Abstract
The study examined the antibacterial effects of both fermented and unfermented ethanolic extracts of Parkia biglobosa seeds against selected entero-pathogenic bacteria. The antibacterial activity was evaluated using agar well diffusion to determine the effect of the extracts against selected entero-pathogenic bacteria; Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus haemoliticus, Escherichia coli, Citrobacter youngae, Klebsiella oxytoca and Acinetobacter haemolyticus. Antioxidant activities of the extracts were quantified by measuring its total flavonoid and total phenol contents, 1,1-diphenyl-2-picrylhydrazyl, 2,2ʹ-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid and ferric reducing antioxidant power. Phytochemical screening and Gas Chromatography–Mass Spectrometry were carried out on both samples using standard methods. The sensitivity test revealed that E. coli, S. aureus, C. youngae, A. haemolyticus and K. oxytoca were more susceptible (25.0 mm, 24.0 mm, 18.0 mm, 27.0 mm, and 17.0 mm) at 100 mg/ml to the crude ethanolic extract of fermented seeds, while S. aureus, E. coli, and A. haemolyticus were more susceptible to the unfermented extract. The seed extracts of fermented and unfermented P. biglobosa were found to be high in phytochemicals such as alkaloids, flavonoids, cardiac, steroids, glycosides, saponins, and tannins. Results showed that calcium, magnesium, sodium, potassium, zinc, iron, phosphorus, manganese, and copper were present in fermented and unfermented samples. The findings indicated that P. biglobosa seeds had considerably high and dose-dependent DPPH radical scavenging and ferric-reducing properties comparable with respective standards. The various constituents of P. biglobosa extracts enhanced its antibacterial activities. This study concluded that P. biglobosa can be a potential source of bioactive compounds and antioxidants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plessas (2022) maintained that fermentation is a production of low food processing and storage method that has been used for centuries to incorporate the transformation and energy metabolism actions of various beneficial microbes, resulting in the bioconversion of fresh produce. Fermentation is a method that uses microorganisms to turn large natural molecules into smaller molecules. Yeast enzymes, for example, turn sugars and starches into alcohol, whereas proteins are turned into peptides/amino acids. Microbes or metabolic enzymes' actions on food products often seem to ferment food, resulting in preferable chemical reactions that are responsible for substantial food alteration (Sharma et al. 2020).
Food plants commonly eaten by man has long been used as the source of medicine to cure various ailment in the human and animal population respectively, this is due to their various constituent compounds which elicit antimicrobial properties against most microbial infections (Akinyemi et al. 2005).
Parkia biglobosa, also known as the African locust bean (West African names: dodongba, netetou, néré, iru, or sumbala), is a Fabaceae family perennial deciduous tree. It grows in a variety of surroundings throughout Africa and is mainly cultivated for its shells, which have lovely tissue and vital seeds. Fermenting and crushing these seeds is a significant financial exercise in the areas where the tree is grown (Osuntokun et al. 2020). P. biglobosa has been widely used as a source of food and natural medicine in West African countries for centuries. The bark, roots, leaves, flowers, fruits, and seeds are commonly used in traditional medicine to treat a wide variety of complaints, both internally and externally, and are sometimes combined with other medicinal plants (Muhammed et al. 2021). Medicinal applications include therapies for parasitic infections, circulatory system disorders such as arterial hypertension, and respiratory, digestive, and skin disorders (Ayo-Lawal et al. 2014).
Parkia biglobosa is a plant with chemotherapeutic potential; a variety of medicinal applications for this plant have been reported (Abioye et al. 2013). Traditional uncontrolled fermentation of seeds produces commonly fermented P. biglobosa seeds, which are used as a condiment of food in countries in Africa such as Nigeria Burkina Faso, Benin, Senegal, and Ghana among others (Camara et al. 2016; Nitiema-Yefanova et al. 2020). Unfermented seeds had more alkaloids, tannins, and phytate than fermented seeds, which contained more flavonoids and saponins (Okwunodulu et al. 2021).
Herbs and spices' antimicrobial activity is determined by the kind of essential oil, the kind of food, and the type of microorganisms (Gutierrez et al. 2008). The chemical structure of essential oils derived from herbs and spices determines their effectiveness, specifically the presence of hydrophilic functional groups such as hydroxyl groups (Muhammad and Syeda 2017).
Enteropathogens are organisms that can cause diseases in the gastrointestinal tract (Parekh and Chanda 2007). A healthy gut microbiome requires a delicate balance of microbial groups. Microbiota deviations have been linked to an increased risk of specific diseases such as inflammatory bowel disease, irritable bowel disease, antibiotic-associated diarrhea, and diabetes (Adetutu et al. 2011). Entero-pathogenic infections are fast becoming difficult to treat due to the development of resistance to various antibiotics used in their treatment. To develop antimicrobial agents, efforts have been made from natural sources to achieve better chemotherapeutic effects with fewer side effects. The antibacterial potentials of P. biglobosa against enteropathogenic bacteria, the proximate, mineral, and phytochemical composition, as well as the antioxidant potentials of unfermented and fermented P. biglobosa seeds, were investigated in this study.
