Abstract
Plant growth promoting bacterial strains were used as bioinoculants on cereal crops to improve plant growth and plant productivity. Crop responses to inoculation are complex because bacteria are not compatible with each other. Therefore, it is necessary to increase our understanding of the microbial ecology of crop rhizosphere under various agricultural techniques. In tropical agriculture, cattle manure is used as an organic fertilizer to increase soil fertility, however use of microbes as consortium have found as sustainable method for the enhancement of crops productivity. The purpose of this study was to evaluate the effects of three potential plant growth-promoting rhizospheric and endophytic bacterial strains EU-C3ST.R1, IARI-JR-44, and IARI-S-45 and organic fertilizers (cattle manure) individually and as consortia on maize (Zea mays L.) under both in-vitro and in-vivo conditions. A total of 123 bacterial strains were sorted out and screened for nitrogen fixation, phosphorus, and potassium solubilization. The potential N2-fixing; P and K solubilizing bacterial strains were identified using 16 S rRNA gene sequencing as Pseudomonas sp. EU-C3ST.R1, Micrococcus indicus IARI-JR-44, and Bacillus horikoshii IARI-S-45 respectively. The inoculation of these three strains on maize as microbial consortium and individual inoculum significantly increased the growth characteristic including height and biomass of the plants, as well as physiological characteristics i.e., chlorophyll, carotenoids, flavonoids, phenolics, and total soluble sugar content of the plant with respect to chemical fertilizers, cattle manure, and untreated control plant. The consortia were found to be more effective with respect to individual inoculants, cattle manure, and uninoculated control plants, so it can be utilized as biofertilizers for inoculation of cereal crops growing in hilly regions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The performance of intensive farming depends on the extensive use of pricy off-farm agrochemical fertilizers, which produce a lot of environmental pollutants (Horrigan et al. 2002). However, in order to meet the demand of an expanding population and the need for food, chemical fertilizers, pesticides, and insecticides must be introduced to the soil, but these substances disturb the soil’s physiochemical properties, including its texture, porosity, and ability to hold water, as well as its microbial population. In past few decades, organic farming has attracted more attention due to the detrimental effect of chemical. Organic farming includes the application of genetically modified organisms (GMOs), mineral fertilizers, pesticides cow’s urine, cow’s horn, a dead body of a cow and sewage sludge. Various compounds made from cattle manure and pee can also be used in agriculture practice as fertilizers and pest repellent. Several studies show that organic farming produces much higher grain and higher quality of plant foodstuffs than conventional agriculture (Chemura 2014; Oliveira et al. 2013). The soil quality using animal manure composted has been also shown to be superior then conventional farming in long-term trials conducted in Switzerland (Mäder et al. 2002). Organic farming is also the best technology that avoids or largely excludes the use of chemical fertilizers or pesticides. Composting (the practice of farmers to stack straw and dung) is a common approach in organic agriculture; however, it takes a lot of time.
Therefore, scientific community is focusing on beneficial microbes which could be used as biofertilizers to reduce chemical fertilizers and maintain soil fertility. Bacteria are found in different regions including rhizospheric, endophytic, and epiphytic promotes the plants growth via direct and indirect mechanism including fixation of nitrogen; solubilization of phosphorus, potassium, zinc and selenium, production of siderophores, hydrogen cyanide, ammonia, and indole-3-actic acid (IAA), hydrolytic enzymes and numerous plant growth promoting (PGP) bioactive compounds (Han et al. 2005; Rodrı́guez and Fraga 1999). Microorganisms have the potential for the enhancement of plant growth and development when they are applied individually and as microbial consortia. Microbial consortia are becoming more popular as a means of ensuring agricultural sustainability. Consortia are more efficient for growth of plants and crop productivity over the individual microbial inoculants. Several reports have been available in which microbial consortia inoculated on different cereal and vegetables crops which has found to more effecient. In a report, consortium of N2-fixer, phosphorus, and potassium solubilizers, Chryseobacterium arthrosphaerae EU-LWNA-37, Erwinia sp. EU-B2SNL1, and Pseudomonas gessardii EU-MRK-19 on barley significantly improved the growth and physiological parameters over to the individual inoculants and chemical fertilizers and uninoculated plants (Kaur et al. 2022). Similarly, microbial consortia of N2- fixer and P, K solubilizers Pseudomonas extremorientalis EU-B1RTR1, Erwinia persicina A3SK3, and Halomonas aquamarina EU-B2RNL2 was evaluated in chilli crop and the consortium was enhancing the growth and physiological parameters over the single inoculants (Devi et al. 2022).
The present study deals with characterization of beneficial bacteria from rhizospheric soil and plant roots. Isolated bacteria were screened for the mineral solubilizing and other plant growth promoting traits, including N2-fixer, P and K solubilization, and selected strains were used to prepare the consortia for the evaluation on maize crop with the combination of vermicomposting and chemical fertilizers.
