Abstract
Banana is considered an important fruit crop in the livelihood of many people in tropical and subtropical countries. However, in India, the banana crop is threatened by Fusarium wilt disease incited by the pathogen Fusarium oxysporum f. sp. cubense (Foc), a soil-borne fungus. A purposive sampling survey was conducted in 2018 in various districts of Kerala to study the extent of disease incidence and its severity. The study revealed that the disease incidence ranged from 1.52 to 43.65 percent with 20.41 to 49.57 percent severity. During the survey, it was observed that the major cultivating varieties such as Rasthali/Poovan, Kadali, Njalipoovan, and Chenkadali were infected by the disease which causes serious economic loss to the farmers. Hence, the research was carried out to create an integrated management package against the pathogen causing Fusarium wilt disease in bananas by testing various chemical fungicides and biocontrol agents under both as well as field conditions. Among the various treatments used, an integrated package including sucker treatment with biocontrol agent Pseudomonas fluorescens followed by the soil application of Arbuscular Mycorrhizal Fungi and Trichoderma viride (biocontrol agent) enriched cow dung at the time of planting and soil drenching with a triazole fungicide tebuconazole at 2 and 4 months after planting was recorded best for the disease management. As a result, the management of Fusarium wilt of banana may be accomplished by the employment of a combined strategy of biocontrol agents and chemical fungicides applied at the prescribed rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Banana is an important fruit crop in tropical and subtropical countries and is the major diet for around 400 million people in several regions of the world. It produces parthenocarpic fruits whereas, the wild diploid banana produces seeds (Li et al. 2013). Its production is seriously threatened by several pests and diseases. Higgins (1904) reported that a fungus found in banana plants causes lethal yellowing and wilting of the entire plants. Thereafter in 1908, the fungus associated with this wilting was realised by Smith (1910) in sick plants collected from Cuba and he termed it Fusarium cubense. A detailed explanation of the pathogen was given by Ashby (1913). The Koch postulates of the causal agent was carried out in Gros Michel (AAA), Manzano (AAB), and Bluggoe (ABB) varieties (Brandes 1919). F. oxysporum causing wilt diseases in banana plants was renamed as F. oxysporum f. sp. cubense after the formation of formae specialis system (Snyder and Hansen 1940).
Banana cultivar Kathali in West Bengal was the first cultivar in India on which symptoms of Fusarium wilt disease were detected in 1911 (ProMusa 2020). Then it became the most destructive disease in banana cultivating areas (Thangavelu et al. 1999). The pathogenic isolates of Foc have been traditionally grouped into four physiological races based on pathogenicity to host cultivars under field conditions. Race 1 infects Gros Michel (AAA), causing Gros Michel epidemics in America, it also infects AAB group varieties such as Rasthali, Lady Finger and Silk. Whereas race 2 affects ABB group cooking bananas like Bluggoe and Monthan and Race 3 infects Heliconia sp., a close relative of the cultivar banana. Race 4 has been divided into 2 groups viz., subtropical R4 (SR4) and tropical R4 (TR4). Subtropical race 4 affects the Cavendish group and the varieties susceptible to race 1 and 2 in subtropics. Tropical race 4 pathogens attack banana both in tropical as well as subtropical conditions. The two most prevalent races of Foc affecting bananas in India are Foc races 1 and 4 (Thangavelu et al. 2022). In Kerala, so far race 1 of Fusarium wilt pathogen is reported from banana varieties like Rasthali/Poovan (AAB), Njalipoovan (AB), Kadali (AA), Monthan (ABB) and Karpooravalli (ABB). It causes huge damage to the economy of banana farmers of the state (AICRP 2017).
It is a typical wilt and a lethal vascular disease of banana. In India, it has become one of the most important diseases in all banana-growing states with a report of disease incidence of about 30 percent and 85 percent in the first crop and ratoon crop respectively (Mustaffa and Thangavelu 2011). Foc, the pathogen that causes the disease, originates in the soil and can live there for over 30 years as chlamydospores. Nowadays, biological control of Fusarium wilt diseases using certain microorganisms has been getting more popular as it is an eco-friendly management strategy compared to the indiscriminate use of chemical fungicides (Fravel et al. 2003; Weller et al. 2002). Cherian and Menon (2001) suggested an integrated management package including carbendazim and biocontrol agents for the control of Fusarium wilt disease in banana.