Materials and methods
Collection and authentication of samples
Parkia biglobosa seeds were obtained from the open market in Ikare-Akoko, Akoko North East Local Government of Ondo State (7° 31′ 0′′ N, 5° 45′ 0′′ E). The plant was identified and authenticated in the Department of Plant Science and Biotechnology, Adekunle Ajasin University, Nigeria. The seeds were taken to Adekunle Ajasin University's Microbiology Laboratory in Akungba-Akoko for analysis.
Test organisms
The pathogenic bacteria used were those known to cause intestinal tract diseases in humans. Eight bacterial isolates were obtained from the culture’s stock of organisms at the Adekunle Ajasin University Health Center, Akungba-Akoko. The organisms utilized in the tests were standard strains of pathogenic enteric bacterial isolates. They include Staphylococcus aureus ATCC 25923, S. haemolyticus ATCC 29970, Escherichia coli ATCC 25922, Citrobacter youngae ATCC 29220, Kleibseila oxytoca, Acinetobacter haemolyticus, Acinetobacter baunmannii ATCC 17978, and Pseudomonas auriginosa as they were identified via API staph and microbat™ 24E identification kits.
Standardization of test organisms
McFarland standards of 0.5 were used to standardize all organisms. A 0.2 ml aliquot of 24 h broth culture was dispensed into another sterilized 20 ml Mueller–Hinton broth and incubated for 3 to 5 h. 1 ml of the finished broth contained 0.5 McFarland standards (6 × 108 CFU/ml) (Oyeleke et al. 2008).
Preparation of locust bean seeds for fermentation
Following the method of Achi (2005), P. biglobosa seeds were prepared for fermentation. Three kilograms of the locust bean seeds were measured and foreign particles inside were handpicked and washed to remove impurities before cooking. Raw P. biglobosa seeds were cooked for 4–6 h using a pressure pot to soften the seed’s coat. The cooked bean seeds were dehulled mechanically by pressing them softly with the help of a pestle and mortar. The dehulled bean seeds were washed in water using a local basket to remove fragmented seeds' coats and dirt. The cotyledons were divided into two parts of 3 kg each; the first part was sun-dried and milled into powder and stored before use and the second part was boiled in retort for I hour using a pressure pot, drained, and cooled to 40 °C. The hot seeds were spread in wide calabash trays (10 cm deep) which were pre-stacked with an absorbent sheet (sterile polythene sheet). Trays were stacked together and wrapped with jute bags after which it was incubated (35 °C) for 36 h for natural fermentation. The fermented bean seeds were dried using sunlight for 3 days and milled into powder and packed for analysis.
Preparation of plant extracts
Five hundred grams of fine powdered fermented and unfermented P. biglobosa seeds were weighed into corked bottles containing 1000 ml of absolute ethanol each, and the mixture was vigorously shaken daily for 7 days. The filtrates were collected directly into sterile crucibles after the mixtures were filtered using filter papers. The filtrates were extracted using a Soxhlet extractor, and the residues were stored at − 4 °C. The unfermented sample was treated in the same way (Musa et al. 2016).
Antimicrobial screening of the solid extracts
The method of agar well diffusion was used to screen P. biglobosa seed extracts for antibacterial properties against enteropathogenic isolates. To make a stock concentration of 100 mg/ml, 1 g of each extract was dissolved in 10 ml of Dimethyl sulfoxide (DMSO) diluted with sterile distilled water in a 1:3 ratio. The filtrates were then concentrated into 50 mg/ml, 100 mg/ml, and 25 mg/ml using a dilution formula (C1V1 = C2V2). One milliliter of 4–10 normal saline dilution of 24 h broth culture was mixed with 19 ml of agar in a sterilized universal bottle and allowed to flow into a sterilized petri dish. Before boring wells into the agar plate with a 6 mm cork borer, it was allowed to settle. Each well received 50 µl of each extract concentration and was incubated at 37 °C for 24 h. The diameters of the zones of inhibition were measured and recorded in millimeters, and the results were interpreted using Clinical Laboratory Standard Institute (CLSI) guidelines (CLSI 2016). As a control, 0.125 mg/ml tetracycline was used.
