly et al. 2005). Eretmocerus eremicus Rose and Zolnerowich are native to the Americas (Rose and Zolnerowich 1997). In Iran, this parasitoid has been reported from Gilan Province (Shahbazvar et al. 2010). In greenhouse crops, polyphagous predatory insects of the genus Orius (Heteroptera: Anthocoridae) are also used to control B. tabaci (Sohrabi et al. 2013). Orius albidipennis Reuter, a common predator in several regions of Iran, has been reported as a promising generalist biocontrol agent in greenhouses and fields (Salehi et al. 2016). Therefore, in programs for control of B. tabaci in greenhouse crops, the concurrent use of E. mundus or E. eremicus and O. albidipennis is expected to decline the pest population.
A limited number of studies have investigated IGP by generalist predators on specialist parasitoids against whiteflies. For instance, Sohrabi et al. (2013) reported IGP by adults and the 5th instar nymphs of Orius majusculus (Reuter) (Heteroptera: Anthocoridae) on Encarsia formosa (Gahan) (Hymenoptera: Aphelinidae), a parasitoid of B. tabaci (Sohrabi et al. 2013). The IGP of Geocoris punctipes (Say) (Hemiptera: Lygaeidae) on E. eremicus was assessed while the parasitoid developed on the nymphs of Trialeurodes vaporariorum Westwood (Hemiptera: Aleyrodidae) (Velasco-Hernández et al. 2013). In another study, the interactions of Macrolophus pygmaeus (Rambur) with Nesidiocoris tenuis Reuter (Hemiptera: Miridae) and E. mundus and their effects on B. tabaci were studied on tomato plants (Moreno-Ripoll et al. 2014). To the best of our knowledge, there is no information regarding IGP by O. albidipennis on E. mundus and E. eremicus or other parasitoid species on B. tabaci. The aim of this study is to investigate the ability of O. albidipennis to prey on parasitized B. tabaci. After proving such an ability, we investigate the predator feeding preference on parasitized and non-parasitized whitefly nymphs.
Materials and methods
Plants
Commercial seeds of cucumber, Cucumis sativus L. (C.V. F1), were sown in plastic pots (9 cm high and 8 cm diameter) into compost. The pots were maintained in cages (120 × 120 × 60 cm3) covered with a white nylon mesh of 210 μm aperture. These cages were in a laboratory at 16–25 °C, 40–50% R.H., and a 14:10 (L:D) photoperiod. When the plants reached 4 to 6 leaves of development, they were used for whitefly rearing.
Insects
A cucumber field in the Mollasani region, Ahvaz, Iran, was used for collecting B. tabaci individuals to initiate colony in September 2015. The collected whiteflies were released on the leaves of cucumber plants in the cages similar to those described in the above lines. When the plants withered, new ones were added to the cage.
The pupae of E. mundus were collected from the same cucumber field to establish a colony in September 2015. E. eremicus wasps were purchased as pupae from Gyah Company, Iran, which was originally produced at Koppert Co. Then, the pupae of both wasps were put into separate petri dishes (9 cm diameter) inside different cages containing cucumber plants infested by B. tabaci. After emergence, the adult wasps started parasitizing B. tabaci nymphs, and the colonies of E. mundus and E. eremicus were created.
Orius albidipennis adults were collected from an unsprayed sunflower field in the experimental plots of Agricultural Sciences and Natural Resources University of Khuzestan, Iran. Then, the females were isolated in a plexiglass container (18 cm height and 7.5 cm diameter) covered with a fine gauze on the top for ventilation. Bean pods also were supplied an oviposition substrate. One male was selected among the offspring and identified by the keys of Pericart (Péricart 1972). After identification, the glasses with O. albidipennis were maintained for building a colony, and other species were removed. For feeding of O. albidipennis, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) eggs and corn pollen were used. To avoid cannibalism, crumpled wipe papers were placed at the bottom of the plexiglass cylinders (Salehi et al. 2016). An incubator was used for rearing insects, which were set at 25 ± 1 °C, 65 ± 5 R.H., and 16:8 h (L: D).
