Abstract
Phalsa (Grewia subinequalis L.) berries also known as Dhamani/Star apple are dark purplish in colour, tiny, tender and acidic in taste. These berries are highly perishable and have poor storage life (< 2 days) under ambient conditions. Different combinations of edible coating materials based on polysaccharides, protein and lipids were applied to Phalsa to extend their shelf life. The use of locally available materials like Aloe vera, guar gum, and aonla juice was also considered. Besides these, two layer-by-layer coatings (LBL) were also applied on berries and kept under refrigerated conditions (4 ± 2 °C) for storage upto 9 days. All coating treatments were found significantly effective in minimizing quality deterioration compared to control. Our findings suggest that LBL coatings are more effective in maintaining quality whilst coatings from locally available resources can also help in extending their shelf life moderately (6 days). LBL based coatings yielded effective results, providing phalsa fruits with good appearance, reduced physiological loss in weight (9.3% compared to 14.11% in control) and respiration rate (5.7% lower). Higher firmness (52%), reducing sugars (32.38%) and better colouration were recorded for LBL coated fruits over conventional coatings. LBL coating based on soy protein isolate-CMC could effectively extend the shelf life upto 7 days under refrigerated conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phalsa (Grewia subineaqualis L.) is an underutilized minor fruit crop indigenous to South Asia and belongs to Malvaceae family. The genus Grewia includes 150 species which are small to medium shrubs. Out of that, G. subineaqualis and G. asiatica species produce edible fruits predominantly found in sub-tropical, arid and semi-arid regions of India like Rajasthan, Punjab, Gujarat and Haryana with an average area of 30 ha and production of 190 MT (Qamar et al. 2020). The berries are well known for their medicinal properties from ancient times and are mentioned in Ayurveda for potent activities like antihyperglycemic, antioxidant, hepatoprotective, radioprotective, antifungal and antiviral activity since they are bestowed with bioactive components like flavonoids, anthocyanins, tannins, proteins, amino acids and phenolic components (Ray and Bala 2019; Sinha et al. 2015). However, phalsa berries are highly perishable in nature having poor shelf life of 48 h at room temperature. These delicate berries senesce quickly and are highly prone to damages caused during handling and transportation like bruising, mishandling, postharvest decay by fungi such as Penicillum, Botrytis leading to soft rot. Higher temperature and lower relative humidity in arid regions where it is cultivated leads to excessive moisture loss, resulting in faster shriveling and weight loss. Thus, improper storage leads to postharvest loss of these valuable fruits (Kumar et al. 2017). Hence, marketability of these prized phalsa berries has become a challenging task.
Among various postharvest technologies available till date, edible coatings have emerged as a promising, cost effective and eco-friendly technique for extending the shelf life of most of the fresh and fresh-cut fruits like apple, melon, papaya, and pineapple. Most of the components of edible coatings are based on proteins, lipids, or polysaccharides. Application of edible coating over phalsa berries may generate a modified atmosphere by creating a semi-permeable barrier against O2, CO2, moisture, and solute movement, thus reducing rate of respiration, water loss, and oxidation reaction rates. Since, arid regions have good native production of nutritive crops such as aloe vera, cluster bean (provides guar gum) and aonla, their utilization as the edible coating resource was explored. Use of guar gum and aloe vera gel, known to be rich in antioxidants and antimicrobial compounds while being non-toxic, biodegradable and readily available can be a practical (Saha et al. 2017), natural and organic raw material for coating on fruits. Braich et al. (2022) have recently demonstrated efficacy of aonla essential oil to enhance shelf life of aonla fruits. Being rich in ascorbic acid, antioxidants and phenolics, it has great potential for use in edible coatings. Harnessing such locally abundant natural resources for postharvest treatments can provide potentially cheaper, natural, sustainable and farmer friendly solutions towards reducing loss of product.