Mineral determination and proximate composition
According to AOAC (2005), the sample composition of proximate was determined, which included the moisture content, ash content, crude fiber, crude protein, crude fat, and carbohydrate. An atomic absorption spectrophotometer was used to determine the iron, calcium, potassium, sodium, and magnesium contents of the samples (Perkin Elmer 500 and Varian AA 475). The concentration of each element was calculated and expressed as percentage on dry matter basis.
Quantitative phytochemical analysis
The concentration of flavonoids in the extract was estimated spectro-photometrically according to the procedure of Sun et al. (1998). The estimation of cardiac glucosides (Borntrager’s Test) was determined using the method described by Fabricant and Farnsworth (2001). The aqueous extract was screened for the presence of tannin, saponin, steroid, phlobatanin, terpenoid, alkaloid, and anthraquinone was determined quantitatively by the methods described by Daramola (2015).
Antioxidant level determination of P. biglobosa seeds
The antioxidant level of the unfermented and fermented seeds of P. biglobosa was determined using various Spectrometric techniques of AOAC (2016) which rely on the reaction of a radical and complex with an antioxidant molecule capable to donate a hydrogen atom. The antioxidant parameters (Total Phenol, Total flavonoid, ferric reducing property, free radical scavenging ability, NO radical scavenging ability, Fe2+ Chelation, ABTS scavenging ability and Superoxide anion scavenging activity assay) were determined using the following procedures: the total phenol content of the extract was determined by the method of Singleton et al. (1999). 0.2 ml of the extract was mixed with 2.5 ml of 10% Folin ciocalteau’s reagent and 2 ml of 7.5% Sodium carbonate. The reaction mixture was subsequently incubated at 45 °C for 40 min, and the absorbance was measured at 700 nm in the spectrophotometer, gallic acid was used as standard. The total flavonoid content of the extract was determined using a colorimeter assay developed by Bao et al. (2005). 0.2 ml of the extract was added to 0.3 ml of 5% NaNO3 at zero time. After 5 min, 0.6 ml of 10% AlCl3 was added and after 6 min, 2 ml of 1 M NaOH was added to the mixture followed by the addition of 2.1 ml of distilled water. Absorbance was read at 510 nm against the reagent blank and flavonoid content was expressed as mg rating equivalent.
The reducing property of the extract was determined as described by Pulido et al. (2002), 0.25 ml of the extract was mixed with 0.25 ml of 200 mM of Sodium phosphate buffer pH 6.6 and 0.25 ml of 1% KFC. The mixture was incubated at 50 °C for 20 min., thereafter 0.25 ml of 10% TCA was also added and centrifuged at 2000 rpm for 10 min, 1 ml of the supernatant was mixed with 1 ml of distilled water and 0.1% of FeCl3 and the absorbance was measure at 700 nm. The free radical scavenging ability of the extract against DPPH (1, 1-diphenyl-2-picryhydrazyl) using Gyamfi et al. (1999) method. One milliliter of the extract was mixed with 1 ml of the 0.4 mM methanolic solution of the DPPH, the mixture was left in the dark for 30 min before measuring the absorbance at 516 nm. The Sodium Nitroprusside in aqueous solution at physiological pH spontaneously generates NO, which interacts with oxygen to produce nitrite ions that can be estimated by use of Greiss reagent. Briefly, 5 mM sodium nitroprusside in phosphate- saline was mixed with the extract, before incubation at 25 °C for 150 min. Thereafter the reaction mixture was added to the Greiss reagent. Before measuring the absorbance at 546 nm, relative to the absorbance of a standard solution of potassium nitrate treated in the same way with Greiss reagent (Kumar et al. 2012).
The ability of the extract to chelate Fe2+ was determined using a method of Minotti and Aust (1987) modified by Puntel et al. (2005). Briefly, 150 mM FeSO4 was added to a reaction mixture containing 168 ml of 0.1 M Tris–HCl pH 7.4, 218 ml saline, and after extraction the volume was made up with 1 ml with distilled water. The reaction mixture was incubated for 5 min, before the addition of 13 ml of 1, 10-phenanthroline and then absorbance read at 510 nm. The 2, 2ʹ-azino-bis (3-ethylbenthiazoline-6-sulphonic acid) (ABTS) scavenging ability of the extract was determined according to the method described by Re et al. (1999). The ABTS was generated by reacting a (7 mM). ABTS aqueous solution with K2S2O8 (2.45 mM/l, final conc.) in the dark for 16 h and adjusting the absorbance at 734 nm to 0.700 with ethanol 0.2 of the appropriate dilution of the extract was then added to 2.0 ml of ABTS solution and the absorbance was read at 732 nm after 15 min. The TROLOX equivalent antioxidant capacity was subsequently calculated.
