Abstract
An efficient protocol for micropropagation of Crotalaria longipes Wight & Arn., an endemic and endangered Indian legume, was developed. Nodal explants cultured on Murashige and Skoog (MS) supplemented with Thidiazuron (TDZ) 1.0 mg L−1 and NAA 0.5 mg L−1 showed maximum shoot induction. The multiplied shoots were subsequently sub-cultured every 3 weeks on the same medium. The separated microshoots cultured on half-strength MS basal salts supplemented with 0.5 mg L−1 indole-3-butyric acid (IBA) showed maximum root induction. After 4 weeks of culture, the rooted shoots (> 6 cm) were planted in pots containing coco peat and garden soil (1:1) covered with plastic domes and maintained at 25 ºC for 2 weeks. After 2 weeks, the potted plants were transferred to a glasshouse, maintained at 25 °C with a relative humidity of 80%. The acclimatized plants were then successfully established in soil with an 80% survival rate. Clonal fidelity assessment of acclimatized plantlets was confirmed using inter simple sequence repeat (ISSR). All the in vitro developed plants showed monomorphic banding pattern similar to the mother plant, thus ascertaining the true-to-type nature of the in vitro raised plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The genus Crotalaria (Fabaceae), is the largest genus of legumes in India with 97 species (Sanjappa 2001). Crotalaria longipes is a herbaceous plant endemic to Andhra Pradesh and Tamilnadu. This species is listed under the threatened category (Nayar and Sastry 1987). Jayanthi (2012) reported that the distribution of C. longipes is restricted only in Kolli hills, Southern Eastern Ghats of Tamil Nadu. However, our field studies in various areas of Nilgiris, the Southern Western Ghats; and Kolli and Shevaroys hills of Southern Eastern Ghats of Tamil Nadu revealed the occurrence of Crotalaria longipes. However, the distribution is very scarce, invariably in all the areas surveyed, which is mainly due to deforestation activities. Plant tissue culture techniques provide a suitable alternative for mass multiplication of this threatened species. A protocol for the in vitro propagation through multiple shoot induction in Crotalaria longipes is not currently available. Ex situ conservation of threatened plant species using plant tissue culture techniques may lead to show somaclonal variation (Ilezuk and Jacygrad 2016; Larkin and Scowcroft 1981), especially while conserving them for a longer duration under in vitro condition. Screening of in vitro developed cultures is, therefore, necessary to remove any mutated plants to maintain the genetic integrity of the germplasm. Polymerase chain reaction (PCR) based deoxyribonucleic acid (DNA) markers, especially ISSR are more preferable for confirming genetic integrity of the micropropagated plants (Mao et al. 2018; Rani and Raina 2000) because it is cost-effective, technically simple, and does not require sequence information of the template DNA (Kumar et al. 2009). The applications of ISSR markers to ascertain the genetic fidelity of the in vitro developed clones is evident from different plant systems viz., Psidium guajava L. (Kamle et al. 2014), Albizia procera (Mohammad et al.2016), Cornus alba L. (Ilczuk and Jacygrad 2016), Rhododendron wattii (Mao et al. 2018). Therefore, in the present study, ISSR markers were used to ascertain the genetic integrity of the in vitro developed seedlings of C.longipes and to ensure mass multiplication of genetically stable true-to-type plants of this species before being introduced in their natural habitat. The present study was initiated firstly to develop a suitable protocol for the rapid micropropagation of Crotalaria longipes and secondly to ascertain the genetic fidelity of the acclimated plants using ISSR markers.
Material and methods
Plant material and culture initiation
Plant material for tissue culture was obtained from the wild plants of Crotalaria longipes (Fig. 1a) grown in the garden premises of National Orchidarium and Experimental Garden, Botanical Survey of India, Southern Regional Centre, Yeraud, Salem District, Tamilnadu, India.