Materials and methods
Enrichment, isolation and enumeration of bacteria
To isolate endophytic bacteria, plant’s root samples of different cereal and pseudocereals crops (finger millet, barley, foxtail millet, maize, and wheat) were collected in sterile bags from diverse location of Baru Sahib, Sirmaur, Himachal Pradesh, India. All the samples were collected in triplicate and transported to the lab. The endophytic bacteria from roots were isolated using surface sterilization method described by Conn and Franco (2004). The surface sterilization was done by sequential washing of roots (water rinsed) with 50% ethanol for 1 min, followed by 2% sodium hypochlorite for 3 min, 50% ethanol for 30 s and sterile distilled water. The surface sterilized root samples were crushed, serially diluted and 0.1 mL sample was spread onto different selective and non-selective growth media including Azotobacter media, Jensen’s agar, T3A agar, King’s B agar, nutrient agar, ammonium mineral salt, tryptic soy agar, and yeast mannitol agar. The plate’s visible colonies were re-streaked using respective media agar plate for purification. The rhizospheric bacterial samples were also obtained from the Division of Microbiology, Institute of Indian Agricultural Research Institute (IARI), Delhi, India. The purified colonies were preserved on nutrient agar slants and in glycerol stock (25%) at -80 ºC and 4ºC, respectively for future analysis.
Screening of bacteria for mineral solubilizing and other plant growth promoting attributes
All the isolates (rhizospheric and endophytic) bacteria were screened for mineral solubilizing attributes including solubilization of P, K and Zn; and on the basis of zone and quantitative analysis, mineral solubilizing bacteria were selected for further screening of other PGP attributes such as acetylene reduction assay; production of siderophores, HCN, ammonia, and IAA.
Screening of bacteria for mineral solubilization attributes
According to Pikovskaya (1948); Hu et al. (2006); and Fasim et al. (2002), the qualitative analysis of the P, K, and Zn solubilization were carried out on 0.5% tricalcium phosphate (TCP) supplemented Pikovskaya agar, 0.2% potassium aluminosilicates amended Aleksandrov agar and 0.1% insoluble zinc compounds (ZnO, ZnS, Zn3(PO4)2, and ZnCO3) containing nutrient agar, respectively. The qualitative analysis was done using spotting method and inoculated plate were incubated at 30 ºC for 2–4 days. The isolates exhibiting minerals solubilization attributes in a qualitative analysis, were further screened for quantitative analysis of P, K, and Zn using Pikovskaya, Aleksandrov and nutrient broth supplemented with 0.5% tricalcium phosphate (TCP), 0.2% potassium aluminosilicates and 0.1% insoluble zinc compounds (ZnO, ZnS, Zn3(PO4)2, and ZnCO3), respectively. The quantitively analysis of P, K, and Zn was carried out by method explained in Murphy and Riley (1962); Hu et al. (2006); and Sugumaran and Janarthanam (2007).
Screening of mineral solubilizing bacteria for other plant growth promoting attributes
Acetylene reduction assay
The selected mineral solubilizing bacteria (rhizospheric and endophytic) were screened for nitrogen fixation attribute using acetylene reduction assay (ARS) method (Han and New 1998). The selected bacterial isolates were inoculated on to nitrogen free bromothymol (NFB) blue medium and kept in BOD incubator at 30 °C for 5–7 days. Each test tube’s cotton plugs were replaced with Suba seal and gas phase was replaced by 10% of nitrogen, air, and acetylene gas mixture at a ratio of 90:10:10, v/v. The test tube containing isolates and 10% of gas mixture were again incubated at 30 °C for 24 h. The production of ethylene was quantified via gas chromatography equipped with a flame ionization detector and a capillary column. To estimate of C2H4 production, the chromatogram was utilized to integrate the regions of the curve of C2H2 and ethylene (Holguin et al. 1992).
Indole-3-actetic acid production
Indole-3-actetic acid production was detected by the modified method of Bric et al. (1991). Quantitative analysis of IAA was performed by using 10% of tryptophan solution. Bacterial cultures were grown for 48 h at 30ºC in LB broth. Fully grown bacterial cultures were centrifuged at 3000 rpm for 30 min. A 50 mL Salkowski reagent (35% perchloric acid, and 1 mL of 0.5 M FeCl3 solution) was prepared and 2–3 drops were added to the supernatant (2 mL). After the development of the pink color and optical density was determined using spectrophotometer at 600 nm (Patten and Glick 2002).
Siderophores, hydrogen cyanide and ammonia production
The selected bacterial isolates were screened for the indirect PGP attributes i.e. siderophores (Schwyn and Neilands 1987), HCN (Bakker and Schippers 1987), and ammonia (Cappucino and Sherman 1992).
Molecular characterization of selected mineral solubilizing and PGP microbial isolates
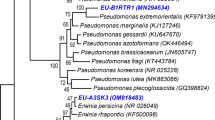
The extraction of genomic DNA of selected bacterial strains was performed according to the method described by Verma et al. (2015). The exacted gDNA samples were subjected to PCR amplification of the 16 S rRNA gene using the primers pA and pH (Yadav et al. 2016). Purified PCR products were sent to the Science of the Genome (SciGenome), Chennai, India for sequencing. The obtained sequences were subjected to BLASTn tool available at GenBank for the identification of selected bacteria. After identification, phylogenetic tree was constructed by neighbor-joining (NJ) method using MEGA 4.0 software. The sequences were submitted to GenBank for the generation of the accession number.
Development of microbial consortia
The three potential bacteria EU-C3ST.R1, IARI-JR-44, and IARI-S-45 having the multifarious PGP traits including fixation of nitrogen, solubilization of P, and K, respectively were developed as microbial consortium. Before the development of microbial consortium compatibility test was conducted by the cross-streaked experiment described by Kaur et al. (2022). The streaked plate was kept for incubation at 28ºC for 2–3 days in BOD incubator and compatibility was confirmed with no inhibition of growth. After compatibility testing, the consortia were developed by growing isolates individually in nutrient broth at 28 °C for 24 h. CFU of the bacterial isolates was estimated as 2.24 × 107 CFU/mL (EU-C3ST.R1), 3.56 × 107 CFU/mL (IARI-JR-44), 2.65 × 107 CFU/mL (IARI-S-45). In the preparation of the microbial consortia, an equal amount of bacterial culture suspension was mixed for the evaluation on maize crop.