Furthermore, the superoxide anion radicals were produced in 2 ml of phosphate buffer (100 mM, pH 7.4) with 78 µM β- nicotinamide adenine dinucleotide (NADH), 50 µM nitro blue tetrazolium chloride (NBT) and test samples at different concentrations. The reaction mixture was kept for incubation at room temperature for 5 min. and added with 5-methylphenazinium methosulphate (PMS) (10 µM) to initiate the reaction and incubated for 5 min at room temperature. The colour reaction between superoxide anion radical and NBT was read at 560 nm. Gallic acid was used as a positive control agent for comparative analysis (Kumar et al. 2012).
Gas chromatography and mass spectroscopy (GCMS)
The GC–MS analysis of ethanolic and ethyl acetate extract of fermented and unfermented N-hexane, acetone, and ethanol-extracted oil of P. biglobosa seeds was performed using the method of Oladipupo et al. (2013), a GC Clarus 500 Perkin Elmer system, with an AOC-201 auto-sampler and as chromatograph interfaced to a mass spectrometer instrument. The column used was an Elite 1 fused silica capillary column, operating in electron impact mode at 70 cV. Helium was used as the carrier gas at a constant flow of 1 ml/min and an injection volume of 0.5 ul was employed. The injector temperature was set at 250 °C and the ion-source temperature at 280 °C. The oven temperature was programmed from 110 to 280 °C, with an increase of 10 °C/min, and then held at 280 °C for 9 min. Mass spectra were taken at 70 eV and a scanning interval of 0.5, with fragments from 40 to 550 Da.
Statistical analysis
Statistical significance was determined using ordinary one-way analysis of variance (ANOVA) and two-way ANOVA, while multiple comparisons between means were made by Tukey’s or Sidak’s multiple comparisons test. Analysis was performed using GraphPad Prism software (GraphPad Software Inc. La Jolla, CA, USA). All data are expressed as means of triplicates ± SEM or SD and values of p < 0.05 were considered significant, where “n” represents independent experiments.
Results and discussions
Sensitivity screening of crude ethanol extracts of fermented and unfermented Parkia biglobosa seeds on clinical isolates
Results presented in Table 1 show the zones of inhibition of fermented and unfermented crude ethanol extract of P. biglobosa seeds on entero-pathogenic bacteria at varying concentrations of 100 mg/ml, 50 mg/ml, 25 ml/ml, 12.5 mg/ml, and 6.25 mg/ml respectively with A. haemolyticus and E. coli having zones of inhibition of 27.0 mm and 25.0 mm at 100 mg/ml while C. youngae still has a 3.0 mm inhibition at a lower concentration of 12.5 mg/ml for fermented extracts while Gram-negative organisms i.e. E. coli and K. oxytoca show more sensitivity to the extracts with clear zones of 28.0 mm and 20.0 mm respectively at 100 mg/ml to the fermented extract although the organisms are sensitive to the extract at the lowest concentration of 12.5 mg/ml for fermented and unfermented extracts. The minimum inhibitory concentration observed in P. aeruginosa for the fermented sample was high as compared to the unfermented sample for both solvents. However, Komolafe et al. (2014) reported a MIC of 3.125 mg/ml for stem bark extract against Shigella sp. and Millogo-Kone et al. (2007) reported a MIC of 0.315 mg/ml for P. biglobosa stem bark extract, both of which are within the range of the MIC observed in both extracts.
Quantitative phytochemical analysis of fermented and unfermented Parkia biglobosa
Figure 1 shows the quantitative phytochemical analysis of fermented and unfermented P. biglobosa seeds analyzed with N-hexane, Ethyl acetate, and acetone respectively. N-hexane extracted phytochemicals show tannins with the highest bar of 6.20 µg in 100 g of the fermented sample and 5.40 µg in the unfermented sample followed by flavonoids having 5.55 µg/100 g of the sample and 4.80 µg in the unfermented sample. Phenol shows a total of 5.20 µg/100 g in unfermented and increased drastically to 6.10 µg/100 g in the fermented sample, alkaloid, tannins, saponin, and flavonoid also shown to be in high concentrations as compared to the unfermented sample which is less than the fermented sample constituents. The chemical properties of the essential oil of Parkia biglobosa play a key role in its solubility in N-hexane.
