Abstract
Piper chaba Hunter belonging to the Piperaceae family, is mainly used as a spice in Bangladesh and West Bengal in India. It is an ethnobotanical-supportive plant. No biological and fatty acid composition study on its’ leaves’ extracts has been found previously. The present study is on the antioxidant activity and fatty acid analysis of P. chaba leaves. The methanol extract of the leaves and its’ different soluble fractionates i.e. n-hexane, dichloromethane, chloroform, ethyl acetate, and aqueous were subjected to screening for antioxidant activity. The highest free radical scavenging activity by the DPPH assay method was shown by dichloromethane fractionate (radical scavenger) with an IC50 value of 8.84 µg/mL which is close to the IC50 value of the standard. Chloroform fractionates showed a significant capacity of 216 mg/g (expressed as ascorbic acid equivalents) by the Phosphomolybdenum assay method. The analysis of fatty acids content showed that the leaves contain the highest proportion of caprylic acid (40.31%) and the lowest proportion of lauric acid and linolenic acid (0.39%) as bound form and the highest proportion of caprylic acid (34.43%) and the lowest proportion of linolenic acid (1.08%) as free form. A good number of unsaturated fatty acids such as palmitoleic, oleic, linolenic, and erucic acids were present in the leaves of this plant. About 24.02% palmitoleic acid was found as the free form which indicated that the antioxidant activity of leaves was significant as per the obtained result. This study recommends using the leaves as a potent source of antioxidants and fatty acids. Extraction of bioactive compounds using methanol extract from the leaves will open the root to new drug discovery as it is a great source of alkaloids, flavonoids, terpenes, etc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The production of free radicals and lipid peroxidation are two primary causes of illness and aging in humans and animals (Oskoueian et al. 2011). An antioxidant is a chemical compound that is very helpful because it can stop or slow the production of free radicals and lipid peroxidation in the bodies of humans and animals. It aids in delaying the aging process and protects against various diseases linked to the heart, kidneys, lungs, circulatory system, muscles, and brain (Karimi et al. 2013).
Typically, fatty acid molecules are bound to other molecules, such as sugars, glycerol, or phosphate head groups, to create lipids. Lipids are essential components of cell structures, such as cell membranes, which are phospholipids’ primary constituents, and energy reserves, which are frequently composed of lipids. Free fatty acids, which have a wide range of powerful biological actions, are created when fatty acids are liberated from lipids, usually by the activity of enzymes (Desbois and Smith 2010). Free fatty acid biological activities play a part in the host’s defense mechanisms against potentially harmful or opportunistic bacteria. Fatty acids are frequently recognized as the active components in traditional and herbal remedies (McGaw et al. 2002; Yff et al. 2002).
There is a great emphasis on discovering biologically active natural products from higher plants that are better than synthetic chemicals and are much safer, from a health and environmental point of view (Balunas and Kinghorn 2005). Plants are an essential source of chemical compounds. Plant-based treatment is becoming more popular with patients and physicians due to its potent medicinal effects, lower cost, and fewer side effects than modern allopathic medicines (Dias et al. 2012; Popović et al. 2016). So considering the beneficial effects of plants, it is necessary to isolate the molecules or bioactive agents that have been considered potential prototypes for the design and development of novel classes of drugs. Besides, almost 80% of available drugs are either directly derived from nature or their modified analogs. Thus, the importance of natural product chemistry is increasing day by day (Alam et al. 2021).
Piper chaba Hunter (P. chaba H) is a flowering vine in the family of Piperaceae native to south and southeast Asia. P. chaba H is called Chuijhal or Choijhal (Islam et al. 2020) in the Khulna-Jessore region of Bangladesh, Tripura (India), and West Bengal (India). In Bangladesh, the use of Choijhal is unique, because the twigs, stems, or roots of P. chaba H are used as a spice. It is a relatively expensive spice in Bangladesh, and the roots are usually more expensive than the stems because of their stronger aroma.