Dave et al. (2016) have studied the effect of different concentrations of edible coating materials comprising of soy protein isolate (SPI), olive oil, hydroxypropyl methyl cellulose (HPMC) and potassium sorbate for phalsa berries. They suggested that combination of SPI 3.45%, HPMC 0.40%, olive oil 1% and potassium sorbate 0.25% as the most suitable formulation for coating of phalsa berries. In recent years, layer-by-layer (LBL) edible coating is gaining more attention in coating of fruits (Sowmyashree et al. 2021). LBL technique employs polysaccharide based coating materials like alginates, CMC, chitosan, pectin mainly based on electrostatic deposition of oppositely charged natural polyelectrolytes. LBL edible coating containing alginate and chitosan was found to improve the quality of fresh cut melon fruits (Poverenov et al. 2014) by providing beneficial properties of both the materials. CMC and chitosan bilayer coating showed significant effect on firmness of citrus fruits like oranges, grapefruit and mandarins (Arnon et al. 2014). CMC-chitosan coating also maintained higher firmness by slowing the primary and secondary metabolism in strawberries during storage at 0 °C for eight days (Yan et al. 2019). However, most LBL coating studies have used CMC-chitosan materials, which can be a deterrent for vegetarian consumers. Hence, use of alternate electrostatic materials like alginate-pectin; SPI-CMC could offer vegan options for coatings on fruits to extend their shelf-life and maintain post-harvest quality. Thus, the current study was conducted with the objectives to apply conventional coatings based on locally sourced materials and compare their efficacy with two vegan layer-by-layer coatings.
Materials and methods
Fruit material
Well mature, firm phalsa (reddish to purple) berries were harvested freshly in early morning hours from the experimental blocks of ICAR-Central Institute for Arid Horticulture, Bikaner, Rajasthan and brought to Central Research Laboratory, Bikaner, washed thoroughly to remove dirt from the fruits and dried under fan immediately.
All chemicals: l-ascorbic acid, guar gum, calcium chloride, sodium alginate, pectin, soy protein isolate (SPI), carboxy methyl cellulose (CMC) and reagents used for this experiment were of analytical grade and procured from SRL Chemicals Pvt. Ltd. New Delhi.
Preparation of coating solutions
Conventional coatings: for T1, Aloe vera gel was extracted from freshly harvested leaves and blended with water in 1:1 ratio, ascorbic acid was added to it at 5% level. For T2, Aloe vera gel (50%) was combined with freshly extracted Aonla juice (diluted with water in equal proportion to extract juice). For T3 coating, solution of 1% of Guar gum with 1% calcium chloride was used.
LBL coatings: for T4, separate solutions of Sodium alginate-Pectin–calcium chloride (1% each) each were prepared by using magnetic stirrer. For T5 coating, two separate solutions of SPI (3.45%) and CMC (0.4%)-Sesame oil (1%)-Tween-20 (0.2%) were prepared. All gums were hydrated overnight prior to application.
Coatings application
Phalsa berries were washed and drained for 1 h at room temperature. Then, the berries were immersed in the coating solutions for 30 s. For LBL coatings, the berries were immersed sequentially in each solution for 30 s. Followed by coating application, residual solution was allowed to drip off and the fruits were dried under fan at room temperature (24 ± 2 °C) for 2 h, weighed 100 g each, placed in punnet boxes (200 mL) and stored in refrigerator (4 ± 2 °C), 70–75% RH based on previous studies (Ray and Bala 2016). The coated as well as control fruits were evaluated during storage every alternate day upto 9 days to determine the quality attributes like PLW, firmness, respiration rate, TSS, titratable acidity, ascorbic acid, PME, and anthocyanin content.
Physiological loss in weight (%)
Phalsa berries were weighed at the beginning of the experiment (i.e. 0 day) and at each storage interval. The difference between the initial and final weight of the fruit was considered as a total weight loss and the results were expressed as the percentage loss of the initial weight as per the standard method (Sowmyashree et al. 2021)
Total soluble solids and titratable acidity
The TSS content of the fruit was determined using digital refractometer (Atago Co., Tokyo, Japan). Samples were squeezed and the filtrate was placed on the testing area of the refractometer and a direct reading was taken. The results were expressed as degree Brix (°B) at 20 °C. The total titratable acidity was determined by visual titration method (Ranganna, 1986). In brief, five gram phalsa pulp was crushed with distilled water and the volume was made up to 100 mL. 10 mL aliquot of filtrate was taken in a conical flask and titrated against 0.1 N NaOH using phenolphthalein indicator.
Ascorbic acid
Ascorbic acid content was determined by 2, 6-dichlorophenol indophenol (DPIP) method (AOAC, 967.21). Five gram phalsa pulp was crushed with 3% metaphosphoric acid, filtered and volume was made up to 100 mL using 3% metaphosphoric acid, from which 10 mL was taken for titration against DPIP. Ascorbic acid content was calculated as mg of ascorbic acid equivalents per 100 g of fresh weight.
