Abstract
Recent studies indicated new perspectives on the morphology and architecture of the quinoa root system, its intraspecific diversity and plasticity in response to drought. This study therefore aimed to evaluate wherether promoted development of root system due to the plasticity triggered by drought stress would contribute to increased growth, and yield of quinoa. The experiment was conducted with randomized complete block design with 6 replications. The main plots were Green (G1) and Red (G2) varieties and sub-plots were three soil moisture contents (SMC, w/w): 30% (W1) as control, 20% SMC (W2), and 15% SMC (W3). The results showed that the growth of varieties was significantly affected by the different soil water regimes. The root traits such as total root length, total nodal root length, total lateral root length, and nodal root numbers under drought treatments (W2 and W3) were significantly higher as compared with those under control. Furthermore, the root plasticity was expressed in both G1, G2 varieties, which resulted in significantly increased water use, shoot dry matter, and consequently increased yield and yield components. In addition, the positive and significant relationships were observed among measured traits (total root length and water uptake, water uptake and shoot dry weight, and shoot dry weight and yield) of two varieties under different water regimes. These results proved that in both varieties, root plasticity was triggered by drought, which enhanced root systems development contributing to increased water uptake, shoot dry weight and yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The negative impacts of climate change on livelihoods and food systems across the world aggravate the effects of multiple stress factors faced by millions of predominantly farming households. Changes in rainfall patterns, leading to pronounced drought, is the most serious factor influencing crop production (Rajsekhar and Gorelick, 2017; Siddiqui et al., 2021) and overall threatening food security (Hussain and Sulaimon 2018; Iqbal et al., 2018; Iizumi and Wagai, 2019). Therefore, exploiting drought tolerance in crops is a way to contribute to that stability and has become an important target for plant production in the near future.
Quinoa (Chenopodium quinoa Willd.) is of interest in a number of countries, because of its nutritional value and also because it is also a very highly drought and salt stress tolerant (Jacobsen et al. 2003). Quinoa is a South American crop plant whose seed has become an extremely popular food product in the last 40 years, particularly in Europe, North America, Southern Africa, South America (centre of origin) and Asia. FAO (2013) selected quinoa as one of the crops destined to offer food security in the twenty-first century. Quinoa has been known with excellent nutritive traits such as high energy, high quality and gluten-free protein i.e., quinoa (González et al., 2015). Besides, quinoa was proved to adapt to various abiotic stresses (Jacobsen et al., 2003; Razzaghi et al., 2012; Cocozza et al., 2013; Iqbal et al., 2018; Hinojosa et al., 2018) including drought (Vacher, 1998; Fghire et al., 2013; Sun et al., 2014; Zurita-Silva et al., 2015; Iqbal et al., 2018; Aziz et al., 2018; Hinojosa et al., 2018) and saline stress (Prado et al., 2000; Wilson et al., 2002; Gómez-Pando et al., 2010; Ruiz-Carrasco et al., 2011; Adolf et al., 2012; Eisa et al., 2012; Shabala et al., 2013; Arshadullah et al., 2016). Quinoa represents an invaluable opportunity, both as a potential crop in consideration of present and future climate change challenges, and as an important source of genes with biotechnological applications (Sun et al. 2014). This species was also found to be an outstanding model for the study of intra and interspecific variation in growth and development patterns along with the response of shoots and root architecture to water deficit (Fry et al., 2018).
Root system plays an important role in water and mineral absorption, and in the synthesis of various essential compounds for shoot growth. Roots are known to have high plasticity and this allows them to adjust to environmental changes (York et al., 2016). In upland crops such as bread wheat (Ehdaie et al., 2012), cassava (Kengkanna et al., 2019), maize (Nakamoto, 1993; Gao and Lynch, 2016), millet (Rostamza et al., 2013), soybean (Ye et al., 2018), and tomato (Ekanayake Midmore, 1992), the plasticity in deep rooting under progressive drought is also of tremendous advantage for maintaining growth and yield. In quinoa, although many studies focused on shoot growth responses of quinoa to drought stresses (Fghire et al., 2013; Zurita-Silva et al., 2015; Aziz et al., 2018) and root growth response to drought was reported by Alvarez Flores et al. (2018) which suggested almost null information on roles of quinoa root plasticity under water regimes. Thus, the current study therefore aimed to evaluate wherether promoted development of root system due to the plasticity triggered by drought stress would contribute to increased growth, and yield of quinoa.