Evaluation of microbial consortia under in-vitro and in-vivo conditions
The experiment was conducted on maize crop under controlled and open field conditions with total nine treatments viz. T1 (microbial consortia of EU-C3ST.R1 (N2-fixer), IARI- JR-44 (P-solubilizer), and IARI- S-45 (K-solubilizer)); T2 (N-fixer + 50% DAP and potash ); T3 (P-solubilizer + 50% urea and potash); T4 (K-solubilizer + 50% DAP, and urea); T5 (cattle manure + 50% NPK); T6 (cattle manure); T7 (recommended 100% NPK chemical fertilizer); T8 (50% NPK chemical fertilizer); T9 (uninoculated control). Under both the conditions experiment was done with three replicates. The in vitro experiment was conducted in plastic pots (30 cm × 30 cm ×26 cm) containing 4 kg soil. Pots were kept at an equal distance from one another to prevent cross contamination. Four seeds were sown in each pot and after the germination of seeds, the number of the plants per pot was reduced to two. Two plants were kept till harvesting. The open field experiment was carried out in the Experimental farm of Eternal University, Baru Sahib, Machher, Sirmaur district Himachal Pradesh having plot area of 11.7 m2 (9 × 1.3 m2) with 0.5 m between each bed. The experiment was done in the laid out as a complete randomized design. In both conditions maize seeds treated with individual inoculants and consortia were mixed with a sugar syrup (1:1 ratio) prior to sowing. After 120 days of sowing, the plants were harvested, and different growth and physiological parameters were recorded.
Analysis of plant growth and physiological parameters
The growth parameters of maize plants including root and shoot length, fresh and dry biomass of the shoot and root, and seed weight were recorded. The dry biomass of the plant (shoot and root) was noted by drying in hot air oven at 65 °C. Post-harvest grain yield was recorded and expressed in q ha− 1. Using the standard method of Lichtenthaler (1987) the chlorophyll and carotenoids content in the leaves of maize plants were assessed. The total soluble sugar content of the maize was carried out according to Irigoyen et al. (1992) phenolic and flavonoids content were estimated according to Kim et al. (2003) and Park et al. (2008), respectively.
Statistical analysis
A student t-test was used to determined statistical significance of the data. The least significant difference (LSD) and critical differences test was used to compared mean (P = 0.05). The finding of the standard error (SE) and the LSD, significant differences, CD 5% and 1% were determined.
Results
Enrichment, isolation and enumeration of bacteria
A total 123 root endophytic bacterial strains were isolated foxtail millet, finger millet, local red and local white maize, and wheat. The abundance of endophytic bacterial population ranged from 0.28 × 107 to 3.14 × 107 per gram of plant roots. The bacterial isolates showed the highest diversity on nutrient agar media from wheat. The purified colonies were obtained on the basis of morphotypes.
Screening of bacteria for mineral solubilizing and other plant growth promoting attributes
All isolated and obtained samples of endophytic and rhizospheric were screened for mineral solubilizing i.e. P, K, Zn and other PGP attributes including nitrogen fixation, production of siderophores, HCN, ammonia, and IAA. Among all the isolates, 22 isolates have ability to fix nitrogen (1.20 to 46.45 nmol C2H4 mg− 1 protein hr− 1), 25 (range 46.3 ± 0.02 to 359.18 ± 0.007 mg/L), 24, and 14, were solubilizers of P, K, and Zn, respectively. Twenty one isolates showed siderophore and 16 showed IAA production (Table 1).
Molecular characterization of selected mineral solubilizing and PGP microbial isolates
The BLASTn algorithm was used to compare partial 16 S rRNA gene sequence obtained after sequencing with those found in the NCBI data base. To determination taxonomic affiliation of the bacterial strain, a phylogenetic tree was constructed (Fig. 1). The 16 S rRNA gene sequencing of selected three efficient bacterial strains EU-C3ST.R1, IARI-JR-44, and IARI-S-45 showed < 97% similarity with Pseudomonas sp., Micrococcus indicus, and Bacillus horikoshii, respectively. The 16 S rRNA gene partial sequences were submitted to NCBI GenBank and accession number were assigned as MN294549, KF054993, and JX460835. The isolates EU-C3ST.R1, IARI-JR-44, and IARI-S-45 were submitted at ICAR-National Bureau of Agriculturally Important Microorganisms (NBAIM) culture-collection facility, Mau Nath Bhanjan, Uttar Pradesh, India (Fig. 1).
Evaluation of microbial consortia under in-vitro and in-vivo condition
The three potential bacterial isolates Pseudomonas sp. EU-C3ST.R1 (nitrogen fixer), Micrococcus indicus IARI-JR-44 (P-solubilizer), and Bacillus horikoshii IARI-S-45 (K- solubilizer) were evaluated on the crop of maize as consortia and individual inoculum for plant growth promotion under in-vitro and in-vivo conditions.