Quantitative phytochemical analysis of fermented and unfermented Parkia biglobosa. All the values are repeated in triplicate and reported as Mean ± SD. FN-H fermented N-hexane, UN-H unfermented N-hexane, FEA fermented Ethyl acetate, UEA unfermented Ethyl acetate, FA fermented acetone, UFA unfermented acetone
Moreover, the availability of tannin, flavonoids, steroids, phenols, cardiac glycosides, Saponins, and alkaloids observed in the phytochemical screenings of the Parkia biglobosa (fermented and unfermented) is in agreement with the findings of Ogbole and Akinmoladun (2020) and this explains their therapeutic value. Steroids found in fermented P. biglobosa are accountable for the treatment of certain endocrine disorders, blood sugar regulation, salt imbalance, and microbial infections (Gautam et al. 2013). Furthermore, reducing sugar and anthraquinone are negative for fermented samples while steroids, phenol, and flavonoids were increased after fermentation (Fig. 1). The saponins and alkaloids found in P. biglobosa are effective in the treatment of Syphilis, Rheumatism, and certain skin diseases, including the treatment of abscesses and other swellings, ulcers, and septic wounds (Mallikharjuna et al. 2007). Saponins are in charge of tonic and stimulating Steroids that display analgesic properties (Soetan et al. 2014).
Cardiac glycosides are a type of naturally occurring drug that aids in the treatment of congestive heart failure. Glycosides are drugs that are found as secondary metabolites in medicinal plants and are used to treat congestive heart failure and cardiac arrhythmia (Gautam et al. 2013). Mostly in the Yoruba tribe of southwestern Nigeria, this class of phytoconstituents compound was discovered in P. biglobosa extracts, which aid in the therapies of heart infections as well as other ailments such as dental caries and cough.
Quantitative mineral analysis of fermented and unfermented seeds of Parkia biglobosa
The quantitative analyses of minerals present in the fermented and unfermented samples of Parkia biglobosa are shown in Fig. 2. Magnesium was the most abundant element in the fermented sample with 15.25 µg/100 g of the sample, followed by potassium having 15.16 µg/100 g and Zinc at 14.50 µg/100 g while Sodium was 10.19 µg/100 g, Manganese has 10.12 µg/100 g followed by calcium with 9.70 µg/100 g. Phosphorus and Iron were also abundant in 100 g of the sample with 6.43 µg/100 g and 5.72 µg/100 g respectively. Lead was absent in both the fermented and non-fermented samples. There is an increase in the number of the constituents’ elements in the fermented than the unfermented sample.
Furthermore, the mineral composition showed the presence of beneficial elements in both the fermented and unfermented samples. The result from the study indicated that lead is absent in both fermented and unfermented samples (Fig. 2). Manganese is present in large quantities in both samples and is a microelement that is required for human nutrition which many enzymes are activated by it (Soetan et al. 2010). Copper is a micro-mineral that aids in iron absorption and red blood cell formation. It is also needed for enzyme production and biological electron transfer in the body (Soetan et al. 2010). Manganese aids blood clotting by collaborating with complex vitamins such as K and B vitamins. Manganese also aids in the management of stress. When an expectant mother does not get enough of this essential nutrient, birth defects may occur (Oluwaniyi and Bazambo 2016).
Subsequently, to maintain acid–base balance, the osmotic balance between cells and interstitial fluid, and nerve function, the body requires sodium. Its unavailability initiates mental apathy, muscle cramps, and a loss of appetite (Oluwaniyi and Bazambo 2016). The daily sodium allowance for healthy adults is no more than 2.3 g, even though the WHO recommended a reduction to < 2 g per day (Mente et al. 2021). Calcium is also required for normal cardiac muscle function, blood coagulation and clotting, and cell permeability regulation. Calcium deficiency symptoms include rickets, back pain, osteoporosis, indigestion, irritability, premenstrual tension, and uterine cramping (Oluwaniyi and Bazambo 2016).
Antioxidant analysis of fermented and non-fermented Parkia biglobosa seeds extracted using acetone
The mean deviation of the antioxidant analyses of both fermented and unfermented seeds of P. biglobosa showed that the fermented sample has the highest mean deviation (Table 2). The antioxidant, 2,2-diphenyl-1-picrylhydrazyl has the highest composition in 100 g of the sample with 80.40 ± 0.064b compared to 49.83 ± 0.206a of the unfermented sample. Phenol is measured as mg/g of the sample; it has 31.04 ± 0.025b in fermented and 28.15 ± 1.737a in the unfermented sample. The flavonoid measured in mg/g is 6.69 ± 0.027b in the fermented sample and 4.25 ± 0.034a in the unfermented sample. The Ferric reducing antioxidant power (FRAP) of the sample was reduced after fermentation. Free radical scavenging activity of P. biglobosa samples recorded in mol/g of the sample as 0.0074 ± 0.0002b in fermented and 0.0028 ± 0.0001a in unfermented while Fe+ chelating % is measured as 43.3961 ± 0.5343b in fermented and 8.2592 ± 0.6048a in the unfermented sample.
However, significantly higher phenol content (31.04) in the fermented sample to 28.15 in the unfermented sample may indicate significant antioxidant potential in fermented more than unfermented sample. Phenols are powerful antioxidants and metal chelators, with hepatoprotective, anti-inflammatory, anti-allergic, antithrombotic, anti-carcinogenic, and antiviral properties (Komolafe et al. 2014).