It is an ethnobotanical-supportive plant, which contains a lot of chemical compounds. Piper species have demonstrated the potential for antidiabetic, antihypertensive, immunoprotective, neuroprotective, and anticarcinogenic activities (Biswas et al. 2022; Haq et al. 2021 Singh and Shukla 2024 Islam et al. 2020). The ingestion of piper species is used to cure a wide range of illnesses, including fever, headache, diarrhea, rheumatism, boils, scabies, and stomach problems (Tsai et al. 2005; Chakraborty and Shah 2011; Sharkar et al. 2013; Umoh et al. 2013; Aziz et al. 2015 ) (Figs. 1 and 2).
The plant’s stem bark contains unique carbamide piperine dimer and alkaloids, which have antimicrobial and pharmacological properties (Rukachaisirikul et al. 2002 Rahman et al., 2005). Piperine shows anti-inflammatory activity. It produces a burning sensation because it activates the TRPV1 receptor (Dong et al. 2019; Correa et al. 2010; Chen et al. 2013).
The previous study shows that compounds isolated from the roots and fruits (Morikawa et al. 2004; Naz et al. 2009 Buagaew and Poomipark 2020) of P. chaba H. exhibit pharmacological activities against different health disorders. The 80% aqueous acetone extract of the fruit of P. chaba has hepatoprotective effects on D-galactosamine (D-GalN/lipopolysaccharide-induced liver injury in mice (Matsuda et al. 2008, 2009 Morikawa 2010).
Alkaloid Chingchengenamide: A was isolated from the leaves of the plant for the first time (Shandhi et al. 2020). Further study will ensure the isolation of more biologically active compounds from the leaves of the plant. From this research, an evaluation of the antioxidant profile and fatty acids content of the leaves has been performed, which may consider this plant as potential therapeutics in human health.
Materials and methods
Plant source
The leaves of the plant P. chaba H (Locally known as Chuijhal) have been collected from the northern part of Bangladesh (Sindurmoti union, Rajarhat, Kurigram). The Department of Botany, University of Dhaka confirmed the taxonomy of the plant (Fig. 3).
Solvent extraction
The air-dried fresh leaves (~ 500 g) of P. chaba were exhaustively extracted with Methanol (MeOH) at room temperature. A solid residue (11.11 g) was obtained after the removal of the solvent using a rotary vacuum evaporator and fridge dryer. The dried MeOH extract was then suspended in H2O and partitioned by separating the funnel successively with n-hexane (HEX), dichloromethane (DCM), chloroform (CHCl3), ethyl acetate (EAC), and MeOH by VanWagenen method (VanWagenen et al. 1993). The fractionates were then evaporated to dryness (Rolta et al. 2020, 2022). The amount of different fractionates was n-hexane (2.86 g), DCM (4.0 g), ethyl acetate (0.64 g), methanol (2.7 g), and aqueous (2.4 g).
Free radical scavenging assay
The free radical scavenging activities of the MeOH extract and different soluble fractionates on the stable radical 1, 1-diphenyl-2-picrylhydrazyl (DPPH) were estimated by the method of Brand-Williams et al. 1995. 2.0 ml of a methanol solution of the extract at different concentrations was mixed with 2.0 ml of a DPPH methanol solution (0.1mM). The antioxidant potential was assayed from the bleaching of purple-colored methanol solution of DPPH radical by the plant extract as compared to that of ascorbic acid by UV spectrophotometer (Figs. 4 and 5).
Inhibition of free radical DPPH in percent (I%) was calculated as follows:
(I%) = (1– Asample/Ablank) X 100.
Where Ablank is the absorbance of the control reaction (containing all reagents except the test material), sample concentration providing 50% inhibition (IC50) was calculated from the graph plotted inhibition percentage against extract concentration.
Phosphomolybdenum assay method
The total antioxidant capacity of the MeOH extract and different soluble fractionates were evaluated by the phosphomolybdenum assay method which is based on the reduction of Mo (VI) to Mo (V) and the subsequent formation of a green phosphate-Mo (V) complex in acidic condition. The 0.3 ml of each sample was allowed to mix with 3.0 mL of the reagent solution (0.6 M H2SO4, 28 mM Na3PO4, 4 mM ammonium molybdate). This reaction mixture was incubated at 95oC for 90 min. After letting the solution cool back to room temperature, the absorbance was measured at 695 nm using a spectrophotometer against a blank solution. The total antioxidant capacity was determined and expressed as mg ascorbic acid equivalents per gram of dry sample using the equation obtained from a standard ascorbic acid calibration curve (y = 0.0084x − 0.0141, R2 = 0.9939).