Analysis of plant growth and physiological parameters
In-vitro condition
The experiment was done to evaluate the potential effect of mineral solubilizing and PGP bacterial isolates i.e., Pseudomonas sp. EU-C3ST.R1 (nitrogen fixer), Micrococcus indicus IARI-JR-44 (P-solubilizer), and Bacillus horikoshii IARI-S-45 (K- solubilizer) as consortia and single inoculum on maize crop. The results showed increment in the growth and physiological parameters i.e., length of shoot/root, biomass of the plant (fresh/dry), chlorophyll, carotenoids, flavonoids, phenolics, and total soluble sugar content of the maize. The microbial consortia of Pseudomonas sp. EU-C3ST.R1, Micrococcus indicus IARI-JR-44, and Bacillus horikoshii IARI-S-45 showed 26.19%, 8.53%, and 66.51% increase in the shoot length than the cattle manure + 50% FT (NPK), cattle manure and uninoculated control plants. The increment in the root length was observed up to 1.8, 2.1, and 3.5 fold by the inoculation of microbial consortia over the cattle manure + 50% FT (NPK), cattle manure and uninoculated control. The fresh weight of maize was also increased by the microbial consortia (116.90 ± 1.00 Kg) in comparison to the, cattle manure + 50% FT (NPK) (103.90 ± 1.50 Kg), cattle manure (101.85 ± 0.35 Kg), control (38.65 ± 0.85 Kg) and individual inoculants i.e., EU-C3ST.R1 (103.25 ± 0.35 Kg), IARI-JR-44 (106.65 ± 1.55 Kg), IARI-S-45 (103.10 ± 0.70 Kg). The microbial consortia showed variable effect on dry weight of the maize plant 18.3%, 15.5%, and 82.05% in comparison to cattle manure + 50% FT (NPK), cattle manure, uninoculated control.
The microbial consortia showed positive effects on chlorophyll content of maize plant by as compared to EU-C3ST.R1 (1.08 fold), IARI-JR-44 (1.19 fold), IARI-S-45 (1.19 fold), Cattle manure + 50% FT (NPK) (1.24 fold), cattle manure (1.28 fold), uninoculated control (2.08 fold). The carotenoids content was 1.7 fold higher as compared to the uninoculated. The total soluble sugar content was incremented by microbial consortia (42.03 ± 1.12 µg/g) as comparison to the cattle manure + 50% FT (NPK) (31.07 ± 0.69 µg/g), cattle manure (33.64 ± 0.78 µg/g), recommended full dose of NPK (29.50 ± 0.43 µg/g), half dose of NPK (28.38 ± 0.15 µg/g), and uninoculated control plants (25.97 ± 0.74 µg/g). The phenolics content on the maize were noticed to be enhanced by 2.3 fold by the microbial consortia in comparison to uninoculated control, 1.2 fold over the cattle manure + 50% FT (NPK), 1.5 fold over the cattle manure, 1.0 fold over N-fixing bacteria + 50% FT (DAP + potash) + cattle manure, 1.0 fold than the P-Solubilizer + 50% FT (Urea + potash) + CD, and 1.2 fold over K-Solubilizer + 50% FT ( DAP + urea) + cattle manure. The microbial consortia showed a 40.44% incremented in the flavonoids content as compared to uninoculated control, 7.09% in comparison to recommended full dose of NPK, 18.1% in comparison to half dose of NPK, 11.09% over cattle manure + 50% FT (NPK), and 11.27% as compare to cattle manure (Table 2).
In-vivo condition
In-vivo condition, the mineral solubilizing and PGP bacteria Pseudomonas sp. EU-C3ST.R1 (nitrogen fixer), Micrococcus indicus IARI-JR-44 (P-solubilizer), and Bacillus horikoshii IARI-S-45 (K- solubilizer) was evaluated on maize crop for the better improvement of plant growth and physiological parameters. The microbial consortia of EU-C3ST.R1 (nitrogen fixer), Micrococcus indicus IARI-JR-44 (P-solubilizer), and Bacillus horikoshii IARI-S-45 (K- solubilizer) showed higher shoot length with 14.18%, 4.79% and 39.18% enhancement than the, cattle manure + 50% FT (NPK), cattle manure, and un-treated plant (uninoculated control). Root length of the maize plants were incremented through the inoculation of microbial consortia by 1.51 fold as compare to uninoculated plants (control), 1.30 fold as compared to cattle manure + 50% FT (NPK), 1.21 fold as compared to cattle manure, 1.34 over recommended dose of NPK agro-chemicals, 1.39 than half dose of NPK agro-chemicals. Fresh weight of the maize plant was increased by the inoculation of microbial consortia (404.67 ± 1.43 Kg), as comparison to cattle manure + 50% FT (NPK) (319.70 ± 0.57 Kg), cattle manure (301.67 ± 1.54 Kg), individual inoculants with the half dose of chemical fertilizers including EU-C3ST.R1 (329.53 ± 0.25 Kg), IARI-JR-44 (376.40 ± 2.24 Kg), and IARI-S-45 (320.43 ± 0.69 Kg), and uninoculated control plants (154.33 ± 2.16 Kg). The dry biomass of the plant has been incremented by the microbial consortia by 23.00%, 3.29%, and 98.66% as comparison to cattle manure + 50% FT (NPK), cattle manure, and untreated (control) plants.