Reducing sugars
Reducing sugar content of the samples were estimated by using modified DNSA reagent method (Rudra et al. 2022). For reducing sugars estimation, 100 mg of phalsa pulp was weighed, suspended in 5 mL hot 80% ethanol two times for complete extraction and kept in hot water bath for 2 h. 100 µL of supernatant was taken and volume made up with 3 mL distilled water, 3 mL of DNS reagent was added and kept in hot water bath for 5 min. 1 mL of 40% Rochelle salt solution was added to the warm samples and cooled for a while, intensity of red color was measured by spectroscopic reading at 510 nm
\({\text{Reducing sugars }}(\mathrm{mg}/100\mathrm{g}) =\left\{\frac{{\text{Abs }}\left(\mathrm{sample}\right)\times {\text{Conc. of std. }}\times {\text{Dilution factor }}\times 100 }{{\text{ Abs }}\left(\mathrm{std}\right)\times {\text{Aliquot taken }}\times 1000}\right\}\times 100\)
Anthocyanins
Anthocyanins were extracted and estimated by using pH differential method (Rudra et al. 2022). Two gram phalsa pulp was blended with 15 mL of extracting solvent (80% ethanol) and centrifuged at 4 °C for 20 min at 10,000 rpm. The samples were filtered and 1 mL of supernatant was taken and mixed with KCl buffer and sodium acetate pH buffers (pHs 1.0, 4.5, respectively).The absorbance was recorded at 510 and 700 nm using UV–Vis Spectrophotometer (Varian Cary) using following formula, where MW is 449.2 g/mol for cyanidin-3-glucoside; L = path length in cm; Eta = 26 900 molar extinction coefficient, in L mol–1 cm–1, for cyanidin-3-glucoside, m = weight of sample.
Respiration rate
Respiration of samples was analyzed periodically in a closed and hermetic system. The samples (five gram per container) were randomly distributed in 50 mL plastic containers with a needle inserted through a septum fixed at the center of the lid and kept at ambient temperature (27 ± 2 °C). The needle was connected to CO2/O2 gas analyzer (PBI Dansensor Gas Analyzer, Checkmate II, Denmark).The CO2 percentage was used for calculation of the respiration rate (mL kg−1 h−1), using the following equation:
Firmness
Fruit firmness (N) was determined by measuring the compression force (at 0.05 mm/s) of the berries with spherical probe (0.25S) using texture analyzer (TA-XTplus, SMS, UK) with 5 kg load cell.
Pectin methyl esterase activity
The procedure for extraction and assay of PME was adapted from Lohani et al. (2004). The reaction mixture contained 1 mL pectin solution (0.01%, pH 7.5), 0.2 mL NaCl (0.15 M), 0.1 mL bromothymol blue solution (0.01%), 0.2 mL water and 0.1 mL of fruit extract. Absorbance was measured immediately at 620 nm and again measured after 3 min. The difference in initial absorbance and absorbance after 3 min was used as measure of PME activity, expressed as μmol g−1 min−1 (fresh fruit weight).
Statistical analysis
The experiment was conducted in a completely randomized design (CRD) with three replications. All the analyses were carried out in triplicates and the standard deviation (SD) was calculated. Data analyses were performed by analysis of variance (ANOVA) using SPSS statistical software (version 16.0). Multiple comparisons among the treatments with significant differences tested with ANOVA were conducted by using least significant difference (LSD) at p < 0.05 level. Tukey’s posthoc analysis was used to compare the difference among mean values at different storage intervals.
Results and discussion
Physiological loss in weight (PLW)
PLW is a major physiological indicator indicating postharvest quality of a commodity. Higher perishability of phalsa is due to its greater physiological loss in weight. The intensity of weight loss was more in control (2.01% per day) compared to edible coated fruits because of rapid moisture loss during storage period (Table 1). PLW of control fruits reached 14.11% on 7th day of storage, so even under refrigerated conditions, the berries remained acceptable barely upto 3–4 days. These findings corroborate with those of Dave et al. (2016). Lamo et al. (2020) have also reported PLW of 22.97% after 4 days of refrigerated storage of phalsa. Edible coating materials made up of polysaccharides, protein derivatives could act as moisture barriers and thus help in reduction of weight loss of the treated fruits. Amongst the conventional coatings, lowest PLW was recorded for T3 (guar gum-CaCl2) with an average PLW of 1.67% per day. On similar lines, application of guar gum at 0.4% has shown reduced weight loss in blackberries (64% reduction) compared to control on day 6 (Ascencio-Arteaga et al. 2022).