In vitro propagation Crotalaria longipes (a) C. longipes growing in its natural habitat with opened flower shown as insert. b Multiple shoot induction from nodal explants. c In vitro rooting after 7 wk of culture on MS medium containing 0.5 mg L−1 IBA. d 8-wk-old plantlets grown in polythene bags for acclimation. e Genetic fidelity analysis using ISSR 834 and (f) ISSR 836. Lane M represents 1 kb ladder, Lane MP represents mother plant and Lanes 1—11represents randomly chosen acclimated plants of C. longipes
For culture initiation, young vegetative shoots measuring 30–45 cm in length were collected and thoroughly washed under running tap water for 10 min to remove any adhering surface dirt particles. The nodal segments of 2–3 cm, excised from the vegetative shoots, were rinsed in 1.0% (v/v) Dettol (Reckitt Benckiser, India Ltd.) for 7 min and washed thoroughly in distilled water. The nodal segments were then sterilized with 10% (v/v) sodium hypochlorite (4% concentrated) solution containing 1 mL of Tween 20 per 100 mL for 10 min. After three washes in sterile distilled water, the final sterilization was carried out using 0.1% (w/v) mercuric chloride for 3 min with subsequent washing thrice in sterile distilled water. The nodal explants were trimmed and inoculated onto MS (Murashige and Skoog 1962) medium. Cytokinins viz., thidiazuran (TDZ), 6-benzylaminopurine (BAP), and kinetin (Kn) (0.5–5.0 mg L−1) and auxin, α-naphthalene acetic acid (NAA) (0.5 mg L−1) were used for initial shoot induction (Table 1). After three weeks, the nodal explants with initial axillary shoot induction were transferred to fresh MS medium supplemented with the different combinations of cytokinin and an auxin (Table 1) for shoot elongation. Data for regeneration frequency, shoot number, and shoot length were recorded, four weeks after continuous culture.
In all the experiments, the nutritional media contained MS basal salts, vitamins, 30 g/l sucrose, 0.8% (w/v) agar and supplemented plant growth regulators. The pH was adjusted to 5.8 with 1 N NaOH and 1 N HCl before autoclaving at 121 ℃ at 110 kPa for 20 min.
All macronutrients, micronutrients, vitamins, hormones, sucrose, agar and other chemicals used in the present study were obtained from Himedia Laboratories Pvt. Ltd. Mumbai, India. All the cultures were maintained at 25 ± 2 ℃ with a 16/8 h light/dark cycle. The light intensity was 40 µmol m−2 s−1 provided by the cool white fluorescent tubes (Philips 36 W, Philips, India).
In vitro rooting
The regenerated shoots, 3–4 cm in length, were dissected and cultured on half-strength MS basal salts supplemented with auxins, viz., IBA or NAA or IAA, at different concentrations (0.5–3.0 mg L−1) (Table 2) for root induction. The regenerated shoots cultured on half-strength MS basal salts without auxin served as the control treatment. Rooting percentage, number of roots per shoot, and root length were recorded after seven weeks of culture.
Plant acclimatization to ex vitro condition
The rooted plantlets after 7 week were removed from the culture and were washed thoroughly in distilled water to remove any adhered agar particles. The washed plantlets were placed in transparent plastic containers filled with an autoclaved mixture of garden soil, vermicompost, and sand (1:1:2) and covered with a polythene dome. The plastic containers with plantlets were maintained in the culture room at 25 ± 2 °C with a relative humidity of 75% and 35 µmol m−2 s−1 light intensity emitted from cool-white fluorescent tubes (36 W, Philips India Pvt. Ltd) in a 16-h photoperiod for 4 weeks. During the period of acclimation, the relative humidity was reduced gradually to 60% after 10 days from the beginning of the hardening process. The plantlets were transferred to a glasshouse without the polythene dome and watered every alternate day, for four weeks. The acclimatized plants were then transferred to the field after 8 weeks.