Materials and methods
Plant materials
Two quinoa varieties with Chilean origin as Red (G1) and Green (G2) were used in this study.
Experimental design and treatments
The experiment was conducted under semi-controlled conditions in a greenhouse at the Faculty of Agronomy, Vietnam National University of Agriculture (latitude 20o 60′N, longitude 105o 56′W, altitude ~ 20 m.a.s.l.) from March to July 2019 (dry season, 11h42′ to 13h19′ in day length and 8.6–18.9 MJ/m2/day in daily solar radiation).
The seeds of each variety were soaked in water. Three pre-germinated seeds of each variety were sown in plastic pots (200 mm in bottom diameter, 300 mm in top diameter, and 200 mm in height) with 5 kg dried paddy soil (sandy clay-loam) that was passed through a 3-mm sieve and mixed thoroughly with compound fertilizer (90 kg N + 60 kg P2O5 + 60 kg K2O per ha (Hoang et al., 2015) at the rate of 50 mg N per kg soil with 20% soil moisture content (SMC, w/w) of plastic pot. The experiments were arranged in the Randomized Complete Block Design (RCBD) with six replications (1 pot was used as1 replication). The seedlings were thinned to one plants per pots at the 4–5 leaf stage. Three soil moisture conditions (SMC) were as follows: Well-watered (30% SMC (w/w), W1) as control; Normal-watered (20% SMC, W2); limited-watered (15% SMC, W3) as drought. For well-watered condition, the plant was continuously grown to harvest stage. In the W2 treatment, continuously kept SMC in pot at 20% SMC during experiment. In the W3 (severe drought), drought was imposed by drying the soil from 20 to 15% SMC (w/w) (W3). After that, water was added in every 2 days to set the target SMC.
Each plastic pot was weighed daily by electronic balance OHAUS V71P15T to determine the amount of water lost, which was replenished according to the target SMC and recorded as evapotranspiration. Nine plastic pots without plants representing each water treatment were used to measure the amount of evaporation from the soil. Whole plant transpiration was calculated as the difference between evapotranspiration from each pot with plant and the average evaporation of nine pots without plant. At the milk stage, half of the plants in each treatment (three pots) were sampled for measurement; the remaining plants were grown until at the end of experiment.
Measurements
Growth traits including growth duration, germination rate, plant height (PH), stem diameter (SD) and number of branches (NoB) on main stem were measured at the milk stage and harvest stage. PH was the height of the main plant from the surface of the soil pot to the top of the main stem. SD was measured at 2 cm height. We used axillary bud as thresholds for counting NoB. Shoot was determined after drying samples at 80 °C for three days until constant weight was observed. At harvest, seed yields of each variety were estimated. SPAD index was calculated using SPAD (Konica-Minolta 502, Japan). Leaf area was calculated by weighing method.
Total water uptake (WU) was calculated by weighing the amount of water lost in pots holding plants by electronic balance OHAUS V71P15T and then subtracting the amount of water lost from the pot without plant in each formula and further added the amount of water equal to the amount of water loss in each pot.
For total root length (TRL): each root sample was cut into approximately 1-cm segments and spread on a transparent plastic sheet with minimal overlapping. Digital tiff files were taken using an image scanner (EPSON Expression V700 photo). Scanned images were analyzed for root length using WinRhizo PRO 2012 (Regent Instrument Inc.) (Bouma et al., 2000). Total root length (TRL) was estimated as the total length of roots with less than 0.3 mm in diameter. Total nodal root length (TNRL) was computed as the difference between the total root length and total nodal root length (Yamauchi et al., 1996). Number of nodal roots was counted as the number of nodal roots on the main root.
The plasticity in nodal root number and TLRN, which was expressed under drought conditions was calculated using single replicates from the drought treatment and mean values from the control (WW) treatment as follows:
Root plasticity=\(\left[\frac{\mathrm{XSMF }-\overline{\mathrm{ X}}\mathrm{WW} }{\overline{\mathrm{X}}\mathrm{WW} }\right]\)
where X is the number of nodal roots number, TLRN.