Though internationally significant progress has been made to manage this pathogen, no attempts have been made to control the pathogen isolates from Kerala. Hence, a sustainable approach utilizing disease-free plantlets, cultural practices, new generation fungicides, and eco-friendly bioagents needs to be formulated to limit the further spread of the pathogen and to contain the disease. In view of such concerns, the project entitled “integrated management of Fusarium wilt disease of banana in Kerala” was undertaken to develop an integrated management strategy against the disease.
Materials and methods
Survey for the assessment of disease incidence and severity
Surveys were conducted in selected banana growing districts of Kerala viz., Thiruvananthapuram, Ernakulam, Thrissur, Palakkad, Kozhikode, and Wayanad to assess the incidence of disease. Percent disease severity (PDS) of the Fusarium wilt infected banana plants was assessed with the standard score chart using a 0–4 scale given by Mak et al. (2004). Where, 0–no streaking or yellowing of the plant, the plant appears healthy, 1–slight streaking and/or yellowing of lower leaves, 2–streaking and/or yellowing of most of the lower leaves, 3–extensive streaking and/or yellowing on most or all of the leaves and 4–dead plant.
Preparation of inoculum
The inoculum was prepared in sterilised half-strength potato dextrose broth in 250 ml conical flasks by transferring a mycelial disc of 5 mm of five to seven days old cultured of Foc race 1 grown on PDA. This was incubated at 25 °C until the surface of the medium is completely covered by the mycelial mat (approximately five to seven days). The culture was then shaken in Orbitek shaker for 3 days at 150 rpm (Zuo et al. 2018). The concentration of conidial suspension was finally diluted up to 5 × 106 CFU/ml and used for inoculation.
Pot culture experiment
An experiment was conducted for the management of Fusarium wilt under pot culture conditions in the College of Agriculture, Vellanikkara, Kerala in a completely randomized design (CRD) with ten treatments and three replications. Healthy suckers of Rasthali/Poovan (susceptible to race 1 of Fusarium oxysporum f. sp. cubense) cultivar brought from Banana Research Station Kannara, Kerala were used as the planting materials. The potting mixture was prepared by mixing sand: soil: cow dung in a ratio of 1:1:1. Potting mixture was sterilized using 40 percent formaldehyde before filling the pots. The plants were inoculated by drenching with 100 ml of spore suspension thrice at the one-month interval. Sucker treatment and soil drenching (1 to 3 months after planting) of copper hydroxide (0.2 percent), tebuconazole (0.1 percent), combination of carbendazim and mancozeb (0.2 percent), carbendazim (0.2 percent), Trichoderma viride (2 percent), P. fluorescens (2 percent), 2 percent PGPR Mix II (P. fluorescens, Bacillus megatherium, and Lactobacillus), soil application of arbuscular mycorrhizal fungi (100 g/plant) at the time of planting + Trichoderma enriched cow dung—neem cake mixture (5 kg/plant) + soil drenching of 2 percent P. fluorescens and an integrated approach comprising of P. fluorescens + arbuscular mycorrhizal fungi and Trichoderma enriched cow dung + soil drenching of 0.1 percent tebuconazole were the treatments used for the study (Table 1). An untreated control was also maintained. After three months of planting, percent wilt index and percent vascular wilt index were calculated for each treatment. The percent wilt index was calculated using the 0–4 standard score chart given by Mak et al. (2004). Whereas, percent vascular wilt index was calculated using the 0–5 scale described by Zuo et al. (2018).