Analysis of fatty acids through GC-FID
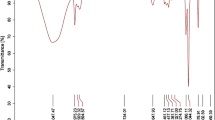
Fatty acid methyl esters (FAMEs) were prepared from the n-hexane soluble fractionate according to the method described by Savage et al. (1997). n-hexane soluble fractionate (0.431 g) of leaves was dissolved in n-hexane (50 mL) and extracted with 5% sodium bicarbonate solution (25mL× 2). The mixture was taken in a separatory funnel and shaken vigorously and allowed to stand overnight. Two layers were obtained. The lower layer (aqueous) was separated and taken to analyze free fatty acid (FFA). The upper layer was separated and taken to analyze bound fatty acids. The FAMEs were determined by GC (Shimadzu 9 A, Column-BP-50, Detector-FID, 105 °C–5 °C/min-150 °C–2 °C/min-280) according to the method reported by Azadmard-Damirchi and Dutta (2006). The FAMEs were analyzed by comparison of their retention times with standard FAMEs and the peak areas are reported as a percentage of the total fatty acids (Figs. 6, 7 and 8).
Result and discussion
Free radical scavenging assay
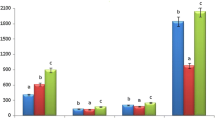
The antioxidant activity of IC50 values in the DPPH method differed in MeOH extract and different fractionates ranging from (8.84 µg/mL) to (68.69 µg/mL). In this investigation, DCM showed the highest free radical scavenging activity with an IC50 value of 8.84 µg/mL as compared to Ascorbic acid at 2.15 µg/mL (Fig. 9). At the same time, the MeOH extract and n-hexane fractionate also exhibited antioxidant potential having IC50 values of 24.80, and 68.69 µg/mL respectively.
Phosphomolybdenum assay method
It is determined through the phosphomolybdenum assay method (Figs. 10 and 11),
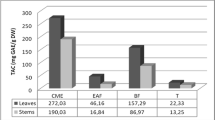
The total antioxidant capacity of the CHCl3 fractionate of the leaves of P. chaba was 216.56 mg/g (expressed as ascorbic acid equivalents), the highest antioxidant capacity compared with other fractionates. On the other hand, the Aqueous fractionate was found to show 21.44 mg/g (as ascorbic acid equivalents) which is the lowest antioxidant capacity compared with others. MeOH extract, n-hexane, DCM, and EAC fractionates showed 122.51, 146.56, 111.08, and 82.27 mg/g antioxidant capacity respectively. The phosphomolybdenum method was based on the reduction of Mo(VI) to Mo(V) by the antioxidant compound and the formation of a green phosphate/Mo(V) complex with maximal absorption at 695 nm.
Fatty acids content
The prepared methyl ester of free fatty acids (FFA), Bound fatty acids (BFA) along with standard fatty acids ester samples were analyzed in GC-FID (Shimadzu 9 A, Column-BP-50, Detector-FID, 105 °C–5 °C/min-150 °C–2 °C/min-280) and their retention time was recorded. The relative percentages of the free fatty acid and bound fatty acids were calculated from the retention time. The fatty acids of the plant were converted to their methyl esters and analyzed by GC-FID. The fatty acids present in the plant were identified by comparison with the retention time of the standard samples. Their relative percentages were also determined.
The analysis of bound fatty acids showed that P. chaba contains the highest proportion of caprylic acid (40.31%), and the lowest proportion of lauric acid and linolenic acid (0.39%). Others, such as palmitoleic, stearic, oleic, arachidic, behenic, and erucic acids are present with intermediate percentages of 9.52, 22.10, 3.08, 0.62, 1.09, and 0.45%.
The analysis of free fatty acids showed that caprylic acid is the most abundant (34.43%) and linolenic acid is the lowest proportion (1.08%) of fatty acid present in free form in the leaves of P. chaba (Figs. 12 and 13).