Data analysis
We used a two-way analysis of variance (ANOVA) to compare the average values within treatments among varieties, and for the variety by treatment interaction. LSD (Least significant difference) test was used to find the means that were significantly different from each other. Correlations regression analysis was also calculated to understand the relationships among measured traits. Data were analyzed using the IRRISTAT 5.0 software.
Results
Responses in growth of two quinoa varieties under different soil moisture conditions
Two quiona varieties possessed similar germination rate at different soil moisture conditions. The total growth duration of quinoa varieties ranged from 90 to 96 days after sowing. Drought conditions prolonged the growth period of quinoa varieties but not significantly (Table 1).
The plant height of quinoa increased gradually over the follow-ups. There were no significant difference between two quinoa varieties but the plant height of both varieties were significantly higher in W2 and W3. At harvest stage, the plant height was significantly highest in G1W3 as compared with another treatments (Table 2).
The number of branches of two quinoa varieties increased gradually during the growth stage. At harvest stage, G2W2 treatment was significantly highest in the number of branches, followed by G1W3 treatment (Table 3).
The stem diameters of two quinoa varieties were not significantly different among soil moisture conditions and the stem diameter ranged from 1.06 cm to 1.42 cm (Table 4).
Root system development responses
The root system developed under drought water treatment was always longer than that under well water treatment. The root system developed of G2 variety was always longer than that on G1 variety at all stages of growth stage but not significantly different (Table 5). The root traits such as total root length (TRL), total nodal root length (TNRL), total lateral root length (TLRL), and nodal root numbers (NRN) under drought treatments (W2 and W3) were significantly higher as compared with those under control.
The different varieties combined with different soil moisture conditions affected the root system development. At all growth stage, G1W3 and G2W2 had a significantly higher in TRL, TNRL, TLRL, NRN than those of another treatments (Table 5).
Responses in physiological characteristics
There were not significant difference of SPAD index between two quinoa varieties at milk and harvest stages. At milk stage, drought treatments increased the SPAD index as compared to control condition (W2). Nevertheless, there were no significantly different in SPAD index among soil moisture conditions at harvest stage (Table 6).
Varieties interactions with SMC affected the SPAD index of quinoa. The period milk at G2W2 gave significantly highest SPAD index and the lowest was at G2W1 during all stages. The SPAD index decreased from milk stage to harvest stage in both G1 and G2 variety (Table 6).
At milk stage, there were not significant difference in leaf area among all SMC and quinoa varieties. But, drought treatments (W2, W3) increased the leaf area of both varieties as compared with control treatment (W1) (Table 6).
There were no significant difference in SDW between two quinoa varieties under milk stage. But, the SDW of G1 variety was significantly higher than that of G2 variety at harvest stage. The SDW under drought water treatments were always significantly higher than that under well-water treatment at all stages. The SDW of G1W3 was significantly highest, followed by G2W2 at harvest stage (Table 7).
Total water uptake of G2 variety was significantly higher than that of G1 variety in all two sampling periods. Total water uptake under drought water treatments (W2, W3) were significantly higher than that under well water treatment. Total water uptake under W2 treatment was significantly highest, and the lowest under W1. Total water uptake increased from W1 to W2 then decreased in W3 (Table 7).
Under different SMC combined with different varieties affected the total water uptake of the two quinoa varieties. Decreased SMC, increased the total water uptake of the quinoa varieties. The total water uptake in the drought water treatments were always higher than that in the well-watered treatment in all two sampling periods of both G1 and G2 varieties. At harvest stage, G2W2 treatment was highest in water uptake but there was no significant difference with G1W3, G2W3 and G1W2 (Table 7).
Responses in yield and yield components
There were no significant difference in P1000 seed between the two varieties under different soil moisture conditions. The individual yield of G2W2 showed the highest value but no significant difference with G1W3 treatment. Individual yield of drought treatments were higher than those of control treatment (Table 8).
Plasticity in nodal root number and total lateral root length
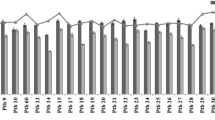
Under drought water treatments (W2, W3), both quinoa varieties (G1 and G2) showed root plasticity in nodal root number. At all growth stages, the G2 variety under W2 condition showed the highest plasticity in nodal root number, followed by G1W3 treatment and the lowest plasticity in nodal root number was shown in G1 variety under W2 condition. Under W3 condition, the plasticity in nodal root number of G1 variety was always higher than G2 variety in all stages while under W2 condition, the plasticity in nodal root number of G2 variety was higher as compared to G1 variety (Fig. 1).