Field experiment
A field experiment to develop a management strategy for Fusarium wilt of banana under natural conditions was conducted in the sick plot at Banana Research Station Kannara. The same treatments used in the pot culture experiment were used for field trials. The planting material used was 3–4 months old disease-free sword suckers of susceptible variety Rasthali/Poovan and the experiment was carried out in randomized block design. Pits of size 50 cm × 50 cm × 50 cm were used for planting the suckers. The suckers were planted at a spacing of 2.1 m × 2.1 m and manuring was done as per the Kerala Agricultural University’s package of practices recommendation, ie 180:180:360 g NPK/plant in two split doses (KAU 2016). A total of ten treatments with three replications were used for the experiment.
Results
Sampling survey
The percent disease incidence (PDI) and percent disease severity (PDS) were recorded from surveyed locations. During the survey, it was observed that the disease incidence ranged from 1.52 to 4.64 percent in Thiruvananthapuram, 19.44–27.40 percent in Ernakulam, 6.40–23.78 percent in Thrissur, 5.33–7.58 percent in Palakkad, 12.30–17.22 percent in Kozhikode and 17.66–43.65 percent in Wayanad district. The PDI was lowest (1.52%) in Thiruvananthapuram and highest (43.65%) in Wayanad district.
The results of the survey revealed that the lowest (20.41%) PDS was recorded in Ernakulam while the highest PDS of 49.57 percent was recorded in Wayanad district. The PDS varied from 21.30 to 38.18 percent, 20.41–42.22 percent, 20.67–32.63 percent, 28.70–34.11 percent, 26.81–29.68 percent and 34.74–49.57 percent in Thiruvananthapuram, Ernakulam, Thrissur, Palakkad, Kozhikode and Wayanad districts respectively.
Pot culture study
Statistically significant differences were noticed in the PDI with various treatments and the treatments were significantly superior to the control plants. The lowest PDI was recorded in plants applied with T6 (IDM – P. fluorescens + AMF and Trichoderma enriched cow dung + tebuconazole) followed by T2 (tebuconazole) and T9 (carbendazim) (Table 2).
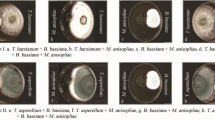
The highest disease severity was observed in control plants (T10) (Table 3). While the lowest disease severity was recorded in T6 (IDM—P. fluorescens + AMF and Trichoderma enriched cow dung + tebuconazole) applied plants (Fig. 1).
Field experiment
The percent disease incidence was recorded at 8 MAP as well as 10 MAP (Table 4). The disease incidence was observed in all treatments. The highest PDI was noticed in control plants whereas the lowest PDI (6.67%) was recorded in plants applied with T6 (IDM – P. fluorescens + AMF and Trichoderma enriched cow dung + tebuconazole). At 10 MAP, the disease incidence has increased in all treatments. During this period also the highest PDI was recorded in control plants (T10) and the lowest PDI was recorded in plants treated with T6 (IDM—P. fluorescens + AMF and Trichoderma enriched cow dung + tebuconazole).
The severity of the disease in the field experiment was assessed by calculating the percent wilt index (PWI) and percent vascular wilt index (PVWI) at 10 MAP (Table 5). The treatments were statistically superior over the control plants. In the field condition also, the maximum disease severity based was noticed in plants kept as control the lowest disease severity was recorded in plants treated with T6 (IDM—P. fluorescens + AMF and Trichoderma enriched cow dung + tebuconazole (Fig. 2).
Discussion
Fusarium wilt of banana causes significant economic loss in the agriculture scenario of tropical and subtropical countries in the world. It is a major threat to banana cultivation with 80 to 90 percent disease severity (Sivamani 1987). In India, the pathogen causes up to 80 percent of disease incidence and yield loss of up to 40 percent in northern states. Whereas, in southern states yield loss up to 90 percent have been reported (Thangavelu et al. 1999). In the present study, it was observed that the PDI ranged from 1.52 percent (Thiruvananthapuram) to 43.65 (Wayanad) percent and thus varied from place to place. The PDS in the surveyed districts varied from location to location. The minimum PDS (20.41%) was noticed in the field at Aluva (Ernakulam) and the maximum PDS (49.57%) was observed at Kenichira field (Wayanad).