Parasitized whiteflies nymphs for experiments
Cucumber plants with 4–6 leaves were used to obtain parasitized nymphs of whiteflies for experiments. First, groups of 10 adult whiteflies collected from the colony were released into clip cages (2 cm diameter) on the bottom side of the leaves. Afterward, they were left to oviposit for 24 h, followed by deleting their clip cages. The plants with whitefly eggs were transferred to incubators that were set at 25 ± 1 °C, 65 ± 5 RH, and 16:8 h (L: D). After about 2 weeks, the eggs developed to the 3rd instar nymphs, which is the favorite stage for E. mundus and E. eremicus to parasitize. Subsequently, groups of 5–6 adults of both parasitoids were moved to a larger clip cage (4 cm diameter) on the whitefly nymphs separately and were left to parasitize for 24 h. Then, the plants were maintained in the mentioned conditions until parasitoid was developed to the desired larval or pupal stage for experiments.
No-choice bioassays
In these experiments, IGP of O. albidipennis on E. mundus and E. eremicus was studied separately for the 5th instar and adults of the predator. Observations were performed in the experimental arena consisting of organdy-vented petri dishes (9 cm diameter). Leaf discs containing parasitized nymphs in larval and pupal stages of parasitoids and the 2nd and 3rd instar nymphs of whitefly were offered separately to each predator stage. Leaves with parasitized or unparasitized whitefly nymphs were taken from plants described above. On each leaf, 20 parasitized or unparasitized nymphs were retained, and other whitefly nymphs were removed by a pin. The petioles of the detached leaves containing nymphs were completely covered in moist cotton to keep the leaves fresh during the experiments. The bottom of the arena was also covered with moistened filter paper completely. One newly molted 5th instar nymph or an adult female of O. albidipennis with no feeding during the past 24 h was introduced into leaf disc. After 24 h, predators were eliminated, and the number of attacked prey was counted on each leaf disc. The number of replications was 10 for each treatment. All the tests were carried out in an incubator with the same condition mentioned for predator rearing.
Choice bioassays
In these bioassays, the same procedure described for the no-choice test was used with some difference in the kind of preys offered to predators in the arena. According to the results of no-choice bioassay, O. albidipennis was able to consume both parasitized and unparasitized whitefly nymphs. Therefore, the following prey combinations were used in choice bioassays:
- 1)
15 parasitized nymphs with the larval stage of parasitoids and 15 unparasitized 2nd instar whiteflies
- 2)
15 parasitized nymphs with the larval stage of parasitoids and 15 unparasitized 3rd instar whiteflies
- 3)
15 parasitized nymphs with the pupal stage of parasitoids and 15 unparasitized 2nd instar whiteflies
- 4)
15 parasitized nymphs with the pupal stage of parasitoids and 15 unparasitized 3rd instar whiteflies
The experimental conditions were similar to those described for no-choice bioassays. The experimental arena was also the same with some modifications. In this experiment, two different leaf discs were used in each petri dish, which one of them contained parasitized nymph and another one with unparasitized whitefly nymphs of the appropriate stage. All the abovementioned tests were carried out separately for the 5th instar nymphs and adult females of O. albidipennis. For each parasitoid, all the experiments were done separately. The predators were released individually into the experimental arena, and the number of prey attacked by the predator was recorded after 24 h. Each combination was replicated 10 times.
Data analysis
No-choice bioassays
To test the differences between predation rates among groups of prey stages and between predator stages, a two-way ANOVA was performed using the procedure GLIMMIX of SAS (SAS Institute 2003). The effect of prey and predator stage together with their interaction was included as fixed effects, and the means were separated using the LSD test at P = 0.05, where applicable. It was assumed that the number of individuals was Poisson-distributed with an unknown overdispersion, which had to be estimated. The natural logarithm was used as a link function. The two-sample t-test was used to compare predation by different predator stages when offered the same prey (SAS Institute 2003).
Choice bioassays
For the choice experiments, the experimental design was a two-way ANOVA including the fixed effects of prey combination and predator stage together with their interaction. It was assumed that the number of individuals chosen was binomially distributed with an unknown overdispersion, which had to be estimated. The logit function was used as a link. A two-sample t-test was used to compare the predation by different predator stages when the same prey combination was offered. The means of whitefly nymphs consumed were separated between prey combinations within each predator stage using the LSD test at P = 0.05, where applicable. All analyses were performed using the GLIMMIX procedure of SAS (SAS Institute 2003).