Genetic fidelity analysis of acclimated plants
The total genomic DNA was extracted from the leaf material (1 g) of both wild plants and 10-weeks-old in vitro raised Crotalaria longipes plantlets using the cetyltrimethyl ammonium bromide (CTAB) method (Doyle and Doyle 1987).
ISSR polymerase chain reaction
Ten ISSR primers (Priority life science, Coimbatore, Tamil Nadu, India) were selected for assessment of polymorphisms (Table 4). Polymerase chain reaction (PCR) was carried out using a DNA thermal cycler (Eppendorf®, Mastercycler™ nexus gradient, Hamburg,Germany) with the final reaction mixture volume of 25 μL contained 10 X PCR reaction buffer (500 mM Tris‒HCl, 160 mM (NH4)SO4 pH 9.2), 1.5 µl MgCl2 (2 mM), 0.5 µl dNTPs (10 mM each of dATP, dGTP, dTTP and dCTP), 1 µL primers, 0.3 µL of DNA Taq polymerase, 1 µL of 25 ng template DNA and sterile distilled water. PCR conditions used amplification consists of an initial denaturation step at 94 °C for 5 min; followed by 35 cycles of 1 min at 94 °C for denaturation, 1 min at 50 °C for annealing, 2 min at 72 °C for extension; and a final extension at 72 °C for 10 min. After amplification, the PCR products (10 µL) per lane were compared by 1.2% (w/v) agarose gel electrophoresis in 1X Tris–acetic acid–EDTA (TAE) buffer containing 0.25 µg/µL, along with 1Kbp DNA ladder (Himedia Pvt. Ltd., India) as size markers. The amplified PCR products were visualized under gel documentation system (Gelstan-1312 series, Medicare, India). ISSR- PCR reactions were analyzed in a binary data scored for presence (1) and absence (0) of banding patterns for each plantlet.
Data analysis
One-way analysis of variance (ANOVA) was used to evaluate the significance of the difference of means of data from various experiments using SPSS statistical software package (Trial version: 16). The values were presented as mean (± SD) and P < 0.05 is considered as significant. All the data pertaining to multiple shoot induction and root induction were taken after seven weeks of continuous culture in vitro.
Results and discussion
Multiple shoot induction
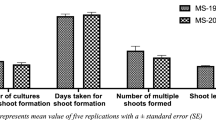
Shoot induction from the nodal explants of C. longipes was achieved on MS basal medium supplemented with various concentrations of three cytokinins (TDZ, BAP and KIN) in combination with an auxin (NAA). Regardless of the combinations of auxin and cytokinins used, the nodal explants showed axillary bud regeneration within 2 weeks after inoculation. Among the different combinations of TDZ (0.5 mg–5.0 mg L−1, Table 1) and NAA (0.5 mg L−1) tested, TDZ (1.0 mg L−1) + NAA (0.5 mg L−1) showed the maximum regeneration frequency (95%) and shoot number (28.6 ± 2.15 per nodal explant) (Fig. 1b). On the other hand, maximum shoot length (4.17 ± 0.55 cm) was attained with TDZ (2.0 mg L−1) + NAA (0.5 mg L−1). However, there was a decline in the regeneration frequency, shoot number and length as the TDZ concentration increased. This trend was invariably true in other PGR combinations viz., BAP + NAA and KIN + NAA. The synergistic effect of TDZ and NAA in promoting shoot induction was reported in Astragalus cariensis leaf explants (Erisen et al. 2011; Murthy et al. 1998). Further it is reported that TDZ when combined with other plant growth regulators showed positive impact on shoot induction as compared to TDZ alone (Huetteman and Preece 1993; Lincy and Sasikumar 2010). Thus the present study is in conformity on the synergistic effect of TDZ and NAA. In a similar trend, as that of TDZ + NAA, the positive effect of BAP in combination with NAA was observed on shoot induction in C. longipes. The maximum regeneration frequency (85%), mean shoot number (7.7 ± 2.05 per nodal explant) was achieved with BAP (1.0 mg L−1) + NAA (0.5 mg L−1). However, maximum shoot length (3.31 ± 0.33 cm) was achieved with BAP (2.0 mg L−1) + NAA (0.5 mg L−1). The positive effect of BAP in combination with NAA was reported in Astragalus cariensis (Erisen et al. 2010). Adlinge et al. (2014) also ascertained that BAP in combination with NAA showed a