In the integrated management strategy of plant diseases, biocontrol agents and chemical fungicides can be used in a scheduled manner (Nel et al. 2007). Fungicides and biocontrol agents have been shown in prior research to be effective at reducing pathogens or enhancing plant disease tolerance (Meyer and Roberts 2002). Minuto et al. (1995) used a chemical fungicide, carbendazim along with antagonistic Fusarium spp. for the effective management of Fusarium wilt of cyclamen. Similarly, other researchers such as Elmer and McGovern (2004) also proved better efficiency of combining biological control agents and chemical fungicides for the control of Fusarium wilt diseases. Thus, the combined application of chemical fungicides and biocontrol agents can be effectively used for the management of Fusarium wilt in banana.
Demethylation inhibitor (DMI) fungicides, which include triazoles are among the most important groups of fungicides (Baldwin and Rathmell 1988). Tebuconazole is an important fungicide that belongs to the group of triazoles, which have DMI mode of action in fungi. Demethylation is an important phase required for sterol synthesis in fungi. Sterol is necessary for fungal cell wall growth. In the present study, the treatment with the application of tebuconazole fungicide showed a higher degree of suppression. Similar results were reported on the use of tebuconazole by Sreeja (2014) for the control of F. oxysporum causing wilt disease in cowpea. Here, the fungicide was applied by means of soil drenching, which is the effective method for the application rather than stem injections in banana (Herbert and Marx 1990). Soil drenching of the fungicides was found very effective in the management of Fusarium oxysporum f. sp. dianthi in carnation (Manasa et al. 2017). Shukla (2019) reported that the maximum inhibition of PWI and PVWI in banana could be achieved by the treatment with the triazole fungicide, tebuconazole.
It is widely known that certain Trichoderma species produce a range of biologically active toxic substances with fungicidal and bactericidal activities. Therefore, a range of phytopathogens, including Fusarium species, can be controlled with this (Sood et al. 2020). The application of biocontrol agents decreased the incidence of Fusarium wilt in banana and increased the yield (Bubici et al. 2019). The present study was in tune with the experiment conducted by Bubici et al. 2019, who reported that the application of Pseudomonas spp., Trichoderma spp., and arbuscular mycorrhizal fungi could manage the plant diseases. Chawla and Gangopadhyay (2009) revealed that the bioagents such as T. harzianum, T. viride and P. fluorescens in the presence of farmyard manure can be effectively used against F. oxysporum f. sp. cumini. The application of cow dung along with other fertilizers increases the vegetative growth of the plants (Solaiman and Rabbani 2006). The application of AMF enhances the root growth and nutrient uptake of plants. Thus, the plant growth and yield attributes would be increased (Jefwa et al. 2008). The present study was in accordance with the study conducted by Emara et al. (2018) which revealed that the application of AMF in banana enhanced the plant height, pseudostem girth, number of leaves, and root dimensions. Similar observations were also reported by Yano-Melo et al. (1999) and Rodriguez-Romero et al. (2005) in banana plants.
Conclusions
An integrated disease management approach including biocontrol agents and chemical fungicides (P. fluorescens + AMF and Trichoderma enriched cow dung + tebuconazole) at recommended rate could be used for the management of Fusarium wilt of banana. Adopting preventive measures by farmers are also taken into consideration for the effective management of Fusarium wilt of banana.