Amongst the LBL coatings, significantly lower PLW of 9.33% was observed in T5 coating on 7th day of storage. Dave et al. (2016) also applied SPI based coating on phalsa berries, using different variables like olive oil, HPMC in different concentrations to check the efficacy of protein based composite coating. They found an optimum weight loss of 7.06% on 4th day of storage which increased further till 8th day. In our study, locally available sesame oil was included in the coating. The rate of increase of PLW was 1.5% per day while 10% PLW was attained in 7.5 days of storage. Thus, it can be considered that there was shelf life extension of two days upon coating with LBL coating T5. The addition of oil or any wax based material to the protein based films helps in modification of barrier properties against moisture loss. Our coatings with SPI-CMC-Oil (T5) showed lower weight loss (6.17%) on 5th day of storage. Thus, an optimized combination of SPI-CMC-sesame oil could help in attaining a constant and lower weight loss in coated phalsa berries upto 8 days.
The other LBL coating (T4: Sodium alginate-Pectin) recorded 10.05% loss in weight upon 7 days storage. The 10% PLW cutoff was realized on ~ 7th day of refrigerated storage indicating > 24 h increase in shelf life over control. It is worth considering that even a days’ extension of shelf life is appreciable for transporting and storing such delicate tropical berries. Reduction in the rate of PLW of LBL coatings might be due to the increased gel strength, better coverage, uniform spreading over the fruits and the water vapour permeability of coating materials which are different with respect to their structure and composition (Chiabrando and Giacalone 2016) enabling varied interaction with cell wall constituents.
Respiration Rate
The effect of different coatings on respiration rate of phalsa fruits stored at refrigerated condition (4 ± 2 °C) is shown in Fig. 1a. Coated fruits showed decreased rate of respiration during storage period compared to control fruits (988.06 µg/kg/h) on day 7. Lower respiration rate during storage is supported with prolonged shelf life of chitosan coated Japanese plum cv. Santa Rosa owing to partial blockage of pores present in peel of plum fruits (Kumar et al. 2017).
While conventional coatings could lower the respiration rate upto 1–2%, LBL coatings lowered the respiration rates upto 5.72% after 7 days of storage. The lowest respiration rate was recorded for T5 (931.5 µg/kg/h) followed by T4 and T3. It is presumed that edible coating helped to modify the internal atmosphere of the phalsa fruits which significantly delayed the respiration rate. This decrease was directly proportional to the percent of physiological loss in weight. The interactive effect of polysaccharide and protein based coatings on respiration rate is related to their functional ability to create a barrier for oxygen diffusion through the coating (Ramírez et al. 2013). Ruelas-Chacon et al. (2017) have also reported reduced respiration rate of coated Roma tomatoes with gum arabic, alginate or zein and hydroxyl propyl methyl cellulose (HPMC) upon storage.
Total soluble solids (TSS)
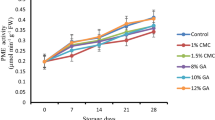
The total soluble solids of phalsa fruits were showing significant variations during storage. An increasing pattern of TSS could be observed in coated and uncoated berries. The significant increase in TSS content of control fruits (23.15 °B) might be attributed not only to rapid breakdown of starch during storage period but also due to desiccation (Table 2). According to Nandane and Jain (2011), increased TSS content might be due to the degradation of the cell wall polysaccharides during ripening and postharvest storage and is directly correlated with hydrolytic changes resulting in starch breakdown and depletion of organic acids as respiratory substrates during ripening in postharvest period. Among coated fruits, lesser increase in TSS was recorded during storage. Similar observations have been made for coated blueberries during storage (Rodríguez et al. 2020) where TSS content is correlated with hydrolytic changes in polysaccharides (hemicelluloses and pectin) during ripening and storage. This clearly indicates the slower metabolic process and starch breakdown. Among conventional coatings, on 7th day, T3 berries showed significant higher TSS (17.95 °B) followed by aloe vera and ascorbic acid coated berries (17.7 °B: T2). The application of aloe vera in combination with aonla juice or ascorbic acid, which is natural antioxidant, might have resulted in decreased respiration rates that slowed down the synthesis and use of metabolites resulting in lower TSS (Saleem et al. 2021). Among the LBL coatings, the lowest TSS was recorded for T5 (16.95 °B). Higher efficacy of T5 could be due to the hydrophobic nature and colloidal interactions between the coating materials especially with carboxy methyl cellulose, which is a widely used biopolymer known for its binding, gel strength and interlocking capacity in addition with soy protein isolate which has excellent antioxidant properties thus acting as a natural preservative. de Oliveira et al. (2021) have also recorded effectiveness of SPI-CMC coatings for formation of effective edible coatings for retaining soluble solids in fruits during storage.