Myristic, palmitoleic, stearic, oleic, arachidic, behenic, and erucic acids are the other fatty acids present with an intermediate percentage of 1.41, 24.02, 4.45, 7.77, 1.72, 4.07 and 1.39%. A small amount of fatty acid was present in the n-hexane extract of the plant in a free state compared with bound fatty acid.
Conclusion
The plant P. chaba H is a medicinal plant with significant pharmacological activity. Leaves of this plant could be a good source of antioxidant agents. This study was not performed previously and should be carried out further to determine this plant’s medicinal importance. DPPH assay method showed that DCM fractionate had the highest free radical scavenging activity with an IC50 value of 8.84 µg/mL which was very significant in comparison with antioxidant agent Ascorbic acid. On the other hand, the CHCl3 fractionate showed a significant capacity of 216 mg/g (expressed as ascorbic acid equivalents) which was higher than many antioxidant sources. P. chaba H leaves are a prominent source of fatty acids that have not been researched previously. It contained the highest proportion of caprylic acid (40.31%) and the lowest proportion of lauric acid and linolenic acid (0.39%) as bound forms of fatty acids. The analysis of free fatty acids showed that caprylic acid was the most abundant (34.43%) and linolenic acid was the lowest proportion (1.08%). Myristic, palmitoleic, stearic, oleic, arachidic, behenic, and erucic acids were the other fatty acids that were also present in the leaves of this plant, which are precious fatty acids for living beings. From this research it can be concluded that leaves of P. chaba H are a potent antioxidant source, they can be used for making antioxidant agents. The antioxidant profile of this plant is too rich, which will open the root of isolation of potent pharmacological active compounds by attending phytochemical investigation of this plant. The fatty acid content of the leaves is also significant. The leaves are a potent source of fatty acids which are very important for living beings. Many unsaturated fatty acids were found and palmitoleic was present in a good quantity, that’s why the antioxidant activity of the leaves was significant. The researcher should emphasize their research interest in this potent medicinal plant. In the future, this plant will contribute a lot to the medicinal sector as a potent drug discovery agent if it is studied properly by dedicated researchers.
Data availability
The different sets of data that have been generated or used and examined to write the present manuscript are available from the corresponding author upon reasonable request.
Abbreviations
- DCM:
-
Dichloromethane
- EAC:
-
Ethyl acetate
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- AQ:
-
Aqueous
- HEX:
-
n-hexane
- D-GalN:
-
D-galactosamine
- MeOH:
-
Methanol
- GC-FID:
-
Gas chromatography-flame ionization detector
- BFA:
-
Bound fatty acid
- FFA:
-
Free fatty acid
- FAME:
-
Fatty acid methyl ester
References
Alam S, Emon NU, Shahriar S, Richi FT, Haque MR, Islam MN, Ganguly A (2020) Pharmacological and computer-aided studies provide new insights into Millettia Peguensis Ali (Fabaceae). Saudi Pharm J 28(12):1777–1790
Alam S, Rashid MA, Sarker MMR, Emon NU, Arman M, Mohamed IN, Haque MR (2021) Antidiarrheal, antimicrobial and antioxidant potentials of methanol extract of Colocasia gigantea hook. f. leaves: evidenced from in vivo and in vitro studies along with computer-aided approaches. BMC Complement Med Ther 21:1–12
Azadmard-Damirchi S, Dutta PC (2006) Novel solid-phase extraction method separates 4-desmethyl-, 4-monomethyl-, and 4, 4′-dimethylsterols in vegetable oils. J Chromatogr A 1108(2):183–187
Aziz DM, Hama JR, Alam SM (2015) Synthesising a novel derivatives of piperine from black pepper (Piper nigrum L). J Food Meas Charact 9:324–331
Balunas MJ, Kinghorn AD (2005) Drug discovery from medicinal plants. Life Sci 78(5):431–441. https://doi.org/10.1016/j.lfs.2005.09.012
Bangladesh Academy of Sciences (1991); 15(2):133–138
Biswas P, Ghorai M, Mishra T, Gopalakrishnan AV, Roy D, Mane AB, Dey A (2022) Piper longum L.: a comprehensive review on traditional uses, phytochemistry, pharmacology, and health-promoting activities. Phytother Res 36(12):4425–4476
Brand-Williams W, Cuvelier ME, Berset CL (1995) Use of a free radical method to evaluate antioxidantactivity. LWT-Food Sci Technol 28(1):25–30.