The microbial consortia inoculation on the maize crop, results in increased the chlorophyll content as compare to uninoculated control plants (2.04 fold), Pseudomonas sp. (1.02 fold), Micrococcus indicus (1.12 fold), and Bacillus horikoshii (1.18 fold); cattle manure + 50% FT (NPK) (1.32 fold), and cattle manure (1.40 fold). Carotenoids content was improved by the microbial consortia showed (7.38 ± 0.30 g/L) in comparison to untreated control (0.55 ± 0.04 g/L), cattle manure + 50% FT (NPK) (6.00 ± 1.09 g/L), cattle manure (3.83 ± 0.39 g/L), recommended full dose of agro-chemicals NPK (5.17 ± 2.02 g/L), recommended half dose of agro-chemicals NPK (3.09 ± 0.17 g/L). The treatment of microbial consortia incremented the total soluble sugar content in maize leaves by 2.32 fold over untreated plant, 1.01 fold over cattle manure + 50% FT (NPK), 1.21 fold than cattle manure, 1.22 fold in comparison to recommended full dose of agro-chemicals NPK, and 1.92 fold over recommended half dose of agro-chemicals NPK. The phenolics content in the maize leaves were detected to be increased by the bacterial consortia (0.91 ± 0.01 µg/g) as compared to uninoculated control (0.44 ± 0.03 µg/g), cattle manure + 50% FT (NPK) (0.77 ± 0.05 µg/g), and cattle manure (0.83 ± 0.05 µg/g). Flavonoids content was incremented by consortia up to 60.37% as compared to uninoculated control, 26.86% over cattle manure + 50% FT (NPK), 15.48% over cattle manure, 24.26% than the recommended full dose of agro-chemicals NPK, and 29.57% as compared to recommended half dose of agro-chemicals NPK. The weight of cob was incremented by 1.35 and 3.32 fold as compare to cattle manure and uninoculated control by the consortia (Table 3).
Discussion
The present state of agriculture is greatly reliant on the use of agro-chemical based fertilizers that affect the nutritional quality, health status and productivity of the crops. Additionally, the continued release of these chemical inputs leads to the accumulation of harmful substances in the soil, including metals, and pass into the plants over a long period of time, which has ultimately affected human health. Therefore, it becomes important to develop alternatives to agro-chemicals in order to increase agricultural crop production. Organic farming is also the best option to reduce chemical fertilizers from the soil and protect the environment. The rhizospheric and endophytic mineral solubilizing microorganisms, are the greatest alternatives to chemical fertilizers for promoting plant growth by providing necessary plant nutrients while minimizing environmental impact. The rhizosphere and endophytic region of the plants is an important place in which microorganisms live in abundance. Beneficial microbial strains can be used as an inoculant and microbial consortia to improve plant growth. A microbial consortium is a collection of bacteria that work together to improve their effectiveness and suppress soil-borne phytopathogens while enhancing agricultural output. The development of a prospective blend of organisms for enhanced output is a recent trend in agriculture research. When bacteria are introduced into the soil as a single inoculants and microbial consortia, they interrelate with the host plant and can help the crop to flourish by stimulating the natural soil conditions (Sarma et al. 2015). Several researches have been reported in which microbial associations using a combination of bacteria (dual, triple, tetra, penta and hexa) as biofertilizers for plant growth and to perform biocontrol activities which have been found to be more efficient method over the manure, and single cultured biofertilizers (Verma et al. 2013).
There is only little information available on mixtures of bacteria with various PGP characteristics and their use in plant growth enhancement. In present study, Micrococcus indicus IARI-JR-44, Bacillus horikoshii IARI-S-45, and Pseudomonas sp. EU-C3ST.R1, were isolated from interior part of the plant and rhizospheric soil. In a study, Pseudomonas sp. was reported as endophytes and isolated from Coffee arabica L. (Baker et al. 2012). Micrococcus indicus was reported as rhizospheric bacteria, isolated from rhizospheric soil of wheat plants (Verma et al. 2016). In present investigation, bacterial isolates Micrococcus indicus IARI-JR-44, Bacillus horikoshii IARI-S-45, and Pseudomonas sp. EU-C3ST.R1 exhibited mineral solubilizing attributes i.e., P and K solubilization; fixation of nitrogen, respectively. In a study Micrococcus sp. was reported to show phosphorus solubilization, and also have other PGP ability like IAA, ACC deaminase activity, and siderophores production (Jha and Saraf 2012). Similarly, Micrococcus sp. were isolated from rhizosphere soil of Western Ghat forest in west coast of India and reported for phosphorus solubilization (122.4 µg of Ca3PO4 mL–1), siderophore production and IAA production (109 µg mL–1) (Dastager et al. 2010). In the present investigation, Bacillus horikoshii reported with PGP attributes i.e., K solubilization based on plate assay. In a report, Bacillus horikoshii reported for the potassium solubilization (22.7 ± 0.9 mg mL− 1) and other plant growth promoting attributes including zinc solubilization (3.6 ± 0.5 mg L− 1) and IAA production (Verma et al. 2016). In this study, Pseudomonas sp. reported as nitrogen fixer (46.45 nmoles C2H4 mg− 1 protein hr− 1. In a report, Pseudomonas sp. sorted out from kallar grass roots in Pakistan and was reported for the fixation of nitrogen (Mirza et al. 2006).