Proximate analysis of fermented and unfermented Parkia biglobosa seeds
The analysis of proximate indicated that fermentation positively affects the contents of nutrients in the P. biglobosa seeds sample (Table 3). Rises were detected in the content of Moisture (8.14 ± 0.27–15.00 ± 0.23) %, fat content (27.88 ± 0.36–29.52 ± 0.37) % and the crude protein (38.54 ± 1.95–44.95 ± 0.28) % after fermentation.
Moreover, the present study adequately explained the approximate composition of plant extracts. The extract contained different percentages of ash, moisture, crude protein, fat fiber, and carbohydrate. Because of the metabolic activities of microorganisms during the fermentation period, the moisture content of the fermented sample increases. The unfermented seeds' low moisture content (8.1367%) is comparable to that reported by Oluwaniyi and Bazambo (2016), (8.67 ± 0.70%), and Omafuvbe et al. (2004), (8.6 ± 0.6%). The fermented seed's high moisture content (51.54%) is comparable to that reported by Omafuvbe et al. (2004) (52.0 ± 5.0%).
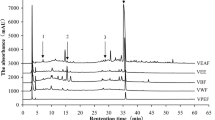
Figure 3 showed the GCMS spectra of the compounds identified in the essential oil extracts of fermented P. biglobosa with a main peak of about 37.83% area yield and match the library mass spectra to 9,12-Octadecadienoic acid (methyl ester). Figure 3 also indicated P. biglobosa seed extracts contain essential oil with specific organic compounds such as Oleic acid and Palmitic acid which have been reported to be soluble in N-hexane and hence explained the solubility of Tannins and Flavanoids in N-hexane (Cao and Sofic 2006). Figure 4 revealed the GCMS spectra of the compounds identified in the essential oil extracts of unfermented P. biglobosa seeds, with the main peak of about 19.90% of the total area yield and matches the library mass spectra to 14-Pentadecanoic acid. Ten compounds were detected in unfermented and fermented P. biglobosa seed essential oil to be of a peak, with 9,12-Octadecadienoic acid (methyl ester), Pentadecanoic acid, and Stearic acid being identified as marker compounds.
Parkia biglobosa essential oil extract showed the presence of large 7–8 peaks including linoleic acid, methyl ester, pentadecanoic acid, arachidic acid, oleic acid chloride, Oleic anhydride, Vitamin E, Palmidrol, Urea, Palmitic acid, L-Ascorbic acid monostearate, and Glycerol 1,2 dipalmitate (Table 4). Fatty acids with antibacterial activities have been isolated from several plants using bioassay-guided fractionation, McGaw et al. (2002) described the antibacterial activity of linoleic and oleic acids isolated from the leaves of Helichrysum pedunculatum which also proved why P. biglobasa has active inhibitory effects against similar organisms as reported. Linoleic and oleic acids inhibited the growth of Gram-positive B. subtilis, Micrococcus kristinae, and S. aureus, and linoleic acid also showed activity against Bacillus cereus and Bacillus pumilis (McGaw et al. 2002). Parkia biglobosa seed essential oil contains various non-polar organic compounds such as fatty acids, alkanes, terpenoids, and esters (Table 4). These non-polar compounds are highly soluble in non-polar solvents such as N-hexane which means they lack polar functional groups like hydroxyl (–OH) or carboxyl (–COOH) (Uche-Ikonne et al. 2019).
Conclusion
The study concluded that P. biglobosa seed contains bioactive compounds that can be used to treat enteropathogenic bacterial infections and could also be used as an antioxidant source. The study concluded that the seeds extract of fermented and unfermented P. biglobosa were high in phytochemicals such as glycosides, flavonoids, tannins, cardiac, steroids, saponins, and alkaloids. The study also concluded that fermentation enhanced the nutritional content of seeds of fermented P. biglobosa, which is needed to pave the way for the availability of nutrients and make the seeds eatable and its essential oil helped against bacterial activities as well.
Recommendation
It is recommended that P. biglobosa seed oil should be further studied phytochemically to elucidate the active principle in the fermented and unfermented seeds which can be used as a leading antibacterial agent in addition to its uses as food condiments.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Abdel-Rahman MA, Yukihiro T, Kenji S (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotech Adv 31(2013):877–902
Abioye EO, Akinpelu DA, Aiyegoro OA (2013) Preliminary phytochemical screening and antibacterial properties of crude stem bark extracts and fractions of Parkia biglobosa (Jacq.). Molecules 18(7):8459–8499
Achi OK (2005) The upgrading of traditional fermented foods through biotechnology. Afr J Biotech 4:375–380
Adetutu A, Morgan W, Corcoran O (2011) Ethnopharmacological survey and in vitro evaluation of wound-healing plants used in South-western Nigeria. J Ethnopharm 137(1):50–56
Ajaiyeoba EO (2002) Phytochemical and antibacterial properties of Parkia biglobosa and Parkia bicolor leaf extracts. Afr J Biomed 5:125–129
AOAC (2005) Official method of analysis, 18th ed. Association of Official Analytical Chemists, Washington D.C.