Titratable acidity (TA)
Significant decrease in titratable acidity was recorded with storage duration in all treatments without much difference. Application of different coating materials helped in preventing faster degradation of organic acids, as organic acids’ breakdown occurred at a slower rate compared to control fruits. It is noteworthy that coatings exerted their effect even on the first day of storage, with significant differences among control, conventionally- and LBL coated berries. (Table 2). The rate of decrease in acidity was highest for control berries (@ 0.07% per day) while T4 and T5 LBL coated berries exhibited decrease @0.026 and 0.023% per day, respectively. Decrease in acidity was also found less in case of aloe vera-aonla juice coated berries (T1) i.e. 0.026% per day and it was at par with LBL coatings. The effect of aonla juice phenolics was evident with better retention in quality of aloe vera-aonla juice coated fruits as well as aloe vera-ascorbic acid coated fruits. Aonla juice and ascorbic acid are the functional components playing a major role in prolonging the shelf life of phalsa berries. T3 coating based on guar gum was found least effective with increase @0.028% per day.
Findings for acidity of berries are in line with lower rate of respiration. This decrease might be associated with physiological activities such as the breakdown of complex polysaccharides and decomposition of sugars during postharvest storage period. The amount of organic acids present in the fruit may be expected as a result of metabolic changes in fruit. A slight decreased TA in all coated fruits during storage period has been reported since coatings reduce the rate of respiration and therefore delay the utilization of organic acids (Ascencio-Arteaga et al. 2022). Retention of TA has also been reported for blackberries coated with aloe vera-based coatings (Ramirez et al. 2015) and cassava starch based coatings (Rodriguez et al. 2020).
Ascorbic acid
Ascorbic acid available in fruits is one of the most abundant antioxidant, helps to scavenge free radicals. Phalsa berries are known to be a good source of ascorbic acid. Table 2 represents the effect of coatings on ascorbic acid content of phalsa fruits in comparison with control. Irrespective of the treatments, an increase followed by decrease in ascorbic acid content has observed. The initial increases in ascorbic acid content may be attributed to synthesis of ascorbic acid from monosaccharides, since in plants most synthesis starts with preformed d-glucose through non-inversion pathway because of respiration activity during storage (Nunes et al. 1998). Better retention of ascorbic acid found in coated fruits over control can be clearly observed. All coated fruits have showed a higher retention of ascorbic content of the phalsa fruits due to slower degradation of organic acids. The initial ascorbic acid content of the phalsa fruit was 3.85 mg/100 g. After 9 days of storage, T2 samples showed significantly higher amounts of ascorbic acid (18.8%) compared to control (6.25 mg/100 g) owing to utilization of organic acids in the respiration process (Nandane and Jain 2011). Similar results have been reported in strawberries by Hazarika and Marak (2022). Among the varied coatings, T2 maintained higher level ascorbic acid during the storage period. The effect could be due to antioxidant properties of aloe vera and ascorbic acid which may help in reduction of oxygen diffusion inside the fruits and reduced respiration thus protecting the ascorbic acid content from deteriorative oxidation reactions (Ramirez et al. 2015). Thus, the coating of fruits effectively helped to preserve the ascorbic acid content during storage.
Reducing sugars
Decrease in reducing sugar content of coated and uncoated phalsa fruits was observed during storage upto 7th day (Fig. 2a). Considering 10% PLW as benchmark, on day 5 lowest reducing sugar content was observed in control fruits (12.35 mg/100 g). On this day among conventional coatings, T3 showed higher reducing sugars (15 g/100 g). T5 LBL coating demonstrated relatively better performance over T4 in deceleration of reduction in reducing sugars. The reducing sugars level was higher (16.35 mg/100 g) in T5 and (15.95 mg/100 g) in T4 on day 5. The level of reducing sugars on day 9 for T5 was equivalent to that on day 7 of control berries. Rate of decrease of reducing sugars in T5 berries was 2 mg/day compared to 2.4 mg per day in control berries, depicting enhanced shelf life.
Such decrease is mainly due to conversion of reducing sugar to other metabolites. Dave et al. (2016) stated that reducing sugars content was found lower in control phalsa on 4th day of storage. This clearly shows that increased rate of respiration in control phalsa had resulted in faster utilization of sugar compared to coated fruits.