In a present investigation, Pseudomonas sp. EU-C3ST.R1, Micrococcus indicus IARI-JR-44, and Bacillus horikoshii IARI-S-45 used for the preparation of microbial consortia and previously these bacteria have not developed as consortium. The consortia of Pseudomonas sp., and Rhizobium sp. were evaluated on Phaseolus vulgaris crop (Sánchez et al. 2014). In another study, development of microbial consortia using Pseudomonas sp. KW1 and Bacillus sp. YW4 was found to be efficient in nitrate reduction (Rajakumar et al. 2008). Similarly, Aspergillus ochraceus NCIM-1146 and Pseudomonas sp. SUK1 were also prepared as microbial consortia that decolorized adsorbed dyes from textile wastewater during solid-state fermentation (Kadam et al. 2011). In a study, Pseudomonas sp. TR15a and Bacillus aerophilus TR15c have been used for the development of a microbial consortia (Kumar et al. 2021). The strain of Serratia liquefaciens DDS-1, S. marcescens DDS-2, and Pseudomonas sp. DDS-3 has been utilized for the development of microbial consortia (Cycoń et al. 2009). The bacterial isolates of Pseudomonas sp. ASDP1, Burkholderia sp. ASDP2, and Rhodococcus sp. ASDP3 were used as microbial consortia (Vaidya et al. 2017). Similarly, Peribacillus sp. P10, Pseudomonas sp. P8, and Streptomyces sp. X52 has been used for the microbial consortia development (Peng et al. 2021).
The combination of P solubilizing (Micrococcus indicus IARI-JR-44), K solubilizing (Bacillus horikoshii IARI-S-45), and N fixing (Pseudomonas sp. EU-C3ST.R1) strains were used as a microbial consortia and individual inoculants were found to improve the growth parameters like length of shoot and root, and biomass of the maize crop (fresh/dry). Similar findings have been reported, in which co-inoculation of Rhziobium phaseoli and Pseudomonas sp. on common bean boosted dry weight of shoot (Stajkovic et al. 2011). The same study was reported, in which rhizospheric microbial mixture of Peribacillus sp. P10, Pseudomonas sp. P8, and Streptomyces sp. X52 inoculated on maize crop were capable of enhancing plant growth of maize under saline soil conditions (Peng et al. 2021). In a report, two PGP microbial consortia have been reported in which, MC-1 included Bacillus sp. MML2551, B. licheniformis MML2501, Pseudomonas aeruginosa MML2212, and Streptomyces fradiae MML1042, whereas MC-2 included Bacillus sp. MML2551, B. licheniformis MML2501, and P. aeruginosa MML2212 both were used on to sunflower. MC-1 has improved the seed germination by 24.4%, plant height by 61.3%, parameters of yield by 936 kg/ha, whereas MC-2 was improved the seed germination by 20.14%, plant height by 43.14%, head size by 41.14%, head weight by 32.24%, number of seeds per head by 26.9%, seed weight per head by 51.44%, 1000 seeds weight by 36.74%, seed yield per plot by 41.74%, and total seed yield/ha by 32.0% according to the farmers’ practice and uninoculated plant (control) (Srinivasan and Mathivanan 2009).
In another report, the inoculation of bacterial mixture of Acinetobacter calcoaceticus, B. licheniformis, Brevibacillus brevis, and Micrococcus sp. were evaluated on Jatropha curcas plant, and significantly increased yield of the plant was found (Jha and Saraf 2012). Similarly, bacterial consortia that included Azospirillum, Leuconostoc mesenteroide, Pseudomonas sp., and P. striata increased the height of plant and biomass of brinjal, chili and tomato seedling (Jayashree and Jagadeesh 2017). Krishnamoorthy et al. (2020) inoculated the single and consortium of endophytic bacteria Micrococcus sp. PB001, Methylobacterium sp. PB005, Methylorubrum sp. PB009, and Pseudomonas sp. PB002 on rice crop and increment in the fresh and dry weight of the plant, length of root and shoot, chlorophyll content up to 34.06%, 38.77%, 182.87%, 16.59% and 33.52, respectively over control plant (uninoculated) has been observed. Co-inoculation of B. licheniformis MS3, and Micrococcus sp. MS4 was evaluated in Jatropha curcas L., and increased weight of root and shoot, total biomass of the plant, length of root and shoot, total content of chlorophyll, grain yield, and width of shoot was recorded (Jha and Saraf 2012). In an experiment conducted by Moreira et al. (2021), the co-inoculation of Bacillus sp. and Trichoderma asperellum were evaluated in the banana seedlings and it showed the enhancement of total leaf area and carotenoids content in bananas leaf. The similar study have been reported by Kapadia et al. (2021) in which microbial consortium of Achromobacter sp., Bacillus sp., Delftia sp., and Enterobacter sp., were assessed for its effects on tomato growth and mineral uptake under salt stress and normal soil conditions. In contrast to the control, bacterized seedlings sown in saline soil significantly increased the number of leaves by 75.68%, 92.95% of shoot length, 146.14% of root length, 91.23% secondary roots, and content of chlorophyll by 61.49%. In another report, the sugar content increased by 5.33% over the control in sweet pepper by the inoculation of microbial consortia of B. cereus AR156, B. subtilis SM21, and Serratia sp. XY21 (Zhang et al. 2019). In another report, the flavonoids and phenolic content have been enriched by the co-inoculation of both rhizospheric, and endophytic bacteria of Mesorhizobium sp. and Pseudomonas sp. in chickpea plant (Nagpal et al. 2021).
In conclusion, the consortium developed by using different microbial strains i.e., Micrococcus indicus IARI-JR-44, Bacillus horikoshii IARI-S-45, and Pseudomonas sp. EU-C3ST.R1 have ability to solubilize phosphorus, potassium and nitrogen fixation, boosted the growth of plant more over to individual inoculation, cattle manure, agro-chemicals fertilizers, and uninoculated control. Organic farming is also the best technique for the reduction of chemical fertilizers from the soil, which will not only decrease the cost of maize cultivation but also reduce environmental pollution. The use of microbial consortia can be an excellent bioformulation to meet the macronutrient needs of the plants. In the forthcoming, the microbial consortia could be used for inoculation (in liquid and powder form) for increasing crop quality and productivity of horticultural and cereal crops.