The assessment of prey preference was based on the formula for Manly’s preference index (Manly 1974) for each predator stage and each prey combination:
where β1 is the preference for prey type 1, e1 and e2 are the numbers of prey 1 and 2 remaining after the experiments, respectively, and A1and A2 are the number of prey types 1 and 2 offered, respectively. The values of preference index (β1) vary within the range of 0 to 1, where the values higher than 0.5 indicate a preference for prey type 1. Differences between prey preference indices including the fixed effects of prey combination and predator stage together with their interaction were analyzed using two-way ANOVA. The one-sample t-test was applied to represent a significant difference in each preference index from the value of 0.5 using SAS 9.0 (SAS Institute 2003).
Results
No-choice bioassays
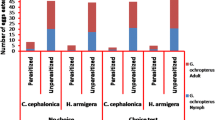
According to the results, both 5th instar nymphs and adults of O. albidipennis are capable of preying on different stages of unparasitized nymphs of B. tabaci and nymphs parasitized by E. eremicus and E. mundus (Table 1). The predation rate was significantly influenced by prey stage (F = 36.67, df = 5108; P < 0.0001), predator stage (F = 45.00, df = 1108; P < 0.0001), and the interaction between predator and prey stage (F = 3.07, df = 5108; P = 0.0125). Adult O. albidipennis had the most predation on the parasitized nymphs containing larval stage of E. eremicus. The 5th instars had the highest predation on parasitized nymphs of B. tabaci containing larval or pupal stages of E. eremicus with no significant difference between these two prey types. Predation by adults was significantly higher than predation of 5th instar nymphs on all offered preys, except on the pupae of E. eremicus such that both adults and the 5th instar nymphs showed the same predation rate (Table 1).
Choice bioassays
The results of choice tests of O. albidipennis adults and the 5th instar nymphs between unparasitized nymphs of B. tabaci and parasitized nymphs by E. eremicus are shown in Table 2. Predation rate varied significantly by predator developmental stages (F = 19.23; df = 1,72; P = 0.0001), while the effects of prey combination (P = 0.09) and the interactions between predator stage and prey combination (P = 0.61) on predation rate were not significant. Adults of O. albidipennis consumed more than the 5th instar nymphs in the combination of unparasitized 2nd instar B. tabaci nymphs and pupae of E. eremicus and unparasitized 3rd instar nymphs of B. tabaci and larvae of E. eremicus (Table 2). The results also showed that the predator stage (P = 0.69), prey combinations (P = 0.96), and the interactions between predator stage and prey combinations (P = 0.97) had no significant effects on preference indices. Both adults and the 5th instar nymphs of O. albidipennis showed no positive preference for parasitized over unparasitized whitefly nymphs (Table 2), and all calculated preference indices were not significantly different from 0.5 (P > 0.05).
In choice experiments with unparasitized nymphs of B. tabaci and nymphs parasitized by E. mundus, predation rates were significantly affected by prey combination (F = 2.86; df = 3, 72; P = 0.042) and predator stage (F = 4.29; df = 1, 72; P = 0.042) but not by the interaction between prey combination and predator stage (P = 0.62). Adults of O. albidipennis showed significantly more predation than the 5th instar nymphs in prey combination of the 3rd instar nymphs of B. tabaci and pupae of E. mundus (Table 3). The results also showed that the predator stage (P = 0.65), prey combinations (P = 0.31), and the interactions between predator stage and prey combinations (P = 0.48) had no significant effects on preference indices. None of the adults and the 5th instar nymphs of O. albidipennis had a recognizable preference for parasitized over unparasitized whitefly nymphs (Table 3), and none of the preference indices was significantly different from 0.5 (P > 0.05).
Discussion
The compatibility between whitefly parasitoids and predators has a significant impact on the effectiveness of biological control programs against B. tabaci. Biological control can be more efficient when useful agents have synergistic or additive effects. In some studies, an increase in the diversity of natural enemies on crops has caused a growing decline in population density of herbivores (Snyder and Ives 2003). However, in some other cases, intraguild predation may result in less successful biological control programs (Rosenheim 1998; Rosenheim et al. 1995; Rosenheim 2005).