The root plasticity in lateral root length was expressed in both G1 and G2 varieties. At all stages, the G2 variety showed higher plasticity of lateral root length than that of G1 variety under drought water treatment (W2, W3). At all stages, the G2 variety under W3 condition showed the highest plasticity in lateral root length, followed by G2W2 treatment. The plasticity in lateral root length of G1 variety under W2 condition was lowest (Fig. 2).
The results of correlation regression analysis
There were a positive and significant correlation between TRL and WU in both quinoa varieties and soil moisture conditions. The TRL was increased, then the total water uptake was also increased in both of G1 and G2 varieties (Fig. 3).
The same trends was found in the correlations between WU and SDW, SDW and yield in both quinoa varieties and soil moisture conditions (Fig. 4, 5).
From these correlation, we seen under SMC different the TRL is increasing, the yield of quinoa is also increasing in both of G1 and G2 varieties.
Discussion
Quinoa growth was significantly reduced by stress effects (Iqbal et al., 2018; González et al., 2011). Stikic et al. (2015) pointed out that drought stress significantly reduced leaf area, leaf, stem diameter and root dry weight, but was not significant for plant height. Yang et al. (2016) also stated that quinoa´s shoot biomass and plant height were decreased under deficit irrigation conditions. Our results of G2 varieties are in harmony with the result of previous studies which highlighted the reductions in plant height, number of branches, number of leaves, shoot diameter, shoot dry weight and seed yield (Table 1–8). However, quinoa is regarded as a drought tolerant crop as G1 showed better growth performances under W3 as compared to those under W2. Interestingly, the growth traits of both varieties showed the lowest values under W1 as compared to other water regimes. These results in current study agreed with González et al. (2009) who suggested that the several negative effects of excessive water on plant and root dry weights, chlorophyll contents; and high amounts of soluble sugars and starch.
Roots are the first organ to sense moisture changes and water stress plays a direct role by changing root system architecture (Fry et al., 2018; Gupta et al., 2020). Plasticity in deep root system development has been observed under progressive drought conditions (Nakamoto, 1993; Ehdaie et al., 2012; Rostamza et al., 2013; Suralta et al. 2016) as the plasticity in deep rooting under progressive drought is also of tremendous advantage for maintaining growth and yield (Ekanayake and Midmore, 1992; Comas et al. 2013). Quinoa´s drought tolerance is based at least partially on the high plasticity of its root system. Alvarez-Flores et al. (2018) detected that quinoa changed root architecture under water deficit through faster elongation along with abundant and longer external root´s branching improving their foraging capacity compared to Chenopodium hircinum, quinoa´s wild ancestor. Gámez et al. (2019) proposed that greater root development and a better stomatal opening adjustment could help in maintaining seed yield of Rainbow quinoa under drought stress. In our study, variation in root plasticity of quinoa plant were also found under W2 and W3 as compared to W1 (Figs. 1, 2).
Higher plasticity in root development is important for promoting water uptake (WU) (Palta et al., 2011). The results in this study indicated a positive and significant correlation between TRL and WU in both quinoa varieties and soil moisture conditions (Fig. 3). The TRL was increased, then the total water uptake was also increase in both of G1 and G2 varieties. These results suggest that by a combined analysis of root growth and its association with water can be used for more mechanistic understanding of factors involved in quinoa root responses to drought.
In conclusion, we found the root plasticity was expressed under water-deficit conditions, which resulted in significantly increased WU, SDW, and consequently increased yield and yield components. In addition, the positive and significant relationships were observed among measured traits (TRL and WU, WU and SDW, and SDW and yield) of two varieties under different water regimes. These results prove that in both varieties, root plasticity was triggered by drought, which enhanced root systems development contributing to increased water uptake, shoot dry weight and yield.
