Abstract
Plant disease resistance (PDR) is an imperative element for the effective and timely management of foliar blights in cereals crops. Because of variations in disease pressure and staggered flowering of wheat germplam material, it is hard to identify cultivars with stable resistance to spot blotch disease caused by Bipolaris sorokiniana across seasons and locations. To overcome these problems, current study was conducted to optimise and validate a rapid and reliable detached leaf assay (DLA) as a phenotyping method to screen wheat genotypes against spot blotch disease. Fifteen wheat genotypes were evaluated based on partial disease resistance (PDR) components: Incubation Period (IP) and Latent Period (LP), to study whether DLA could be comparable to field and greenhouse screening against spot blotch of wheat. The time taken by DLA to successfully screen the genotypes was very less (30 days) as compared to field (80 days) and greenhouse (41 days) experiments. Disease severity obtained in DLA was positively correlated with greenhouse (r = 0.97, P = 0.02) and field experiments (r = 0.94, P = 0.01). Assessments of the host genotypes indicated that the genotype DBW 39 exhibited resistance, while susceptible reaction to the disease was observed in genotype K 1317 and PBW 703. A negative correlation was observed between IP and LP with the disease severity. So, it can be concluded that DLA could be considered as an alternative and reliable way of phenotyping against spot blotch of wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat (Triticum aestivum L.) is a dominant staple crop cultivated in an area of around 219 million hectares, across a wide range of latitudes covering around 89 countries of the world (FAOSTAT 2020). Although wheat production reached a record 103.5 million tonnes in India alone in 2019–20 (FAOSTAT 2020), the annual yield is compromised by a plethora of biotic and abiotic stresses, spot blotch of wheat being one of them.
Out of the total area under wheat cover globally, an estimated 25 million hectare is infected by spot blotch disease, of which Indian subcontinent shares 40% (Joshi et al. 2007). Spot blotch of wheat is prevalent throughout the wheat growing belts of the country and there is an estimated yield loss with every 1% increase in disease severity of spot blotch (Devi et al. 2018). Spot blotch (SB) of wheat is caused by Bipolaris sorokiniana (Sacc.) Shoem. (syn. Helminthosporium sativum, teleomorph Cochliobolous sativus) which has a broad host range, including 29 crop species and several grasses (Acharya et al. 2011). The disease is characterised by elongated to oval dark brown lesions which turn light brown to tan colour, surrounded by a dark ring, upon maturity. Severe infections lead to complete defoliation, resulting in shrunken and shrivelled grains (Zhang et al. 2020; Debnath et al. 2021). High relative humidity with moderate to high temperature (18–32 °C) provides the most conducive conditions for pathogen growth. Prolonged periods of leaf wetness, caused by irrigation, fog, rainfall, or dew during crucial phenological growth stages facilitated the pathogen development further (Kashyap et al. 2022a; Devi et al. 2017; Kumar et al. 2019; Kumar et al. 2020; Mahapatra et al. 2020; Tamang et al. 2021).
Among the various strategies used for managing spot blotch, resistance breeding forms a major component attributable to its economic feasibility and environmental viability. Besides this, development of resistant wheat cultivars also ensures the stability of production and offers sanitary and epidemiological safety in the wheat field (Kumar et al. 2022). Since spot blotch is particularly devastating in the Northeastern Plain Zone (NEPZ) (Kashyap et al. 2022b; Roy et al. 2023), owing to its high temperature and humidity, a number of resistant varieties for NEPZ such as HD 2733, HD 2888, K 0307 and K 8027 (Mahapatra et al. 2020) have been recommended for cultivation in areas vulnerable to spot blotch disease. However, breeding for resistance is time consuming as it requires more than 2 years in field evaluation followed by stability analysis considering the variability by genotype, environment and years of interaction (Kumari et al. 2018; Mahapatra et al. 2020; Marak et al. 2018; Mudi et al. 2010; Singh et al. 2015). Hence, the development of a technique which is precise, fast as well as less labour intensive, as compared to the field and greenhouse phenotyping, is the need of the hour. Under such circumstances, detached leaf assay (DLA) could be an alternate way of phenotyping for disease resistance (Liatukas and Ruzgas 2009; Al-Sadi 2016), as demonstrated in the case of net blotch of barley (El-Mor et al. 2018), Fusarium head blight of barley (Kumar et al. 2011) and leaf rust of wheat (Patial et al. 2017).