The current study confirms that both predator stages, adults, and the 5th instar nymphs of O. albidipennis are able to prey upon parasitized nymphs of B. tabaci in both choice and no-choice tests. Similar results were reported in several studies. O. majusculus adults and late instars nymphs had the ability to prey on B. tabaci parasitized by E. formosa (Sohrabi et al. 2013), Serangium parcesetosum Sicard (Coleoptera: Coccinellidae) preyed on B. tabaci parasitized by E. mundus (Kutuk et al. 2011), and Delphastus catalinae (Horn) (Coleoptera: Coccinellidae) readily feed on whitefly nymphs parasitized by Encarsia sophia (Girault & Dodd) (Hymenoptera: Aphelinidae) (Zang and Liu 2007).
In no-choice tests, adults of predator consumed more on the larval stage of both parasitoids than their pupae. Similar results have been reported in the no-choice test by O. majusculus on different developmental stages of E. formosa (Sohrabi et al. 2013). In these experiments, adults of O. albidipennis showed the least predation on the 2nd and 3rd instar nymphs of B. tabaci and E. mundus pupae, compared with larval and pupal stages of E. eremicus and larval stage of E. mundus. In the case of the 5th instar nymphs, the least predation was recorded on the 2nd and 3rd instar nymphs of B. tabaci and larval and pupal stages of E. mundus. Previous studies have shown that some predators only consume unparasitized hosts while others may prefer parasitized hosts (Brodeur and Rosenheim 2000; Colfer and Rosenheim 2001). The reason why O. albidipennis in the current study consumed fewer unparasitized B. tabaci nymphs is the fact that unparasitized nymphs might use defense mechanisms against predators to avoid predation (Meisner et al. 2011). Moreover, a lower rate of predation on the pupal stage of E. mundus compared to the larval stage was also seen in the adult predator stage. Some other reports are in parallel with our results. For example, D. catalinae consumed fewer whitefly nymphs with E. sophia pupae (Zang and Liu 2007), or the parasitoid pupae of Psyllaephagus bliteus Riek (Hymenoptera: Encyrtidae) were largely free from the attack of Anthocoris nemoralis (Fabricius) (Heteroptera: Anthocoridae) (Erbilgin et al. 2004). Furthermore, both adults and the 4th instar S. parcesetosum strongly avoided B. tabaci nymphs containing pupae of E. mundus (Kutuk et al. 2011). Several reasons could be presumed for this phenomenon including hardening of whitefly cuticle induced by parasitism (Hoelmer et al. 1994; Kutuk et al. 2011), introducing air around the developing parasitoid, physical and chemical alterations in cuticle during the pupal development (Hoelmer et al. 1994), and morphological changes in parasitized prey (Kutuk et al. 2011).
In the choice tests of the present study, O. albidipennis did not exhibit a clear preference for B. tabaci nymphs or parasitized nymphs by E. mundus and E. eremicus. The same trend was reported for D. catalinae (Zang and Liu 2007), which did not avoid parasitized or unparasitized whitefly nymphs significantly. Anthocoris nemorum L. also are involved in IGP with Aphidius colemani Viereck and did not discriminate between unparasitized and parasitized aphids and mummies (Meyling et al. 2004). According to Brodeur and Rosenheim (2000), for unparasitized aphids with active behavioral defense mechanisms, there is a higher chance of predation on mummified prey. Since unparasitized nymphs of B. tabaci are almost as sedentary as parasitized nymphs, the lack of prey preference can be explained by the above argument. Nevertheless, in a previous study on O. majusculus, Sohrabi et al. (2013) showed that the IG-predator prefer preying upon B. tabaci nymphs parasitized by E. formosa. In these cases, differentiated size and color of the parasitized nymphs were considered as a reason for the IG-predator preference (Naranjo 2007). In another study, however, Geocoris punctipes showed preference to non-parasitized nymphs of the whitefly Trialeurodes vaporariorum rather than nymphs parasitized by E. eremicus during the choice test (Velasco-Hernández et al. 2013). In such cases, some factors like mechanical aspects (Hoelmer et al. 1994), physiological or chemical changes (Chen 1966; Gelman et al. 2002), and prey species (Roger et al. 2000; Williamson 1980) potentially affect IG- predator responses.
In conclusion, as the parasitized hosts are not avoided by a predator, the presence of O. albidipennis could have an undesirable effect on the fitness of both parasitoids. However, to obtain more reliable results, future efforts should be directed toward greenhouse experiments.