References
AICRP (All India Coordinated Research Project on Fruits) (2017) Proceedings of fourth group discussion, 4–7 January 2017, IIHR, Bangalore. 65p
Ashby SF (1913) Banana diseases in Jamaica. Bulletin of the Department of Agriculture Jumaica volume 2, Department of Agriculture, Jumaica, 95p
Baldwin BC, Rathmell WG (1988) Evolution of concepts for chemical control of plant disease. Annu Rev Phytopathol 26:265–283. https://doi.org/10.1146/annurev.py.26.090188.001405
Brandes EW (1919) Banana Wilt. Phytopathol 9:339–383
Bubici G, Kaushal M, Prigigallo MI, Gomez-Lama Cabanas C, Mercado-Blanco J (2019) Biological control agents against Fusarium wilt of banana. Front Microbiol 10:1–33. https://doi.org/10.3389/fmicb.2019.00616
Chawla N, Gangopadhyay S (2009) Integration of organic amendments and bioagents in suppressing cumin wilt caused by Fusarium oxysporum f. sp. cumini. Indian Phytopathol 62:209–216
Cherian AK, Menon R (2001) Integrated management of panama wilt disease of banana caused by Fusarium oxysporum f. sp. Cubense. In: Proceedings of National Symposium on Ecofriendly Appoaches for Plant Disease Managenment, 22–24 January 2001, Chennai. University of Madras, Chennai, 31p
Elmer WH, McGovern RJ (2004) Influence of combining biological products with chemical fungicides for control of Fusarium wilt of cyclamen. Crop Prot 23:909–914. https://doi.org/10.1016/j.cropro.2004.01.012
Emara HA, Nower A, Hmza E, Saad M, El Shaib F (2018) Role of mycorrhiza as biofertilization of banana grand naine on nursery stage. Int J Curr Microbiol App Sci 7:805–814 https://doi.org/10.20546/ijcmas.2018.710.089.
Fravel DR, Olivain C, Alabouvette C (2003) Fusarium oxysporum and its biocontrol. New Phytol 157:493–502. https://doi.org/10.1046/j.1469-8137.2003.00700.x
Herbert JA, Marx D (1990) Short-term control of Panama disease in South Africa. Phytophylactica 22:339–340
Higgins JE (1904) The banana in Hawaii. Hawaii Agricultural Experiment Station Bulletin No.7, Hawaiian Gazette Company Ltd, Honolulu, 51p
Jefwa J, Vanlauwe B, Coyne D, Van Asten P, Gaidashova S, Rurangwa E, Mwashasha M, Elsen A (2008) Benefits and potential use of arbuscular mycorrhizal fungi (AMF) in banana and plantain (Musa spp.) systems in Africa. In: IV International Symposium on Banana: International Conference on Banana and Plantain in Africa: Harnessing International partnership to increase research impact, 5–9 October 2008, Kenya. International society for horticultural science, Belgium, pp. 479–486
KAU (Kerala Agricultural University) (2016) Package of Practices Recommendations: Crops (15th Ed.). Kerala Agricultural University, Thrissur, 393p
Li CY, Mostert G, Zuo CW, Beukes I, Yang QS, Sheng O, Kuang RB, Wei YR, Hu CH, Rose L, Karangwa P (2013) Diversity and distribution of the banana wilt pathogen Fusarium oxysporum f. sp. cubense in China. Fungal Genom Biol 3:1–6. https://doi.org/10.4172/2165-8056.1000111
Mak C, Mohamed AA, Liew KW, Ho YW (2004) Early screening technique for Fusarium wilt resistance in banana micropropagated plants. In: Jain SM and Swennen R (Eds), Banana Improvement: Cellular, Molecular Biology, and Induced Mutations. Science Publishers, Enfield, USA, p
Manasa BG, Somashekara YM, Shankara K, Swamy C (2017) Efficacy of fungicides in control of Fusarium oxysporum f. sp. dianthi, the cause of wilt in carnation. Int J Curr Microbio App Sci 6:10
Meyer SL, Roberts DP (2002) Combinations of biocontrol agents for management of plant-parasitic nematodes and soilborne plant-pathogenic fungi. J Nematol 34:219–227
Minuto A, Migheli Q, Garibaldi A (1995) Evaluation of antagonistic strains of Fusarium spp. in the biological and integrated control of Fusarium wilt of cyclamen. Crop Prot 14:221–226. https://doi.org/10.1016/0261-2194(95)00008-A
Mustaffa MM, Thangavelu R (2011) Status of fusarium wilt in India. In: Bergh IVD, Smith M, Swennen R, Hermanto C (Eds), Proceedings of International ISHS-ProMusa Symposium on Global Perspectives on Asian Challenges, 14–18 September 2009, Guangzhou. ISHS Acta Horticulturae, pp 323–329
Nel B, Steinberg C, Labuschagne N, Viljoena A (2007) Evaluation of fungicides and sterilants for potential application in the management of Fusarium wilt of banana. Crop Prot 26:697–705. https://doi.org/10.1016/j.cropro.2006.06.008
ProMusa (2020) Fusarium wilt of banana [on-line]. Available: http ://www.promusa.org/Fusarium+wilt [12 January 2020].