Buagaew A, Poomipark N (2020) Protective effect of piperine from Piper chaba fruits on LPS-induced inflammation in human intestinal cell line. J Med Plants Res 14(9):438–444. https://doi.org/10.5897/JMPR2020.6996
Chakraborty D, Shah B (2011) Antimicrobial, antioxidative and antihemolytic activity of Piper betel leaf xtracts. Int J Pharm Pharm Sci 3(3):192–199.
Chen CY, Li W, Qu KP, Chen CR (2013) Piperine exerts anti-seizure effects via the TRPV1 receptor in mice. Eur J Pharmacol 714(1–3):288–294
Correa EA, Högestätt ED, Sterner O, Echeverri F, Zygmunt PM (2010) In vitro TRPV1 activity of piperine derived amides. Bioorg Med Chem 18(9):3299–3306
Desbois AP, Smith VJ (2010) Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol 85:1629–1642. https://doi.org/10.1007/s00253-009-2355-3
Dias DA, Urban S, Roessner U (2012) A historical overview of natural products in drug discovery. Metabolites 2(2):303–336
Dong Y, Yin Y, Vu S, Yang F, Yarov-Yarovoy V, Tian Y, Zheng J (2019) A distinct structural mechanism underlies TRPV1 activation by piperine. Biochem Biophys Res Commun 516(2):365–372
Haq IU, Imran M, Nadeem M, Tufail T, Gondal TA, Mubarak MS (2021) Piperine: a review of its biological effects. Phytother Res 35(2):680–700
Islam MT, Hasan J, Snigdha HSH, Ali ES, Sharifi-Rad J, Martorell M, Mubarak MS (2020) Chemical profile, traditional uses, and biological activities of Piper Chaba Hunter: a review. J Ethnopharmacol 257:112853
Karimi E, Jaafar HZ, Ahmad S (2013) Antifungal, anti-inflammatory and cytotoxicity activities of three varieties of labisia pumila benth: from microwave obtained extracts. BMC Complement Altern Med 13:1–10
Matsuda H, Ninomiya K, Morikawa T, Yasuda D, Yamaguchi I, Yoshikawa M (2008) Protective effects of amide constituents from the fruit of Piper chaba on d-galactosamine/TNF-α-induced cell death in mouse hepatocytes. Bioorg Med Chem Lett 18(6):2038–2042. https://doi.org/10.1016/j.bmcl.2008.01.101
Matsuda H, Ninomiya K, Morikawa T, Yasuda D, Yamaguchi I, Yoshikawa M (2009) Hepatoprotective amide constituents from the fruit of Piper chaba: structural requirements, mode of action, and new amides. Bioorg Med Chem 17(20):7313–7323
McGaw LJ, Jäger AK, Van Staden J (2002) Antibacterial effects of fatty acids and related compounds from plants. South Afr J Bot 68(4):417–423
Morikawa T (2010) Search for TNF-alpha sensitivity degradation principles from medicinal foods-hepatoprotective amide constituents from Thai natural medicine Piper Chaba. Yakugaku Zasshi: J Pharm Soc Japan 130(6):785–791
Morikawa T, Matsuda H, Yamaguchi I, Pongpiriyadacha Y, Yoshikawa M (2004) New amides and gastroprotective constituents from the fruit of Piper Chaba. Planta Med 70(02):152–159
Naz T, Mosaddik A, Haque ME (2009) Antimicrobial and cytotoxic activities of root extracts of Piper Chaba. J Sci Res 1:138–144
Oskoueian E, Abdullah N, Hendra R, Karimi E (2011) Bioactive compounds, antioxidant, xanthine oxidase inhibitory, tyrosinase inhibitory and anti-inflammatory activities of selected agro-industrial by-products. Int J Mol Sci 12(12):8610–8625
Popović Z, Matić R, Bojović S, Stefanović M, Vidaković V (2016) Ethnobotany and herbal medicine in modern complementary and alternative medicine: an overview of publications in the field of I&C medicine 2001–2013. J Ethnopharmacol 181:182–192