References
Brodeur J, Rosenheim JA (2000) Intraguild interactions in aphid parasitoids. Entomol Exp Appl 97:93–108
Chen PS (1966) Amino acid and protein metabolism in insect development. Adv In Insect Phys 3:53–132
Colfer RG, Rosenheim JA (2001) Predation on immature parasitoids and its impact on aphid suppression. Oecologia 126:292–304
Erbilgin N, Dahlsten DL, Chen P (2004) Intraguild interactions between generalist predators and an introduced parasitoid of Glycaspis brimblecombei (Homoptera: Psylloidea). Biol Control 31:329–337
Gabarra R, Zapata R, Castañé C, Riudavets J, Arnó J (2006) Releases of Eretmocerus mundus and Macrolophus caliginosus for controlling Bemisia tabaci on spring and autumn greenhouse tomato crops. IOBC WPRS Bulletin 29:71
Gardiner MM, Landis DA (2007) Impact of intraguild predation by adult Harmonia axyridis (Coleoptera: Coccinellidae) on Aphis glycines (Hemiptera: Aphididae) biological control in cage studies. Biol Control 40:386–395
Gelman DB, Blackburn MB, Hu JS (2002) Timing and ecdysteroid regulation of the molt in last instar greenhouse whiteflies (Trialeurodes vaporariorum). J Insect Phys 48:63–73
Gerling D, Alomar Ò, Arnò J (2001) Biological control of Bemisia tabaci using predators and parasitoids. Crop Prot 20:779–799
Herrick NJ, Reitz SR, Carpenter JE, O’Brien CW (2008) Predation by Podisus maculiventris (Hemiptera: Pentatomidae) on Plutella xylostella (Lepidoptera: Plutellidae) larvae parasitized by Cotesia plutellae (Hymenoptera: Braconidae) and its impact on cabbage. Biol Control 45:386–395
Hoelmer KA, Osborne LS, Yokomi RK (1994) Interactions of the whitefly predator Delphastus pusillus (Coleoptera: Coccinellidae) with parasitized sweet potato whitefly (Homoptera: Aleyrodidae). Environ Entomol 23:136–139
Kutuk H, Yigit A, Alaoglu O (2011) Intraguild predation of Serangium parcesetosum (Coleoptera: Coccinellidae), on whitefly Bemisia tabaci (Homoptera: Aleyrodidae) parasitized by Eretmocerus mundus (Hymenoptera: Aphelinidae). Biocontrol Sci Techn 21:985–989
Meisner M, Jason P, Harvey CT, Ives AR (2011) Intraguild predation on the parasitoid Aphidius ervi by the generalist predator Harmonia axyridis : the threat and its avoidance. Entomol Exp Appl 138:193–201
Meyling NV, Enkegaard A, Brødsgaard H (2004) Intraguild predation by Anthocoris nemorum (Heteroptera: Anthocoridae) on the aphid parasitoid Aphidius colemani (Hymenoptera: Braconidae). Biocontrol Sci Techn 14:627–630
Moreno-Ripoll R, Gabarra R, Symondson WOC, King RA, Agustí N (2014) Do the interactions among natural enemies compromise the biological control of the whitefly Bemisia tabaci? J Pest Sci 87:133–141
Naranjo SE (2007) Intraguild predation on Eretmocerus sp. nr. emiratus, a parasitoid of Bemisia tabaci, by three generalist predators with implications for estimating the level and impact of parasitism. Biocontrol Sci Techn 17:605–622
Naranjo SE, Ellsworth PC (2005) Mortality dynamics and population regulation in Bemisia tabaci. Entomol Exp Appl 116:93–108
Oliveira MR, Henneberry VTJ, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 20:709–723
Péricart J (1972) Hémiptères Anthocoridae, Cimicidae et Microphysidae de l’ouest-paléarctique. Fauna l’Europe du Bassin Mediterraneen 7:1–404
Polis GA, Myer CA, Holt RD (1989) The ecology and evolution of intraguild predation: potential competitors that eat each other. Annu Rev Ecol Syst 20:297–330
Roger C, Coderre D, Boivin G (2000) Differential prey utilization by the generalist predator Coleomegilla maculata lengi according to prey size and species. Entomol Exp Appl 94:3–13
Rose M, Zolnerowich G (1997) Eretmocerus Haldeman (Hymenoptera: Aphelinidae) in the United States, with descriptions of new species attacking Bemisia (tabaci complex) (Homoptera: Aleyrodidae). Proc Entomol Soc Wash 99:1–27