Detached leaf assays (DLA) are acknowledged as one of the most rapid, low-cost laboratory-based techniques to screen for resistance against various diseases (Al-Sadi 2016). Generally, in such types of assays, viable leaf tissue is plated onto culture media and then inoculated with a test pathogen. A major merit of such techniques is the requirement of a very minute quantity of leaf material for each genotype (i.e. approximately 6 cm2 leaf area or less), which in turn allows rapid screening of a large number of genotypes in a relatively small area, under controlled conditions with minimal planting space and resources. Wheat breeding programs aiming to devise SB resistant wheat germplasm would benefit if DLA will be made available with comparable results to greenhouse (WPAP) or field (WPAF) screening experiments. Therefore, attempts have been made to test the hypothesis that detached leaf assay (DLA) is a reliable substitute for the whole-plant greenhouse (WPAP) or field (WPAF) screening for wheat resistance to B. sorokiniana. The major objectives of the study include developing DLA method and drawing a comparison with conventional methods (WPAP and WPAF), using wheat genotypes with different resistance levels to SB.
Materials and methods
Fungal and plant material
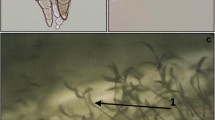
The pure culture of a highly virulent isolate of B. sorokiniana BS7 (NCBI gene accession: MT804348) was used for the development of a rapid leaf detached assay for the phenotyping of SB of wheat (Fig. 1). Briefly, test isolate (BS7) was grown on Potato Dextrose Agar (PDA) amended in Petri plates and pure culture of the fungus was harvested by flooding the plates with sterilised distilled water and rubbing the culture with a sterilised glass rod. 1% TWEEN®20 solution was added to suspension to aid the emulsification of the spores. The obtained suspension was filtered through three-layered muslin cloth, to obtain a dirt free, uniform suspension of fungal spores/mycelial bits. The concentration of the inoculum was adjusted at 2 × 105 spores ml−1 with the aid of a haemocytometer prior to inoculation. The plant materials used in present study composed of 15 wheat genotypes (Table 1), obtained from Indian Institute of Wheat and Barley Research (IIWBR), which were recommended for Eastern zone of India. The genotypes were assessed under field conditions, in a greenhouse and in a detached leaf assay (Fig. 2).
Whole plant assay under field conditions (WPAF)
The field experiments were conducted at Instructional Farm, Jaguli of Bidhan Chandra Krishi Viswavidyalaya (BCKV), Nadia, West Bengal, India, situated at 23.5 ºN and 89.0 ºE, at an elevation of 9.75 m above sea level during rabi season of 2019–20. Each genotype (Table 1) was grown in 2 rows of 1 m long plots with 23 cm distance between rows, following recommended agronomic practices. Highly virulent Bipolaris sorokiniana isolate BS7 (NCBI gene accession: MT804348) was used for the development of a rapid detached leaf assay for the phenotyping of spot blotch of wheat. The methodology of Kumar et al. (1998) was used for inoculating the pathogen suspension (2 × 105 spores ml−1) uniformly on the wheat plants using a hand sprayer on 3-week-old plants and sprayed again after 15 days. After inoculation, the plants were regularly observed for symptom development. Upon disease development, disease severity was recorded for the genotypes till 80 DAS after sowing.
Whole splant assay under pot conditions (WPAP)
Same 15 wheat genotypes, used in WPAF, were grown in pots containing nutrient rich soil, under greenhouse conditions. The pots were placed in Randomised Block Design (RBD), with 3 replications. A mixture of N:P:K (14:14:14) was added to the soil @ 350 g per 30 kg of soil medium. The seeds were sown in a circular fashion with 10 seeds per pot (15 × 13 × 11 cm), at a depth of 6 cm. Irrigation was given as per requirement.