Rodriguez-Romero AS, Guerra MSP, Jaizme-Vega (2005) Effect of arbuscular mycorrhizal fungi and rhizobacteria on banana growth and nutrition. Agron Sustain Dev 25:395–399 https://hal.archives-ouvertes.fr/hal-00886313
Shukla DN (2019) Studies on variability and management of Fusarium oxysporum f. sp. cubense isolates causing Panama wilt of banana. Ph.D. thesis, Dr. Rajendra Prasad Central Agricultural University, Pusa, Samastipur, 142p
Sivamani E (1987) Wilt diseases of banana in Tamil Nadu. Studies on the biology and control of Panama wilt caused by Fusarium oxysporum f. sp. cubense (E.F. Smith) Snyder ed. Hansen. Ph.D. Thesis. University of Madras, Chennai, India
Smith EF (1910) A Cuban Banana Disease Sci 31:754–755
Snyder WC, Hansen HN (1940) The species concept in Fusarium. Am J Bot 27(2):64–67 https://doi.org/10.2307/2436688. .
Solaiman ARM, Rabbani MG (2006) Effects of NPKS and cow dung on growth and yield of tomato. Bull Inst Trop Agr Kyushu Univ 29:31–37
Sood M, Kapoor D, Kumar V, Sheteiwy MS, Ramakrishnan M, Landi M, Araniti F, Sharma A (2020) Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 9:762. https://doi.org/10.3390/plants9060762
Sreeja SJ (2014) Integrated management of Fusarium wilt and anthracnose of vegetable cowpea (Vigna unguiculata sub sp. sesquipedalis (L) Verdcourt) using new generation fungicides. Ph.D. (Ag) thesis, Kerala Agricultural University, Thrissur, 182p
Thangavelu R, Sundararaju P, Sathiamoorthy S, Reghuchander T, Velazhahan R, Nakkeeran S, Palanisamy A (1999) Status of Fusarium wilt of banana in India. In: Proceedings of the International Workshop on Banana Fusarium Wilt Disease, 18–20 October 1999, Malaysia. INIBAP- ASPNET, Philippines, pp 58–63
Thangavelu R, Edwinraj E, Gopi M, Pushpakanth P, Sharmila K, Prabaharan M, Loganathan M, Uma S (2022) Development of PCR-based race-specific markers for differentiation of Indian Fusarium oxysporum f. sp. cubense, the causal agent of Fusarium Wilt in Banana. J Fungi 8:53
Weller DM, Raaijmakers JM, McSpadden-Gardener BB, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Ann Rev Phytopathol 40:309–348. https://doi.org/10.1146/annurev.phyto.40.030402.110010
Yano-Melo AM, Maia LC, Saggin OJ, Lima-Filho JM, Melo NF (1999) Effect of arbuscular mycorrhizal fungi on the acclimatization of micropropagated banana plantlets. Mycorrhiza 9:119–123. https://doi.org/10.1007/s005720050009
Zuo C, Deng G, Li B, Huo H, Li C, Hu C, Kuang R, Yang Q, Dong T, Sheng O, Yi G (2018) Germplasm screening of Musa spp. for resistance to Fusarium oxysporum f. sp. cubense tropical race 4 (Foc TR4) Eur J Plant Pathol 151:723–734 https://doi.org/10.1007/s10658-017-1406-3
Author information
Authors and Affiliations
Contributions
We certify that all co-authors and the relevant administrators at the institute where the study was done have given their consent for the publication.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lishma, N.P., Cherian, K.A., Louis, V. et al. Integrated management of Fusarium wilt disease of banana in Kerala, India. Vegetos 37, 117–124 (2024). https://doi.org/10.1007/s42535-023-00576-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-023-00576-7