Anthocyanins
During storage period, both coated and control fruits showed a significant increase in anthocyanin content. A gradual initial increase in the anthocyanin concentration was followed by a spurt in pigmentation of berries until they became very dark purplish color at the end of storage (Fig. 2b). This might be due to the biochemical reactions resulted in synthesis of anthocyanins especially in control or untreated fruits which showed maximum colouration (556.82 mg/L) compared to other edible coated phalsa fruits. Dave et al. (2016) also found that the anthocyanin content of phalsa fruits showed significant increase during storage period since the development of anthocyanin content is linked with changes in color of fruits at different stages of ripening. Thus, a gradual increasing pattern of color development in phalsa fruits shows the process in ripening while higher concentration could also be due to desiccation during storage (Ali et al. 2019). From Fig. 2b, it can be seen that control fruits’ pigmentation reached peak on day 5 followed by plateau. In contrast, T2 and T5 berries showed much lower rate of increase over other coatings till day 9. The suppression of colour development in coated fruits might be due to their barrier properties which could strongly affect the anthocyanin synthesis by creating modified internal atmosphere in fruits. This in turn may retard the biochemical reactions leading to anthocyanin synthesis (Ramirez et al. 2015). Gol et al. (2013) has also reported that the chitosan coated strawberries synthesized anthocyanins at a slower rate. The lower rate of colour development however did not altogether suppress pigmentation of berries which maintained their red colour (Fig. 3).
Pectin methyl esterase
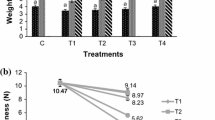
Phalsa berries showed a significant increase in PME activity initially with high spurt till day 7 followed by decrease in later days (Fig. 1b). While all coated fruits maintained lower PME activity than control fruits during storage, on day 7 PME activity of T4 and T5 was 34.7 and 43% lower than control fruits, respectively. Efficacy of guar gum-CaCl2 (T3) was also found appreciable in maintaining lower activity of PME (40% lower) at par with sodium alginate-pectin (T4). In contrast, the aloe vera based T1 and T2 coatings were not found as effective as other coatings. Kumar et al. (2017) found that coating treatments in plum fruits led to about 82–86% reduction in pectin methylesterase (PME) activity over control fruits. The probable reason could be the ability of protein based films/coatings (Soy protein, whey protein and wheat gluten) to act as barrier for oxygen due to their mechanical properties (Hassan et al. 2018). Effectiveness of SPI-CMC films has been reported previously by several authors (de Oliveira et al. 2021) and Gol et al. 2013 for strawberries) during storage.
Firmness
One of the deciding quality parameters for berries includes firmness, which was found to be improved significantly by the application of edible coatings. The increase in firmness of phalsa fruits during storage (Fig. 1c) is in contrast with other climactic fruits where rapid loss of firmness occurs at ripening and development stage. Firmness of phalsa berries was found to increase until 5 days followed by decrease. This firming up was accompanied by detachment of pulp from the seeds as evidenced upon consumption. Increase in firmness was much higher in control berries compared to coated ones. Similar behaviour has been attributed in blueberries to thickening of the parenchymal cell walls, corrugations of cell walls, increased skin toughness as well as drying and firming in berries (Paniagua et al. 2013).
Significant delay in firming was observed on the fruits coated with polysaccharide based coating materials like CMC, sodium alginate, guar gum, pectin on the 3rd day of storage compared to control. However during extended storage period, higher firmness was recorded for coated berries. This could be due to formation of thicker layer of gum coating on berries. Following 7th day, firmness decreased more rapidly for aloe vera coated berries (T1 and T2) compared to others. Firmness was found significantly higher for T5 (640.02 kg/cm2) followed by T4 (592.74 kg/cm2) and T3 (590.01 kg/cm2) compared to control fruits (326.06 kg/cm2) on 9th day of refrigerated storage. Higher effectiveness of T5 coating could be due to functional properties and integrity associated with SPI and CMC. SPI-based films are generally considered to have good oxygen barrier and tensile strength (Su et al. 2010) and hence may help to slow down the metabolic processes in the fruits to prevent textural loss during storage. Totad et al. (2019) showed similar effect of CMC coating on Misty Blueberry, with 22% higher firmness over control during storage. Li et al. (2017) showed similar results in edible coated strawberry where they showed effectiveness of polysaccharide based edible coating on soft berry like fruits in delaying tissue softening and reducing loss of textural integrity while preserving firmness during storage.