The pathogen suspension of concentration 2 × 105 spores ml−1 was sprayed on 3-week-old plants using a hand sprayer in the evening hours till run-off, according to Mahapatra and Das (2013). After inoculation, the plants were transferred to a humid chamber at 15–18 °C under moist conditions (100% RH) for 14 h, to facilitate pathogen infection. Plants were then transferred to the greenhouse, and seedlings were recorded for three days after inoculation. Disease ratings in the percentage area affected were determined at three DAI and thereafter every 4 days until 20 DAI. Disease estimation was conducted using AUDPC and the whole experiment was conducted twice for confirmation of the results obtained. Disease assessment was done on a 1–9 scale, as given by Liatukas and Ruzgas (2009). As per the scale, 1 = 0% infection, very resistant reaction (VR); 1.1–3.0 = > 0–10.0% infection, resistant reaction (R); 3.1–5.0 = 10.1–50.0% infection, moderately resistant reaction (MR); 5.1–7.0 = 50.1–75.0% infection, moderately susceptible (MS); 7.1–8.0 = 75.1–90.0% infection, susceptible reaction (S); 8.1–9.0 = 90.1– 100% infection, very susceptible reaction (VS). The assessment was done every 3 days till 42 days after inoculation (DAI). The virulence was assessed based on the mean disease severity scores in three different stages of the crop (25, 47 and 59 GS).
Detached leaf assay (DLA)
Same set of fifteen wheat genotypes (Table 2) was used to evaluate the partial disease resistance (PDR) components in vitro using a detached leaf assay. The practices for WPAP were followed for growing the wheat seedlings. After 14 days, 5 cm long leaf segments were excised from the first, second and third leaf. The segments were placed on the surface of 0.5% water agar containing 10 mg L−1 kinetin as a senescence retarder, on Petri dishes (90 mm). The leaf segments were inoculated with 10 µL droplet of spore suspension of Bipolaris sorokiniana accessions at a concentration of 2 × 105 spores ml−1 containing 0.08% of Tween 20, as used by Arabi and Jawhar (2013). The inoculated samples were incubated in a laboratory incubator at low temperature (10 ± 2 °C) under continuous white light or room temperature (21 ± 2 °C). Disease severity of the inoculated leaf samples was assessed from 2 to 14 DAI based on percentage of leaf area affected. The scoring was done according to the scale given by Liatukas and Ruzgas (2009).
Evaluation of inoculated samples for PDR components was conducted daily by using a compound microscope (40X). PDR components assessed were the incubation period (days from inoculation to symptom development in the plant) and the latent period (days from incubation to development of conidia on wheat leaf). The experiment was conducted twice to minimise the error.
Calculation of disease severity
For estimating the disease severity based on blighted leaf area, the following formula was used, as recommended by Osman et al. (2015). Here, D1 indicates the progress of the disease in the height of the plant canopy from the ground level and D2 refers to the disease severity as diseased leaf area.
In keeping with previous studies (Mahapatra et al. 2020), the observed responses were classified on the basis of their varietal response as percent disease infection into six categories according to Zadok’s scale (Zadoks et al. 1974). The disease severity was assessed by visually scoring the flag (F) and penultimate (F–1) leaves, as per the double-digit scale (00–99), mentioned by Kumar et al. (1998). The observations recorded were taken from middle rows to avoid the buffer effect associated with the terminal rows.
Data analysis
The recorded data from the experiments were statistically evaluated by employing SPSS (version 20.0 SPSS Inc., Chicago IL, USA) software package. One way analysis of variance (ANOVA) was performed. Tukey’s test was used for the mean separation of disease severity values. The area under the disease progress curve (AUDPC) is another criterion that represents the spread of the disease in the evaluated genotypes. AUDPC was calculated from the three evaluations using the following formula (Campbell and Madden 1990):
where, Y is AUDPC value, Xi = disease severity at time ti, t(i + 1) – ti is the time interval between two disease scores expressed as Days After Sowing (DAS), n is the total number of observations. AUDPC data obtained from all three experiments were analysed together (i.e., DLA, greenhouse, and field). For each genotype, means of transformed AUDPC values from DLA, greenhouse, and field experiments were compared using Pearson’s correlation (α = 0.05).