AOAC (2016) Official methods of analysis, 20th ed. Association of Official Analytical Chemist, Washington D.C.
Ayo-Lawal RA, Osoniyi O, Famurewa AJ, Lawal OA (2014) Evaluation of antioxidant and hypolipidaemic effects of fermented Parkia biglobosa (Jacq) seeds in tyloxapol-induced hyperlipidaemic rats. Afr J Food Sci 8(5):225–232
Bao JY, Cai M, Sun G, Wang C (2005) Anthocyanins, flavonoid and free radical scavenging activity of thines Baybery (Myrial rubia) extracts and their colour properties and stability. J Agric Food Chem 53:2327–2332
Camara F, Soro S, Traore S, Brou K, Dje KM (2016) Centesimal composition and bioactive compounds in African mustards used as condiments in Ivory Coast. Afr J Food Sci 10(7):87–93
Cao G, Sofic E (2006) Priorities in the use of common hydrophilic and lipophilic antioxidant assays in health food evaluation. J Agric Food Chem 54(3):889–896. https://doi.org/10.1021/jf052308+
Clinical, Laboratory Standards Institute (CLSI) (2016) Performance standards for antimicrobial susceptibility testing. In: Twenty-sixth informational supplement. CLSI document M100-S26. Clinical and Laboratory Standards Institute, Wayne
Daramola B (2015) Effects of extraction solvent, morphological parts and ripening stage on antioxidative activity of Solanum anguivi fruit. Int Food Res J 22:2
Edeoga HO, Okwu DE, Mbaebie BO (2005) Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol 4:685–688
Fabricant DS, Farnsworth NR (2001) The value of plants used in traditional medicine for drug discovery. Environ Health Perspect 109:69–75
Gautam RK, Dixit PK, Mittal S (2013) Herbal sources of antidepressant potential: a review. Int J Pharm Sci Rev Res 18:86–91
GraphPad Prism (2018) GraphPad Prism 8th edition. GraphPad Software Inc
Gutierrez J, Barry-Ryan C, Bourke P (2008) The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int J Food Microb 124:91–97
Harborne JB (1998) A guide to modern technique of plant analysis, 3rd edn. Chapman and Hall, London, p 285
Iheke E, Oshodi A, Omoboye A, Ogunlalu O (2017) Effect of fermentation on the physicochemical properties and nutritionally valuable minerals of locust bean (Parkia biglobosa). Am J Food Tech 12(6):379–384
Komolafe K, Olaleye TM, Omotuyi OI (2014) In vitro antioxidant activity and effect of Parkia biglobosa bark extract on mitochondrial redox status. J Acup Merid Stud 7(4):202–210
Kumar RS, Rajkapoor B, Perumal P (2012) Antioxidant activities of Indigofera cassioides Rottl. Ex. DC. using various in vitro assay models. Asian Pac J Trop Biomed 2(4):256–261. https://doi.org/10.1016/S2221-1691(12)60019-7
Mallikharjuna PB, Rajanna LN, Seetharam YN, Sharanabasappa GK (2007) Phytochemical studies of Strychnos potatorum Lf-A medicinal plant. J Chem 4:510–518
McGaw LJ, Jäger AK, Van Staden J (2002) Antibacterial effects of fatty acids and related compounds from plants. South Afri J Bot 68:417–423
Mente A, O’Donnell M, Yusuf S (2021) Sodium intake and health: what should we recommend based on the current evidence? Nutrients 13(9):3232. https://doi.org/10.3390/nu13093232
Millogo-Kone H, Guissou I, Nacoulma O, Traore AS (2007) Antimicrobial effects of the stem bark extracts of Parkia biglobosa (Jacq.) Benth. on Shigellae. Afr J Tradition Complement Alternat Med 4(4):392–396
Minotti G, Aust SD (1987) An investigation into thee mechanism of citrate-Fe2+-dependent lipid peroxidation. Free Radical Biol Med 3(6):379–387
Muhammad SA, Syeda AB (2017) Natural antimicrobials, their sources and food safety. Food Add. https://doi.org/10.5772/intechopen.70197
Muhammed M, Yusuf AA, Odey BO, Alawode AR, Adegbola GA, Agboola RA (2021) A systematic review of domestication, ethnopharmacological use, phytochemistry, nutritional composition, and biological activities of Parkia biglobosa. BIOMED Nat Appl Sci 1(1):01–12
Musa A, Abubakar FK, Uthman A (2016) Effect of different levels of Craseonycteris thonglongyai (bumblebee bat) dung on the concentrations of some phytotoxins in Telfairia occidentalis. Niger J Agric Food Environ 12(1):112–120