Conclusion
This study clearly demonstrates that layer-by-layer edible coating of phalsa berries could extend their shelf life upto 8 days with acceptable textural and physiological quality while control fruits could be stored for 6 days even upon refrigeration. Among conventional coatings, guar gum-calcium cloride coating showed higher acceptance compared to aloe vera based coatings. The phalsa berries coated with soy protein isolate-CMC-sesame oil exhibited effective retention of quality, with lower PLW loss, higher sweetness and firmness. Since phalsa is an under exploited fruit crop, there is a huge scope for harnessing its natural, bioactive components like antioxidants and pigments with suitable postharvest handling practices and processing techniques.
Data availability
All data generated or analyzed during this study are included in this published article however details may be shared by Corresponding author upon reasonable request.
References
Ali S, Khan AS, Malik AU, Anjum MA, Nawaz A, Shah HM (2019) Modified atmosphere packaging delays enzymatic browning and maintains quality of harvested litchi fruit during low temperature storage. Sci Hortic 254:14–20
Arnon H, Zaitsev Y, Porat R, Poverenov E (2014) Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest Biol Technol 87:21–26
Ascencio-Arteaga A, Luna-Suárez S, Cárdenas-Valdovinos JG, Oregel-Zamudio E, Oyoque-Salcedo G, Ceja-Díaz JA, Angoa-Pérez MV, Mena-Violante HG (2022) Shelf life of blackberry fruits (Rubus fruticosus) with edible coatings based on candelilla wax and guar gum. Horticulturae 8(7):574
Braich AK, Kaur G, Singh A, Dar BN (2022) Amla essential oil-based nano-coatings of amla fruit: Analysis of morphological, physiochemical, enzymatic parameters, and shelf-life extension. J Food Process Preserv 46(6):e16498
Chiabrando V, Giacalone G (2016) Effect of chitosan and sodium alginate edible coatings on the postharvest quality of fresh-cut nectarines during storage. Fruits 71(2):79–85
Dave R, Rao TR, Nandane AS (2016) RSM-based optimization of edible-coating formulations for preserving postharvest quality and enhancing storability of phalsa (Grewia asiatica L.). J Food Process Preserv 40(3):509–520
de Oliveira MM, de Souza SK, Mauro MA (2021) Evaluation of interactions between carboxymethylcellulose and soy protein isolate and their effects on the preparation and characterization of composite edible films. Food Biophys 16(2):214–228
Gol NB, Patel PR, Rao TR (2013) Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol Technol 85:185–195
Hassan B, Chatha SAS, Hussain AI, Zia KM, Akhtar N (2018) Recent advances on polysaccharides, lipids and protein based edible films and coatings: a review. Int J Biol Macromol 109:1095–1107
Hazarika TK, Marak T (2022) Salicylic acid and oxalic acid in enhancing the quality and extending the shelf life of grape cv. Thompson seedless. Food Sci Technol Int 28(6):463–475
Kumar P, Sethi S, Sharma RR, Srivastav M, Varghese E (2017) Effect of chitosan coating on postharvest life and quality of plum during storage at low temperature. Sci Hortic 226:104–109
Lamo K, Bhat DJ, Bakshi P, Wali VK (2020) Exogenously applied plant growth regulators enhanced the growth, yield, quality and shelf life of phalsa. J Pharmacogn Phytochem 9(3):640–646
Li L, Sun J, Gao H, Shen Y, Li C, Yi P, Tang Y (2017) Effects of polysaccharide-based edible coatings on quality and antioxidant enzyme system of strawberry during cold storage. Int J Polymer Sci 2017:9746174. https://doi.org/10.1155/2017/9746174
Lohani S, Trivedi PK, Nath P (2004) Changes in activities of cell wall hydrolases during ethylene-induced ripening in banana: effect of 1-MCP, ABA and IAA. Postharvest Biol Technol 31(2):119–126
Nandane AS, Jain RK (2011) Effect of composite edible coating on physicochemical properties of tomatoes stored at ambient conditions. Int J Adv Eng Technol 2:211–217
Nunes MCN, Brecht JK, Morais AMMB, Sargent SA (1998) Controlling temperature and water loss to maintain ascorbic acid levels in strawberries during postharvest handling. J Food Sci 63:1033–1036