Results
Whole plant assay under field conditions (WPAF)
With an increase in growth of the plant, the disease severity also increased simultaneously. In 25 GS, the maximum disease was noticed in PBW 703 (25.93%) which was significantly at par with K 1317 (23.89%), and K 1006 (22.47%) whereas minimum disease was recorded in DBW 39 (2.41%) followed by WB2 (6.65%) and HD2967 (6.82%) and their difference was statistically significant. Similarly, at 47GS, maximum disease severity was observed in PBW 703 (71.82%) significantly at par with K1317 (63.87%) followed by PBW 725 (45.84%), K1006 (42.39%) HD 3086 (40.88%), while DBW 39 (9.12%) recorded minimum disease severity, followed by WB 2 (19.02%). At 59GS, PBW 703 (97.98%) and DBW 39 (13.33%) exhibited maximum and minimum disease severity, respectively (Table 1). The resistant genotype DBW 39 exhibited a mean disease severity of 8.28%, which was lowest among the evaluated genotypes. Statistically, highest severity was recorded in PBW 703, PBW725 and K1317 which indicated a susceptible response to the pathogen. The progress of the disease was observed to be rapid in the susceptible genotypes, while the reverse was observed in resistant genotypes (Fig. 3).
Whole plant assay under pot conditions (WPAP)
The response of wheat genotypes against B. sorokiniana accession was assessed under artificial conditions in a greenhouse. Disease severity varied significantly (P < 0.05) among the genotypes (Table 2). In vitro study revealed that genotype DBW 39 was resistant, 5 genotypes were moderately resistant, viz., WB 2, DBW 187, DBW 14, HD 2967, DBW 221, 8 genotypes were moderately susceptible, viz., PBW 725, PBW 804, DBW 173, DBW 252, K 1317, K 1006, HD 3226, HD 3086, and one genotype (PBW 703) was rated as susceptible to pathogen infection. Among the genotypes, the lowest score was obtained in DBW 39 (> 0–10.0% infection) while the highest score was recorded in PBW 703 (75.1–90.0% infection).
Detached leaf assay (DLA)
The leaf segments were evaluated on a daily basis after inoculation with the pathogen. Symptoms initially appeared in the form of chlorotic lesions, which soon turned necrotic and enlarged in size. However, disease severity varied significantly (P < 0.0001) among the evaluated genotypes. DBW 39 showed the lowest mean severity score (2.5) while PBW 703 had the highest severity score (7.6). The resistance response was similar to one observed in the case of WPAF and WPAP (Table 2).
PDR components evaluated, viz., IP and LP, also varied significantly among the genotypes. All leaves exhibited symptoms with significant pathogen growth from 4 days after inoculation, till 9 days after inoculation. IP values ranged from 4.624 days to 10.138 days in PBW 703 and DBW 39, respectively. LP values were in the range of 8–15 days, with PBW 703 (7.509 days) having the lowest and DBW 39 (14.487) having the highest LP values (Table 2).
A correlation study was drawn between the disease scores and PDR components under DLA (Table 3). In the study, IP and LP values were negatively correlated with the mean disease scores, while PDR components were observed to have significant positive correlations with each other.
Comparison between DLA, WPAP, WPAF
AUDPC obtained under three screening methods is represented in Table 4. It was observed that the values obtained were higher in each individual genotype under WPAF, followed by WPAP and DLA (Table 4). A significantly positive correlation was observed between DLA and WPAP (r = 0.975, n = 15), between WPAP and WPAF (r = 0.937, n = 15) and between DLA and WPAF (r = 0.945, n = 15) (Table 5).