Rolta R, Kumar V, Sourirajan A, Upadhyay NK, Dev K (2020) Bioassay guided fractionation of rhizome extract of Rheum Emodi wall as bio-availability enhancer of antibiotics against bacterial and fungal pathogens. J Ethnopharmacol 257:112867
Rolta R, Goyal M, Sharma S, Bharaj D, Salaria D, Upadhyay NK, Sourirajan A (2022) Bioassay guided Fractionation of Phytocompounds from Bergenia Ligulata: a synergistic approach to treat drug resistant bacterial and fungal pathogens. Pharmacol Research-Modern Chin Med 3:100076
Rukachaisirikul T, Prabpai S, Champung P, Suksamrarn A (2002) Chabamide, a novel piperine dimer from stems of Piper Chaba. Planta Med 68(09):853–855
Salmerón-Manzano E, Garrido-Cardenas JA, Manzano-Agugliaro F (2020) Worldwide research trends on medicinal plants. Int J Environ Res Public Health 17(10):3376. https://doi.org/10.3390/ijerph17103376
Savage GP, McNeil DL, Dutta PC (1997) Lipid composition and oxidative stability of oils in hazelnuts (Corylus avellana L.) grown in New Zealand. J Am Oil Chem Soc 74(6):755–759
Shandhi SP, Roy AC, Rahman H, Chowdhury TA (2020) Isolation of alkaloids Chabamide I, Piperine and Chingchengenamide: a from Piper Chaba H. leaves and their in vitro antimicrobial activities. J Pharmacognosy Phytochemistry 9(5):2811–2814
Sharkar P, Rahman MM, Masum H, Nayeem GZ, Hossen MA, M. M., Azad AK (2013) Ethnomedicinal importance of the plants in villages in kushtia sador and mirpur upozila, Bangladesh. J Herbs Spices Med Plants 19(4):401–417
Singh S, Shukla A (2024) Therapeutic potential of Piperine: a Comprehensive Review. Nat Prod J 14(3):53–67. https://doi.org/10.2174/0122103155273860230928071249
Tsai IL, Lee FP, Wu CC, Duh CY, Ishikawa T, Chen JJ, Chen IS (2005) New cytotoxic cyclobutanoid amides, a new furanoid lignan and anti-platelet aggregation constituents from Piper arborescens. Planta Med 71(06):535–542
Umoh I, Oyebadejo S, Bassey EO, Udoh N (2013) Histomorphological study of the effect of chronic consumption of Abelmoschus esculentus and Piper guineense on the gastric mucosa of albino wistar rats. Int J Pharm Res Allied Sci 2:31–37
VanWagenen BC, Larsen R, Cardellina JH, Randazzo D, Lidert ZC, Swithenbank C (1993) Ulosantoin, a potent insecticide from the sponge Ulosa ruetzleri. J Org Chem 58(2):335–337
Yff BT, Lindsey KL, Taylor MB, Erasmus DG, Jäger AK (2002) The pharmacological screening of Pentanisia prunelloides and the isolation of the antibacterial compound palmitic acid. J Ethnopharmacol 79(1):101–107
Acknowledgements
The author is thankful to the Bangladesh Council of Scientific & Industrial Research, the Committee for Advanced Studies and Research (CASR), the University of Dhaka for providing lab facilities, and Professor Dr. Tofail Ahmad Chowdhury for his valuable advice.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SPS the 1st and corresponding author has done all the lab works and writing, editing the manuscript related to the research work.
Ethics declarations
Ethical approval
Not applicable.
Consent of interests
No, I declare that there is no consent of interests as defined by the journal.
Competing Interests
No, I declare that the author has no competing interests as defined by the journal, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shandhi, S.P. Antioxidant profile, and fatty acids analysis of leaves of medicinal plant Piper chaba H. (Chuijhal): a promising source of antioxidant and fatty acids. Vegetos (2024). https://doi.org/10.1007/s42535-024-00898-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42535-024-00898-0