References
Adolf VI, Shabala S, Andersen MN, Razzaghi F, Jacobsen SE (2012) Varietal differences of quinoa’s tolerance to saline conditions. Plant Soil 357:117–129
Alvarez-Flores R, Nguyen-Thi-Truc A, Peredo-Parada S, Joffre R, Winkel T (2018) Rooting plasticity in wild and cultivated Andean Chenopodium species under soil water deficit. Plant Soil 425:479–492
Arshadullah M, Suhaib M, Usama M, Zaman BU, Mahmood IA, Hyder SI (2016) Effect of salinity on growth of Chenopodium quinoa Wild. Int J Res Agr Forest 3:21–24
Aziz A, Akram NA, Ashraf M (2018) Influence of natural and synthetic vitamin C (ascorbic acid) on primary and secondary metabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol Biochem 123:192–203
Bouma TJ, Nielsen KL, Koutstaal B (2000) Sample preparation and scanning protocol for computerised analysis of root length and diameter. Plant Soil 218:185–196
Cocozza C, Pulvento C, Lavini A, Riccardi M, d’Andria R, Tognetti R (2013) Effects of increasing salinity stress and decreasing water availability on eco-physiological traits of quinoa grown in a Mediterranean-type agro-ecosystem. J Agron Crop Sci 199:229–240
Comas LH, Becker SR, Cruz VM, Byrne PF, Dierig DA (2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4:442
Ehdaie B, Layne AP, Waines JG (2012) Root system plasticity to drought influences grain yield in bread wheat. Euphytica 186:219–232
Ekanayake IJ, Midmore DJ (1992) Genotypic variation for root pulling resistance in potato and its relationship with yield under water-deficit stress. Euphytica 61:43–53
FAO (Food and Agriculture organization) (2013). Home-international year of quinoa 2013. http://www.fao.org/quinoa-2013/en/. Accessed 26 Dec 2013.
Fghire R, Ali OI, Anaya F, Benlhabib O, Jacobsen SE, Wahbi S (2013) Protective antioxidant enzyme activities are affected by drought in quinoa (Chenopodium quinoa Willd). J Biol Agr Health 3:62–68
Fry EL, Evans AL, Sturrock CJ, Bullock JM, Bardgett RD (2018) Root architecture governs plasticity in response to drought. Plant Soil 433:189–200
Gao Y, Lynch JP (2016) Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J Exp Bot 67:4545–4557
González JA, Gallardo M, Hilal M, Rosa M, Prado FE (2009b) Physiological responses of quinoa (Chenopodium quinoa Willd.) to drought and waterlogging stresses: dry matter partitioning. Bot Stud 50:35–42
González JA, Bruno M, Valoy M, Prado FE (2011) Genotypic variation of gas exchange parameters and leaf stable carbon and nitrogen isotopes in ten quinoa cultivars grown under drought. J Agron Crop Sci 197:81–93
González JA, Eisa SSS, Hussin SAES, Prado FE (2015) Quinoa: An Inca Crop to Face Global Changes in Agriculture. In: Murphy K, Matanguihan J (eds) Quinoa: Improvement and Sustainable Production. John Wiley Sons Inc, Hoboken, NJ, USA, pp 1–18
González JA, Gallardo M, Hilal M, Rosa M, Prado FE (2009a). Physiological responses of quinoa (Chenopodium quinoa Willd.) to drought and waterlogging stress: Dry matter partitioning. Bot. Stud. 2009a, 50, 35–42.
Gupta A, Rico-Medina A, Caño-Delgado AI (2020) The physiology of plant responses to drought. Sci 368(6488):266–269
Hinojosa L, González JA, Barrios-Masias FH, Fuentes F, Murphy KM (2018) Quinoa abiotic stress responses: a review. Plants 7:106
Hoang DT, Canh NT, Long NV (2015). Effect of Nitrogen on Growth and Yield of Quinoa Accession. J Sci Devel 13 (2): 173–182 (in Vietnamese, English Abstracts).