Discussion
Resistance and symptom development are influenced by the stage of crop as well as the pathogen load (Kumar et al. 2020). Therefore, it becomes indispensable to devise rapid methods to detect resistance against devastating diseases, such as spot blotch caused by fungus B. sorokiniana. In this context, DLA has been explored as a useful tool to screen crop cultivars against an array of diseases (Liatukas and Ruzgas 2009; Al-Sadi 2016; El-Mor et al. 2018; Kumar et al. 2011; Patial et al. 2017). Similarly, in the current study, attempts have been made to optimize and test a rapid and reliable DLA to screen wheat genotypes for resistance to SB disease. Published literature (Twizeyimana et al. 2007; El-Mor et al. 2018) indicated that DLA has manifold advantages over other conventional disease phenotyping methods. For instance, in the case of DLA, each cycle of disease evaluation can be rapidly completed within 4–5 weeks. Moreover, different disease phenotyping data required for determining the rate of pathogen development and components of resistance in a large number of genotypes can be recorded at a uniform time span on the same experimental leaf. More importantly, all the fungal pathogen handling steps can be performed effortlessly on the detached leaves. Besides this, DLA is a useful tool for studying pathogenic variability that requires pure cultures of the fungus, a version of cross contamination between different fungal cultures, inoculation of a large number of isolates on different genotypes and hosts, along with precise quantification of pathogen inoculum load and their reproduction behaviour, etc. Hence, the study on development, validation and comparison of DLA assay with greenhouse and field screening experimentation with fifteen different wheat genotypes has been conducted.
It has been reported that the preliminary screening is more consistent and time saving approach under controlled conditions (Singh and Rajaram 2008; Twizeyimana et al. 2007). Field screening is resource intensive and time consuming, which makes artificial screening a preferred method for resistance screening, as evident from the results of the present study, where WPAF took more than 80 days to complete field evaluations. In addition, DLA developed and validated in the present investigation permits rapid screening and evaluation of wheat germplasm at a minimal cost in comparison to the cost incurred on the greenhouse or field-based screening of germplasms. Additionally, DLA allows phenotypic screening of a large number of wheat genotypes and therefore, serves as a valuable tool for resistance evaluation against SB fungus. Comparison made in current study regarding the responses of 15 wheat genotypes under DLA, WPAP and WPAF revealed that reliable resistance assessment can be achieved within 4 days after inoculation i.e. approximately 30 DAS, in the case of DLA, whereas WPAP and WPAF evaluations required a minimum of 41 and 80 DAS, respectively. Recently, similar trends have been noticed by Aregbesola et al. (2020), who reported that DLA requires a minimum time span of four weeks for resistance assessment in maize against Bipolaris maydis, when compared with screen house (33 DAS) and field level resistance assessment (72 DAS). In addition, DLA allows for the simultaneous screening of wheat genotypes and can aid in enhancing the breeding process by offering multiple cycles of screening and selection in a single year at a nominal cost. Based on research findings and the essential requirement for high-throughput screening, it has been concluded that DLA is a very promising and advantageous tool for screening wheat for SB resistance.
In the current study, it has been observed that PDR components, viz., Incubation Period (IP) and Latent Period (LP), correlated significantly with the disease ratings observed in the field, greenhouse and DLA. Longer IP and LP were observed in moderately resistant and resistant genotypes, viz., WB 2, DBW 187, DBW 14, HD 2967, DBW 221 and DBW 39. The reverse was recorded in moderately susceptible and susceptible genotypes, viz., PBW 725, PBW 804, DBW 173, DBW 252, K 1317, K 1006, HD 3226, HD 3086, and PBW 703. Similar trends have been noticed by El-Shamy and Mousa (2004) and Li et al. (2010). Kumar et al. (2011) reported that parameters such as lesion size, density of lesions and sporulation at room temperature, in addition to IP and LP, were highly reliable in screening the genotypes for resistance against pathogens. Similar observations were reported by Parlevliet and Ommeren (1975) in barley against leaf rust. However, in the present study, it has been observed that the latent period had a negative correlation with disease scores taken. This suggests that B. sorokiniana development as reflected by lesion expansion, and sporulation on detached leaf sections may not simply be a function of IP and LP, and that components of PDR may be under distinct genetic control. Therefore, further investigation is warranted to determine which individual components are best correlated to SB resistance in whole plants. A similar conclusion has already been drawn by Browne and Cooke (2004), while developing in vitro DLA for pre-screening resistance to Fusarium head blight in wheat.