Nitiema-Yefanova S, Dossa CP, Gbohaïda V, Kanfon RE, Mossi I, Beakou BH (2020) Fermented Parkia biglobosa seeds as a nitrogen source supplementation for bioethanol production from cashew apple juice. Int J Biol Chem Sci 14(9):3441–3454
Ogbole OO, Akinmoladun AC (2020) Phytochemical analysis and antimicrobial activity of Parkia biglobosa seed oil. J Pharm Res Int 32(20):109–115. https://doi.org/10.9734/jpri/2020/v32i2030734
Okwunodulu FU, Friday C, Okwunodulu IN, Chukwudi VI (2021) Comparative study on the phytochemicals, proximates, vitamins and mineral elements compositions of unfermented and fermented seeds of Parkia biglobosa. Biosci Biotechnol J 2(1):7–10
Oladipupo BB, Adebayo-Tayo BC, Sodipo OA (2013) Phytochemical and GC-MS analysis of ethanol and acetone extracts of Parkia biglobosa leaves, stem bark and root bark. IOSR J Appl Chem 4(3):12–17
Oluwaniyi O, Bazambo IO (2016) Nutritional and amino acid analysis of raw, partially fermented and completely fermented locust bean (Parkia biglobosa) Seeds. Afr J Food Agric Dev 16(2):10867–10875
Omafuvbe BO, Falade OS, Osuntogun BA, Adewusi SR (2004) Chemical and biochemical changes in African locust bean (Parkia biglobosa) and melon (Citrullus vulgaris) seeds during fermentation to condiments. Pak J Nutr 3(3):140–145
Osuntokun O, Akele E, Paul D (2020) Evaluation of fermented Parkia biglobosa (African locust bean) and Bombax evaluation of fermented Parkia biglobosa (African locust bean) and Bombax glabra (Malabar chest nut). J Bacteriol Mycol 7:1148
Oyeleke SB, Dauda BE, Boye OA (2008) Antibacterial activity of Ficus capensis. Afr J Biotech 7(10):1414–1417
Parekh J, Chanda SV (2007) In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turkey J Biol 31:53–58
Plessas S (2022) The rendering of traditional fermented foods in human diet: distribution of health benefits and nutritional benefits. Fermentation 8(12):751
Pulido R, Bravo L, Saura-Calixto F (2002) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J Agric Food Chem 48:3396–3402
Puntel RL, Nogueira CW, Rocha JB (2005) Krebs cycle intermediates modulate Thiobarbituric Acid Reactive Species (TBARS) production in rat brain in vitro. Neurochem Res 30:225–235
Re R, Pellegrin N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improve ABTS radication decolourization assay. Free Rad Biol Med 26:1231–1237
Sharma R, Garg P, Kumar P, Bhatia SK, Kulshrestha S (2020) Microbial fermentation and its role in quality improvement of fermented foods. Fermentation 6(4):106–109
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Cioalteau reagents. Methods Enzymo 299:152–178
Soetan KO, Olaiya CO, Oyewole OE (2010) The importance of mineral elements for humans, domestic animals, and plants: a review. Afr J Food Sci 4(5):200–222
Soetan KO, Akinrinde AS, Adisa SB (2014) Comparative studies on the proximate composition, mineral and antinutritional factors in the seeds and leaves of African locust bean (Parkia biglobosa). Ann Food Sci Technol 15(1):70
Sun JS, Tsuang YH, Chen IJ, Huang WC, Hang YS, Lu FJ (1998) An ultra-weak chemiluminescence study on oxidative stress in rabbits following acute thermal injury. Burns J Int Soc Burn Injur 24(3):225–231. https://doi.org/10.1016/s0305-4179(97)00115-0
Uche-Ikonne CO, Ebere CO, Nwachoko N (2019) Essential oil extraction and characterization of Parkia biglobosa. J Pharm Res Int 29(6):1–13. https://doi.org/10.9734/jpri/2019/v29i630259
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
VOI and TKA Conceptualization; DOO, OGO, and TKO writing original draft; VOI, ADD, and OGO writing, review, and editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We authors declare that we have no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ilesanmi, V.O., Adegbehingbe, K.T., Oyeniyi, D.O. et al. In-vitro antibacterial activities of fermented and unfermented Parkia biglobosa seeds against selected entero-pathogens. Vegetos 37, 566–577 (2024). https://doi.org/10.1007/s42535-023-00702-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-023-00702-5