References
Baker S, Sahana S, Rakshith D, Kavitha H, Kavitha K, Satish S (2012) Biodecaffeination by endophytic Pseudomonas sp. isolated from Coffee Arabica L. J Pharm Res 5:3654–3657
Bakker AW, Schippers B (1987) Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp-mediated plant growth-stimulation. Soil Biol Biochem 19:451–457
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Cappucino J, Sherman N (1992) Nitrogen cycle. Microbiology: a laboratory manual, 4th edn. Benjamin/Cumming Publishing Co, California
Chemura A (2014) The growth response of coffee (Coffea arabica L) plants to organic manure, inorganic fertilizers and integrated soil fertility management under different irrigation water supply levels. Int J Recycl Org Waste Agric 3:1–9
Conn VM, Franco CM (2004) Effect of microbial inoculants on the indigenous actinobacterial endophyte population in the roots of wheat as determined by terminal restriction fragment length polymorphism. Appl Environ Microbiol 70:6407–6413
Cycoń M, Wójcik M, Piotrowska-Seget Z (2009) Biodegradation of the organophosphorus insecticide diazinon by Serratia sp. and Pseudomonas sp. and their use in bioremediation of contaminated soil. Chemosphere 76:494–501
Dastager SG, Deepa C, Pandey A (2010) Isolation and characterization of novel plant growth promoting Micrococcus Sp NII-0909 and its interaction with cowpea. Plant Physiol Biochem 48:987–992
Devi R, Kaur T, Kour D, Yadav AN, Suman A (2022) Potential applications of mineral solubilizing rhizospheric and nitrogen fixing endophytic bacteria as microbial consortium for the growth promotion of Chilli (Capsicum annum L). Biologia 77:2933–2943
Fasim F, Ahmed N, Parsons R, Gadd GM (2002) Solubilization of zinc salts by a bacterium isolated from the air environment of a tannery. FEMS Microbiol Lett 213:1–6
Han SO, New PB (1998) Variation in nitrogen fixing ability among natural isolates of Azospirillum. Microb Ecol 36:193–201
Han J, Sun L, Dong X, Cai Z, Sun X, Yang H et al (2005) Characterization of a novel plant growth-promoting bacteria strain Delftia tsuruhatensis HR4 both as a diazotroph and a potential biocontrol agent against various plant pathogens. Syst Appl Microbiol 28:66–76
Holguin G, Guzman MA, Bashan Y (1992) Two new nitrogen-fixing bacteria from the rhizosphere of mangrove trees: their isolation, identification and in vitro interaction with rhizosphere Staphylococcus Sp. FEMS Microbiol Lett 101:207–216
Horrigan L, Lawrence RS, Walker P (2002) How sustainable agriculture can address the environmental and human health harms of industrial agriculture. Environ Health Perspect 110:445–456
Hu X, Chen J, Guo J (2006) Two phosphate-and potassium-solubilizing bacteria isolated from Tianmu Mountain, Zhejiang, China. World J Microbiol Biotechnol 22:983–990
Irigoyen J, Einerich D, Sánchez-Díaz M (1992) Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago Sativd) plants. Physiol Plant 84:55–60
Jayashree C, Jagadeesh K (2017) Testing the effect of the microbial consortium on growth of vegetable seedlings in a farmer’s nursery. Int J Curr Microbiol Appl Sci 6:1636–1639
Jha CK, Saraf M (2012) Evaluation of multispecies plant-growth-promoting consortia for the growth promotion of Jatropha curcas L. J Plant Growth Regul 31:588–598
Kadam AA, Telke AA, Jagtap SS, Govindwar SP (2011) Decolorization of adsorbed textile dyes by developed consortium of Pseudomonas sp. SUK1 and Aspergillus ochraceus NCIM-1146 under solid state fermentation. J Hazard Mater 189:486–494
Kapadia C, Sayyed R, El Enshasy HA, Vaidya H, Sharma D, Patel N et al (2021) Halotolerant microbial consortia for sustainable mitigation of salinity stress, growth promotion, and mineral uptake in tomato plants and soil nutrient enrichment. Sustainability 13:8369
Kaur T, Devi R, Kumar S, Sheikh I, Kour D, Yadav AN (2022) Microbial consortium with nitrogen fixing and mineral solubilizing attributes for growth of barley (Hordeum vulgare L). Heliyon 8:e09326
Kim D-O, Jeong SW, Lee CY (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 81:321–326
Krishnamoorthy A, Agarwal T, Kotamreddy JNR, Bhattacharya R, Mitra A, Maiti TK et al (2020) Impact of seed-transmitted endophytic bacteria on intra-and inter-cultivar plant growth promotion modulated by certain sets of metabolites in rice crop. Microbiol Res 241:126582
Kumar A, Maleva M, Bruno LB, Rajkumar M (2021) Synergistic effect of ACC deaminase producing Pseudomonas sp. TR15a and siderophore producing Bacillus aerophilus TR15c for enhanced growth and copper accumulation in Helianthus annuus L. Chemosphere 276:130038
Lichtenthaler HK (1987) [34] Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In: Packer L, Douce R (eds) Methods in Enzymology, vol 148. Academic Press, pp 350–382
Mäder P, Fliessbach A, Dubois D, Gunst L, Fried P, Niggli U (2002) Soil fertility and biodiversity in organic farming. Science 296:1694–1697
Mirza MS, Mehnaz S, Normand P, Prigent-Combaret C, Moënne-Loccoz Y, Bally R et al (2006) Molecular characterization and PCR detection of a nitrogen-fixing Pseudomonas strain promoting rice growth. Biol Fert Soils 43:163–170