Disease severity responses observed under DLA were similar to the results observed under field conditions as evident from the significant correlation between DLA, WPAP and WPAF data. Similar observations of significant correlations between DLA and greenhouse (r = 0.79; P < 0.0001) and between DLA and field resistance (r = 0.83) across genotypes have been documented by Twizeyimana et al. (2007). It has been further suggested by Singh et al. (1997) that the screening of genotypes under artificial conditions identifies the resistant sources under maximum disease pressure and hence, is highly reliable. Among the fifteen genotypes screened, we observed DBW 39 to exhibit a resistant reaction to spot blotch disease, which was also reported by Kumar et al. (2020). Singh et al. (2017) reported HD 2967 and WB 2 as moderately resistant, while HD 3086 was reported as moderately susceptible by Mahapatra et al. (2020), which is similar to the resistance response observed in the present study. Among the genotypes assessed, DBW 39 exhibited resistance under both natural and artificial conditions. Thus, it could be concluded that the genotype contains several genes responsible for imparting resistance against B. sorokiniana. Under Indian conditions, where the weather is highly conducive to pathogen development, wheat cultivars with improved spot blotch resistance are desirable. However, under limited time and resource availability, an informative detached leaf assay, complemented with reliable PDR components such as the incubation period and latent period, would be an asset to breeding programmes for spot blotch resistance.
In conclusion, our study has established that the detached leaf assay can be utilized as a rapid and reliable screening technique for phenotyping resistance sources of wheat against spot blotch disease. Additionally, the genotype DBW 39 has been observed to exhibit resistance, while a susceptible reaction to the disease has been observed in genotype K 1317 and PBW 703 against B. sorokiniana. However, our study also revealed a negative correlation between the incubation period (IP) and latent Period (LP) with the disease severity. Further research can be conducted on increasing the number of genotypes for resistance screening. DLA can also be optimized for other economically important foliar diseases of wheat.
Data availability
The data that support the findings of this study are available upon request from the corresponding authors.
References
Acharya K, Dutta AK, Pradhan P (2011) Bipolaris sorokiniana (Sacc.) Shoem.: the most destructive wheat fungal pathogen in the warmer areas. Aust J Crop Sci 5(9):1064–1071
Al-Sadi AM (2016) Variation in resistance to spot blotch and the aggressiveness of Bipolaris sorokiniana on barley and wheat cultivars. J Plant Pathol 98:97–103. https://doi.org/10.4454/JPP.V98I1.029
Arabi MI, Jawhar M (2013) A Simple method for determining barley reaction to spot blotch disease. Res Plant Biol 3(2):01–05
Aregbesola E, Ortega-Beltran A, Falade T, Jonathan G, Hearne S, Bandyopadhyay R (2020) A detached leaf assay to rapidly screen for resistance of maize to Bipolaris maydis, the causal agent of southern corn leaf blight. Eur J Plant Pathol 156(1):133–145. https://doi.org/10.1007/s10658-019-01870-4
Browne RA, Cooke BM (2004) Development and evaluation of an in vitro detached leaf assay forc pre-screening resistance to Fusarium head blight in wheat. Eur J Plant Pathol 110(1):91–102. https://doi.org/10.1023/B:EJPP.0000010143.20226.21
Campbell CL, Madden LV (1990) Introduction to plant disease epidemiology. Wiley
Debnath D, Chakroborty S, Mahapatra S (2021) Spot blotch: A journey from minor to major threat of wheat. J Cereal Res 13(3):255–269. https://doi.org/10.25174/2582-2675/2022/112851
Devi HM, Mahapatra S, Dutta S, Das S (2017) Influence of phenological growth stages and meteorological parameters on leaf blight infestation of wheat in Gangetic plains of West Bengal. J Wheat Res 9(2):101–107. https://doi.org/10.25174/2249-4065/2017/71803
Devi HM, Mahapatra S, Das S (2018) Assessment of yield loss of wheat caused by spot blotch using regression model. Indian Phytopathol 71(2):291–294. https://doi.org/10.1007/s42360-018-0036-9
El-Mor IM, Fowler RA, Platz GJ, Sutherland MW, Martin A (2018) An improved detached-leaf assay for phenotyping net blotch of barley caused by Pyrenophora teres. Plant Dis 102(4):760–763. https://doi.org/10.1094/PDIS-07-17-0980-RE
El-Shamy MM, Mousa MM (2004) Detection of partial resistance components of leaf rust in four Egyptian wheat cultivars. J Agric ResTanta Univ 30(2):116–170
FAOSTAT (2020) Food and agriculture organization of the United Nations. https://www.fao.org/faostat/en/#data/QCL. Accessed 11 Oct 2022.