Paniagua AC, East AR, Hindmrsh JP, Heyes JA (2013) Moisture loss is the major cause of firmness change during postharvest storage of blueberry. Postharvest Biol Technol 79:13–19
Poverenov E, Danino S, Horev B, Granit R, Vinokur Y, Rodov V (2014) Layer-by-layer electrostatic deposition of edible coating on fresh cut melon model: anticipated and unexpected effects of alginate–chitosan combination. Food Bioprocess Technol 7(5):1424–1432
Qamar M, Akhtar S, Ismail T, Sestil P, Tawab A, Ahmed N (2020) Anticancer and anti-inflammatory perspectives of Pakistan’s indigenous berry Grewia asiatica Linn (Phalsa). J Berry Res 10(1):115–131
Ramirez JD, Aristizábal ID, Restrepo JI (2013) Blackberry conservation through the application of edible coating of aloe vera mucilaginous gel. Vitae Rev Fac Química Farm 20:172–183
Ramirez ME, Timón ML, Petrón MJ, Andrés AI (2015) Effect of chitosan, pectin and sodium caseinate edible coatings on shelf life of fresh-cut Prunus persica var nectarine. J Food Process Preserv 39(6):2687–2697
Ranganna S (1986) Handbook of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Education, New York
Ray A, Bala K (2016) Effects of storage conditions on sensory attributes of phalsa fruit (Grewia asiatica) of variety sharbati. In: International conference on recent innovation in sciences, management, education and technology, pp 675–680
Ray AB, Bala K (2019) Bioactive compounds and health benefits of phalsa: an underutilized fruit. Food bioactives. Apple Academic Press, Palm Bay, pp 109–128
Rodriguez MC, Yépez CV, González JH, Ortega-Toro R (2020) Effect of a multifunctional edible coating based on cassava starch on the shelf life of Andean blackberry. Heliyon 6(5):e03974
Rudra SG, Singh S, Bollinedi H, Singh KN, Nain L, Singh S, Awasthi OP (2022) Anthocyanin-rich fruit vinegar from Grewia and Cantaloupe fruit blends. Int J Food Sci Technol 57(7):4566–4574
Ruelas-Chacon X, Contreras-Esquivel JC, Montañez J, Aguilera-Carbo AF, Reyes-Vega ML, Peralta-Rodríguez RD, Sanchéz-Brambila G (2017) Guar gum as an edible coating for enhancing shelf-life and improving postharvest quality of roma tomato (Solanum lycopersicum L.). J Food Qual 2017:8608304. https://doi.org/10.1155/2017/8608304
Saha A, Tyagi S, Gupta RK, Tyagi YK (2017) Natural gums of plant origin as edible coatings for food industry applications. Crit Rev Biotechnol 37(8):959–973
Saleem MS, Anjum MA, Naz S, Ali S, Hussain S, Azam M, Ejaz S (2021) Incorporation of ascorbic acid in chitosan-based edible coating improves postharvest quality and storability of strawberry fruits. Int J Biol Macromol 189:160–169
Sinha J, Purwar S, Chauhan SK, Rai G (2015) Nutritional and medicinal potential of Grewia subinaequalis DC. (syn. G. asiatica.) phalsa. J Med Plants Res 9(19):594–612
Sowmyashree A, Sharma RR, Rudra SG, Grover M (2021) Layer-by-Layer coating of hydrocolloids and mixed plant extract reduces fruit decay and improves postharvest life of nectarine fruits during cold storage. Acta Physiol Plant 43(8):1–10
Su JF, Huang Z, Yuan XY, Wang XY, Li M (2010) Structure and properties of carboxymethyl cellulose/soy protein isolate blend edible films crosslinked by Maillard reactions. Carbohydr Polym 79:145–153
Totad MG, Sharma RR, Sethi S, Verma MK (2019) Effect of edible coatings on ‘Misty’ blueberry (Vaccinium corymbosum) fruits stored at low temperature. Acta Physiol Plant 41(12):1–7
Yan J, Luo Z, Ban Z, Lu H, Li D, Yang D, Li L (2019) The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest Biol Technol 147:29–38
Acknowledgements
The authors are thankful to Post Graduate School, IARI for providing fellowship to Sindhu C. We also acknowledge the help of Director, ICAR-CIAH, Bikaner for providing facilities and material for research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no financial or non-financial interests related to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chinnaswamy, S., Rudra, S.G., Reddy, V.R. et al. Shelf life extension of Grewia berries using layer-by-layer edible coatings. Vegetos 36, 1326–1336 (2023). https://doi.org/10.1007/s42535-022-00527-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-022-00527-8