positive impact on the maximum shoot induction in Vigna mungo (L.) Hepper cv. Sarala. In a different set of experiment, the effect of different combinations of Kn + NAA (Table 1) on the maximum regeneration frequency, mean shoot number and mean shoot length was tested. The results showed a maximum regeneration frequency (70%), mean shoot number (5.5 ± 1.43 per nodal explants) and mean shoot length (1.72 ± 0.16 cm) with Kn (1.0 mg L−1) + NAA (0.5 mg L−1). Effect of kinetin on shoot induction was reported by earlier workers in other plant species viz., Caralluma bhupenderiana (Ugraiah et al. 2011), Lens culinaris (Singh and Raghuvanshi 1989; Williams and McHughen 1986), Crotalaria laburnifolia (Rajender et al. 2012). The present study, on the whole, revealed that the combination effect of TDZ + NAA on multiple shoot induction in nodal explants of C. longipes was found to be significantly higher, followed by BAP + NAA and Kn + NAA. Present study is in agreement with the earlier workers to ascertain that the low concentration of an auxin in combination with cytokinins can substantially improve the shoot induction frequency (Kaliamoorthy et al. 2008; Martin 2002, 2003; Sreekumar et al. 2000; Wotavova-Novotna et al. 2007).
In vitro rooting
Shoots more than 3–4 cm were transferred to half strength MS basal salts supplemented with IBA, IAA and NAA (Table 2) for rooting. Root induction was observed in all the treatments including the control. Invariably in all the treatments, the emergence of root primordia at the base of the shoot was observed after two weeks of culture. However, the shoots treated with 0.5 mg L−1 IBA resulted in the maximum rooting rate (90%), mean root number (12.9 ± 0.70 per shoot) and root length (4.26 ± 0.68 cm) (Fig. 1c). The positive effect of IBA on root induction was reported in Vigna mungo (Ignacimuthu et al. 1997), Cajanus cajan (Franklin et al. 2000a), Pisum sativum (Franklin et al. 2000b), Harpagophytum procumbens (Kaliamoorthy et al. 2008), Rhododendrons (Mao et al. 2011, 2018) Cicer microphyllum (Singh et al. 2019). The present study supports the results of earlier workers pertaining to the positive effect of IBA in C. longipes.
Acclimatization
Around 80% of the in vitro raised plants of C.longipes transferred from lab to greenhouse condition survived (Fig. 1d). The successfully acclimatized plants were then transferred to soil beds developed in the garden premises and maintained. A protocol for the in vitro propagation through multiple shoot induction in C. longipes is not available and therefore this is the first report on in vitro regeneration in C. longipes. This efficient and reproducible protocol can be used for in situ and ex situ germplasm conservation.
ISSR fingerprinting
Mass multiplication of an endemic and threatened plant through micropropagation method can be achieved. However, it may not be successful from the conservation point of view, unless genetic fidelity is maintained. During the course of in vitro propagation, especially when cultures are being stored in vitro for longer duration, the chances of genetic mutations are quite high (Rani and Raina 2000). In view of this, genetic integrity of the acclimatized plants of C.longipes was assessed using 10 ISSR primers (Table 3) and compared with a representative mother plant growing naturally in the garden premises of BSI, SRC, Yercaud, Salem District, Tamil Nadu, India. The results yielded a total of 37 amplified products generated from 10 ISSR primers with 10 acclimatized plants and a mother plant. The ISSR markers each generated an average of 3.7 monomorphic bands and their lengths varied from 100 to 2700 base pairs (Table 3) (Fig. 1e, f). The results of genetic fidelity assessment using ISSR markers revealed that all the bands produced by the acclimatized clones were monomorphic and were similar to that of the mother plant growing in the wild. The effective use of ISSR markers in clonal fidelity assessment of micropropagated plants is well documented in various studies (Mao et al. 2018; Teeluck et al. 2016).