Joshi AK, Mishra B, Chatrath R, Ortiz Ferrara G, Singh RP (2007) Wheat improvement in India: present status, emerging challenges, and prospects. Euphytica 157(3):431–446. https://doi.org/10.1007/s10681-007-9385-7
Kashyap PL, Gupta V, Gupta OP, Sendhil R, Gopalareddy K, Jasrotia P, Singh GP (2022a) New horizons in wheat and barley research: crop protection and resource management. Springer, Singapore, p 637. https://doi.org/10.1007/978-981-16-4134-3
Kashyap PL, Kumar S, Sharma A, Kumar RS, Mahapatra S, Kaul N, Khanna A, Jasrotia P, Singh GP (2022b) Molecular diversity, haplotype distribution and genetic variation flow of Bipolaris sorokiniana fungus causing spot blotch disease in different wheat-growing zones. J Appl Genet 63(4):793–803
Kumar J, Singh G, Nagarjan S (1998) A field scale for leaf blight recording. Indian Wheat Newslett 4:3
Kumar K, Xi K, Turkington TK, Tekauz A, Helm JH, Tewari JP (2011) Evaluation of a detached leaf assay to measure Fusarium head blight resistance components in barley. Can J Plant Pathol 33(3):364–374. https://doi.org/10.1080/07060661.2011.590820
Kumar S, Singroha G, Bhardwaj SC, Bala R, Saharan MS, Gupta V, Khan A, Mahapatra S, Sivasamy M, Rana V, Mishra CN (2019) Multi environmental evaluation of wheat (Triticum aestivum L.) germplasm identifies donors with multiple fungal disease resistance. Genet Resour Crop Evol 66(4):797–808. https://doi.org/10.1007/s10722-019-00751-3
Kumar S, Kumar N, Prajapati S, Maurya S (2020) Review on spot blotch of wheat: an emerging threat to wheat basket in changing climate. J Pharmacogn Phytochem 9(2):1985–1997
Kumar S, Kashyap PL, Singh I, Jasrotia P, Singh GP (2022) Field screening and identification of stable resistance sources in wheat germplasm against loose smut disease caused by Ustilago segetum var. tritici. J Cereal Res 14(1):76–81. https://doi.org/10.25174/2582-2675/2022/119668
Kumari J, Kumar S, Singh N, Vaish SS, Das S, Gupta A, Rana JC (2018) Identification of new donors for spot blotch resistance in cultivated wheat germplasm. Cereal Res Commun 46(3):467–479. https://doi.org/10.1556/0806.46.2018.028
Li ZF, Xia XC, He ZH, Li X, Zhang LJ, Wang HY, Meng QF, Yang WX, Li GQ, Liu DQ (2010) Seedling and slow rusting resistance to leaf rust in Chinese wheat cultivars. Plant Dis 94(1):45–53. https://doi.org/10.1094/PDIS-94-1-0045
Liatukas Ž, Ruzgas V (2009) Evaluation of Lithuanian winter wheat breeding lines for Bipolaris sorokiniana resistance by detached leaf. Biologija 55(1):40–45. https://doi.org/10.2478/v10054-009-0008-1. (55:40-45)
Mahapatra S, Das S (2013) Efficacy of different fungicides against spot blotch of wheat in terai region of West Bengal. J Wheat Res 5(2):7–11
Mahapatra S, Devi HM, Kumar S, Mukherjee D, Das S (2020) Performance and stability of different zone specific genotypes evaluated against spot blotch of wheat. Int J Curr Microbiol Appl Sci 9:2968–2981. https://doi.org/10.20546/ijcmas.2020.906.357
Marak T, Mahapatra S, Das S (2018) Stability analysis of disease reactions and yield of green gram [Vigna radiata (L.)Wilczck] against Anthracnose caused by Colletotrichum truncatum. Legume Res 41:919–924. https://doi.org/10.18805/LR-3806