Hussain AO, Sulaimon HOA (2018) Comparative study of food security in Africa amid growing population. Int J Sci Res Publ 8(10):6–12
Iizumi T, Wagai R (2019) Leveraging drought risk reduction for sustainable food, soil and climate via soil organic carbon sequestration. Sci Rep 9:19744
Iqbal H, Yaninga C, Wakasa M, Shareef M (2018) Differential response of quinoa varieties to drought and foliage-applied H2O2 in relation to oxidative damage, osmotic adjustment and antioxidant capacity. Ecotox Environ Saf 164:344–354
Jacobsen SE, Mujica A, Jensen CR (2003) The resistance of quinoa (Chenopodium quinoa Willd.) to adverse abiotic factors. Food Rev Inter 19:99–109
Kengkanna J, Jakaew P, Amawan S, Busener N, Bucksch A, Saengwilai P (2019) Phenotypic variation of cassava root traits and their responses to drought. Appl Plant Sci 7:e1238
Nakamoto T (1993) Effect of soil water content on the gravitropic behavior of nodal roots in maize. Plant Soil 152:261–267
Palta JA, Chen X, Milroy SP, Rebetzke GJ, Dreccer MF, Watt M (2011) Large root systems: are they useful in adapting wheat to dry environments? Funct Plant Biol 38:347–354
Prado FE, Boero C, Gallardo M, González JA (2000) Effect of NaCl on germination, growth, and soluble sugar content in Chenopodium quinoa Willd seeds. Bot Bullet Acad Sinica 41:27–34
Rajsekhar D, Gorelick SM (2017) Increasing drought in Jordan: Climate change and cascading Syrian land-use impacts on reducing transboundary flow. Sci Adv 3(8):e1700581
Razzaghi F, Plauborg F, Jacobsen S-E, Jensen CR, Andersen MN (2012) Effect of nitrogen and water availability of three soil types on yield, radiation use efficiency and evapotranspiration in field-grown quinoa. Agr Water Manag 109:20–29
Rostamza M, Richards RA, Watt M (2013) Response of millet and sorghum to a varying water supply around the primary and nodal roots. Ann Bot 112:439–446
Shabala S, Hariada Y, Jacobsen SE (2013) Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. J Plant Physiol 10:906–914
Siddiqui MdN, Léon J, Naz AA, Ballvora A (2021) Genetic and genomics of root system variation in adaption to drought stress in cereal crops. J Exp Bot 72:1007–1019
Stikic R, Jovanovic Z, Marjanovic M, Dordevic S (2015). The effect of drought on water regime and growth of quinoa (Chenopodium quinoa Willd.) Ratar i povrt 52: 80–84
Sun Y, Liu F, Bendevis M, Shabala S, Jacobsen SE (2014) Sensitivity of two quinoa (Chenopodium quinoa Willd.) varieties to progressive drought stress. J Agron Crop Sci 200:12–23
Vacher JJ (1998) Responses of two main Andean crops, quinoa (Chenopodium quinoa Willd.) and papa amarga (Solanum juzepczukii Buk.) to drought on the Bolivian Altiplano: significance of local adaptation. Agr Ecosys Environ 68:99–108
Yamauchi A, Pardales JrJR, Kono Y (1996). Root system structure and its relation to stress tolerance. In O. Ito, Katayama K, Johansen C, Kumar Rao JV DK,. Adu-Gyamfi JJ, Rego TJ (Eds.), Roots and nitrogen in cropping systems of the semi-arid tropics (pp. 211–234). Tsukuba: Japan International Research Center for Agriculture Sciences.
Yang A, Akhtar SS, Amjad M, Iqbal S, Jacobsen SE (2016) Growth and physiological responses of quinoa to drought and temperature stress. J Agron Crop Sci 202:445–453
Ye H, Roorkiwal M, Valliyodan B, Zhou L, Chen P, Varshney RK, Nguyen HT (2018) Genetic diversity of root system architecture in response to drought stress in grain legumes. J Exp Bot 69:3267–3277
York LM, Carminati A, Mooney SJ, Ritz K, Bennett MJ (2016) The holistic rhizosphere: integrating zones, processes, and semantics in the soil influenced by roots. J Exp Bot 67:3629–3643
Zurita-Silva A, Jacobsen SE, Razzaghi F, Alvarez-Flores R, Ruiz K, Morales A, Silva H (2015). Quinoa drought responses and adaptation. In: Bazile D, Bertero D, Nieto C (Eds.), State of the art report on quinoa around the world in 2013, FAO CIRAD, Roma (2015), pp. 157–171.
Author information
Authors and Affiliations
Contributions
NTND and NVL designed the study. NHH collected and analyzed the data. All authors contributed to manuscript writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, T.N.D., Nguyen, H.H. & Nguyen, V.L. Roles of root plasticity to growth, water uptake and yield of quinoa under water regimes. Vegetos 36, 980–988 (2023). https://doi.org/10.1007/s42535-022-00495-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-022-00495-z