Rosenheim JA (1998) Higher-order predators and the regulation of insect herbivore populations. Annu Rev Entomol 43:421–447
Rosenheim JA (2005) Intraguild predation of Orius tristicolor by Geocoris spp. and the paradox of irruptive spider mite dynamics in California cotton. Biol Control 32:172–179
Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffee BA (1995) Intraguild predation among biological-control agents: theory and evidence. Biological Control 5:303–335
Rosenheim JA, Wilhoit LR, Armer CA (1993) Influence of intraguild predation among generalist insect predators on the suppression of an herbivore population. Oecologia 96:439–449
Salehi Z, Yarahmadi F, Rasekh A, Zandi SN (2016) Functional responses of Orius albidipennis Reuter (Hemiptera, Anthocoridae) to Tuta absoluta Meyrick (Lepidoptera, Gelechiidae) on two tomato cultivars with different leaf morphological characteristics. Entomol Gen 36:127–136
SAS (2003) Statistical analysis system. SAS release 9.1 for windows. SAS Institute Inc., Cary
Schausberger P, Walzer A (2001) Combined versus single species release of predaceous mites: predator–predator interactions and pest suppression. Biol Control 20:269–278
Shahbazvar N, Sahragard A, Manzari S, Hosseini R, Hajizadeh J (2010) A faunal study of whiteflies (Hemiptera: Aleyrodidae) and their parasitoids in Gilan province, Iran. Entomofauna 31:269–284
Snyder WE, Ballard SN, Yang S, Clevenger GM, Miller TD, Ahn JJ, Hatten TD, Berryman AA (2004) Complementary biocontrol of aphids by the ladybird beetle Harmonia axyridis and the parasitoid Aphelinus asychis on greenhouse roses. Biol Control 30:229–235
Snyder WE, Ives AR (2003) Interactions between specialist and generalist natural enemies: parasitoids, predators, and pea aphid biocontrol. Ecology 84:91–107
Sohrabi F, Enkegaard A, Shishehbor P, Saber M, Mosaddegh MS (2013) Intraguild predation by the generalist predator Orius majusculus on the parasitoid Encarsia formosa. BioControl 58:65–72
Stansly PA, Calvo J, Urbaneja A (2005) Release rates for control of Bemisia tabaci (Homoptera: Aleyrodidae) biotype “Q” with Eretmocerus mundus (Hymenoptera: Aphelinidae) in greenhouse tomato and pepper. Biol Control 35:124–133
Velasco-Hernández MC, Ramirez-Romero R, Cicero L, Michel-Rios C, Desneux N (2013) Intraguild predation on the whitefly parasitoid Eretmocerus eremicus by the generalist predator Geocoris punctipes: a behavioral approach. PLoS One. 8:e80679
Williamson CE (1980) The predatory behavior of Mesocyclops edax: Predator preferences, prey defenses, and starvation-induced changes. Limnol Oceanogr 25:903–909
Zandi-Sohani N, Shishehbor P, Kocheili F (2009) Parasitism of cotton whitefly, Bemisia tabaci on cucumber by Eretmocerus mundus: Bionomics in relation to temperature. Crop Prot 28:963–967
Zang LS, Liu TX (2007) Intraguild interactions between an oligophagous predator, Delphastus catalinae (Coleoptera: Coccinellidae), and a parasitoid, Encarsia sophia (Hymenoptera: Aphelinidae), of Bemisia tabaci (Homoptera: Aleyrodidae). Biol Control 41:142–150
Acknowledgments
The authors would like to thank Agricultural Sciences and Natural Resources University of Khuzestan, Iran, for the financial support of this research project.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pirzadfard, S., Zandi-Sohani, N., Sohrabi, F. et al. Intraguild interactions of a generalist predator, Orius albidipennis, with two Bemisia tabaci parasitoids. Int J Trop Insect Sci 40, 259–265 (2020). https://doi.org/10.1007/s42690-019-00075-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-019-00075-w