Mudi N, Mahapatra S, Das S (2010) Screening of barley cultivars against Helminthosporium sativum and the stability of disease reaction and yield. Indian Phytopathol 63(1):91–93
Osman M, He X, Singh RP, Duveiller E, Lillemo M, Pereyra SA, Westerdijk-Hoks I, Kurushima M, Yau SK, Benedettelli S, Singh PK (2015) Phenotypic and genotypic characterization of CIMMYT’s 15th international Fusarium head blight screening nursery of wheat. Euphytica 205(2):521–537. https://doi.org/10.1007/s10681-015-1425-0
Parlevliet JT, Van Ommeren A (1975) Partial resistance of barley to leaf rust, Puccinia hordei. II. Relationship between field trials, micro plot tests and latent period. Euphytica 24:293–303. https://doi.org/10.1007/BF00028194
Patial M, Kumar J, Pal D (2017) Detached leaf assay for evaluating resistance to leaf rust Pst. 104–2 in wheat (Triticum aestivum L.). Indian J Exp Biol 55:789–794
Roy C, He X, Gahtyari NC, Mahapatra S, Singh PK (2023) Managing spot blotch disease in wheat: conventional to molecular aspects. Front Plant Sci 14:1098648. https://doi.org/10.3389/fpls.2023.1098648
Singh RP, Rajaram S (2008) Breeding for disease resistance in wheat. In: Curtis BC, Rajaram S, Gómez Macpherson H (eds) Bread wheat improvement and production. FAO Plant Production and Protection Series No. 30. ISBN 92-5-104809-6
Singh RV, Singh AK, Singh SP (1997) Distribution of pathogens causing foliar blight of wheat in India and neighboring countries. In: Duveiller E, Dubin HJ, Reeves J, McNab A (eds) Helminthosporium blight of wheat: spot blotch and tan spot. Proc. Int., Workshop held at CIMMYT El Batan, Mexico, pp 59–62 (ISBN 970-648-001-3)
Singh DP, Sharma I, Singh I, Jindal MM, Mann SK, Chowdhury AK, Mahapatra S, Singh KP, Kumar J, Srivastava K, Vaish SS (2015) Evaluation of sources of resistance to leaf blight (Bipolaris sorokiniana and Alternaria triticina) in wheat (Triticum aestivum) and Triticale. Indian Phytopathol 68(2):221–222
Singh D, Singh SP, Singh CK, Singh RK, Singh VK, Singh AP (2017) Searching of wheat genotypes for resistance against Bipolaris sorokiniana. J Pharmacogn Phytochem 6(5):2181–2183
Tamang S, Kumar S, Das S, Mahapatra S (2021) Role of abiotic factors on disease progression of Spot blotch of Wheat. Indian Phytopathol 74(1):263–269. https://doi.org/10.1007/s42360-020-00280-5
Twizeyimana M, Ojiambo PS, Ikotun T, Paul CH, Hartman GL, Bandyopadhyay R (2007) Comparison of field, greenhouse, and detached-leaf evaluations of soybean germplasm for resistance to Phakopsora pachyrhizi. Plant Dis 91(9):1161–1169. https://doi.org/10.1094/PDIS-91-9-1161
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weed Res 14(6):415–421. https://doi.org/10.1111/j.1365-3180.1974.tb01084.x
Zhang P, Guo G, Wu Q, Chen Y, Xie J, Lu P, Li B, Dong L, Li M, Wang R, Yuan C (2020) Identification and fine mapping of spot blotch (Bipolaris sorokiniana) resistance gene Sb4 in wheat. Theor Appl Genet 133(8):2451–2459. https://doi.org/10.1007/s00122-020-03610-3
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahapatra, S., Chakraborty, S., Kundu, R. et al. A rapid detached leaf assay for the phenotyping of spot blotch of wheat. Vegetos 37, 1574–1582 (2024). https://doi.org/10.1007/s42535-023-00709-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-023-00709-y