Moreira FM, Cairo PAR, Borges AL, da Silva LD, Haddad F (2021) Investigating the ideal mixture of soil and organic compound with Bacillus sp. and Trichoderma asperellum inoculations for optimal growth and nutrient content of banana seedlings. South Afr J Bot 137:249–256
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Nagpal S, Sharma P, Sirari A (2021) Induction of antioxidant response with compatible combination of Mesorhizobium sp. and Pseudomonas sp. against Fusarium oxysporum sp. ciceris in Chickpea. Bangladesh J Bot 50:359–364
Oliveira AB, Moura CF, Gomes-Filho E, Marco CA, Urban L, Miranda MRA (2013) The impact of organic farming on quality of tomatoes is associated to increased oxidative stress during fruit development. PLoS ONE 8:e56354
Park YS, Jung ST, Kang SG, Heo BG, Arancibia-Avila P, Toledo F et al (2008) Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem 107:640–648
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68:3795–3801
Peng J, Ma J, Wei X, Zhang C, Jia N, Wang X et al (2021) Accumulation of beneficial bacteria in the rhizosphere of maize (Zea mays L.) grown in a saline soil in responding to a consortium of plant growth promoting rhizobacteria. Ann Microbiol 71:40
Pikovskaya R (1948) Mobilization of phosphorus in soil in connection with vital activity of some microbial species. Mikrobiologiya 17:362–370
Rajakumar S, Ayyasamy PM, Shanthi K, Thavamani P, Velmurugan P, Song YC et al (2008) Nitrate removal efficiency of bacterial consortium (Pseudomonas sp. KW1 and Bacillus sp. YW4) in synthetic nitrate-rich water. J Hazard Mater 157:553–563
Rodrı́guez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Sánchez AC, Gutiérrez RT, Santana RC, Urrutia AR, Fauvart M, Michiels J et al (2014) Effects of co-inoculation of native Rhizobium and Pseudomonas strains on growth parameters and yield of two contrasting Phaseolus vulgaris L. genotypes under Cuban soil conditions. Euro J Soil Biol 62:105–112
Sarma BK, Yadav SK, Singh S, Singh HB (2015) Microbial consortium-mediated plant defense against phytopathogens: readdressing for enhancing efficacy. Soil Biol Biochem 87:25–33
Schwyn B, Neilands J (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Srinivasan K, Mathivanan N (2009) Biological control of sunflower necrosis virus disease with powder and liquid formulations of plant growth promoting microbial consortia under field conditions. Biol Control 51:395–402
Stajkovic O, Delic D, Josic D, Kuzmanovic D, Rasulic N, Knezevic-Vukcevic J (2011) Improvement of common bean growth by co-inoculation with Rhizobium and plant growth-promoting bacteria. Rom Biotechnol Lett 16:5919–5926
Sugumaran P, Janarthanam B (2007) Solubilization of potassium containing minerals by bacteria and their effect on plant growth. World J Agric Sci 3:350–355
Vaidya S, Jain K, Madamwar D (2017) Metabolism of pyrene through phthalic acid pathway by enriched bacterial consortium composed of Pseudomonas, Burkholderia, and Rhodococcus (PBR). 3 Biotech 7:29
Verma JP, Yadav J, Tiwari KN, Kumar A (2013) Effect of indigenous Mesorhizobium spp. and plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol Eng 51:282–286
Verma P, Yadav AN, Khannam KS, Panjiar N, Kumar S, Saxena AK et al (2015) Assessment of genetic diversity and plant growth promoting attributes of psychrotolerant bacteria allied with wheat (Triticum aestivum) from the northern hills zone of India. Ann Microbiol 65:1885–1899
Verma P, Yadav AN, Khannam KS, Kumar S, Saxena AK, Suman A (2016) Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J Basic Microbiol 56:44–58
Yadav AN, Sachan SG, Verma P, Saxena AK (2016) Bioprospecting of plant growth promoting psychrotrophic Bacilli from the cold desert of north western Indian Himalayas. Indian J Exp Biol 54:142–150
Zhang LN, Wang DC, Hu Q, Dai XQ, Xie YS, Li Q et al (2019) Consortium of plant growth-promoting rhizobacteria strains suppresses sweet pepper disease by altering the rhizosphere microbiota. Front Microbiol 10:1668
Funding
The authors are grateful to the Department of Biotechnology, Akal College of Agriculture, Eternal University, Baru Sahib and Department of Environment, Science & Technology (DEST), Shimla, HP funded project “Development of microbial consortia as bio-inoculants for drought and low temperature growing crops for organic farming in Himachal Pradesh” for providing the facilities support, to undertake the investigations.
Author information
Authors and Affiliations
Contributions
RD, TK and RN carried out the experimental part and wrote the manuscript; DK and BS made the tables and done statistical analysis; MFA DKAAT, SK, SS, AKR, SR, AK, ASA review the manuscript; and ANY give the hypothesis and supervise the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devi, R., Alsaffar, M.F., AL-Taey, D.K. et al. Effect of indigenous mineral availing microbial consortia and cattle manure combination for growth of maize (Zea mays L.). Vegetos (2024). https://doi.org/10.1007/s42535-024-00897-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42535-024-00897-1