Present study has provided a simple, efficient and reproducible protocol for a high frequency shoot regeneration from the nodal explants of Crotalaria longipes Wight & Arn., an endemic and threatened plant of south India was developed. This protocol could be effectively used for mass multiplication of this endemic and threatened plant species for re-introduction in the wild and also to maintain a germplasm in the botanical gardens.
References
Adlinge PM, Samal KC, Kumara Swamy RV, Rout GR (2014) Rapid in vitro plant regeneration of black gram (Vigna mungo L. Hepper) var. Sarala, an important legume crop. Proc Natl Acad Sci India Sect B Biol Sci 84:823–827. https://doi.org/10.1007/s40011-013-0281-8
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Erisen S, Yorgancilar M, Atalay E, Babaoglu M (2010) Prolific shoot regeneration of Astragalus cariensis Boiss. Plant Cell Tiss Org 100:229–233. https://doi.org/10.1007/s11240-009-9638-3
Erisen S, Atalay E, Yorgancilar M (2011) The effect of thidiazuron on the in vitro shoot development of endemic Astragalus cariensis in Turkey. Turk J Bot 35:521–526. https://doi.org/10.3906/bot-1009-74
Franklin G, Jeyachandran R, Ignicimuthu S (2000a) Factors affecting regeneration of pigeon pea (Cajanus cajan L. Millsp) from mature embryonal axes. Plant Grow Reg 30:31–36. https://doi.org/10.1023/A:1006394402210
Franklin G, Pius P, Ignacimuthu S (2000b) Factors affecting in vitro flowering and fruiting of green pea (Pisum sativum L.). Euphytica 115:65–74. https://doi.org/10.1023/A:1003982900117
Huetteman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss Org 33:105–119. https://doi.org/10.1007/BF01983223
Ignacimuthu S, Franklin G, Melchias G (1997) Multiple shoot formation and in vitro fruiting from cotyledonary nodes of Vigna mungo L. Hepper Curr Sci 73:733–735
Ilczuk A, Jacygrad E (2016) In vitro propagation and assessment of genetic stability of acclimated plantlets of Cornus alba L. using RAPD and ISSR markers. Vitro Cell Dev Biol Plant 52:379–390. https://doi.org/10.1007/s11627-016-9781-6
Jayanthi M (2012) Population ecology, threats, status and conservation of natural populations of Crotalaria longipes-an endangered plant. J Biodivers Environ Sci 2:1–9
Kaliamoorthy S, Naidoo G, Achar P (2008) Micropropagation of Harpagophytum procumbens. Biol Plant 52:191–194. https://doi.org/10.1007/s10535-008-0043-2
Kamle M, Kumar P, Bajpai A, Kalim S, Chandra R (2014) Assessment of genetic fidelity of somatic embryogenesis regenerated guava (Psidium guajava L.) plants using DNA-based markers. New Zeal J Crop Hort 42:1–9. https://doi.org/10.1080/01140671.2013.814574
Kumar P, Gupta VK, Misra AK, Modi DR, Pandey BK (2009) Potential of molecular markers in plant biotechnology. Plant Omics 2:141–162
Larkin PJ, Scowcroft WR (1981) Somaclonal variation a novel source of variability from cell cultures for plant improvement. Theor Appl Genet 60:197–214. https://doi.org/10.1007/BF02342540
Lincy A, Sasikumar B (2010) Enhanced adventitious shoot regeneration from aerial stem explants of ginger using TDZ and its histological studies. Turk J Bot 34:21–29. https://doi.org/10.3906/bot-0805-6
Mao AA, Kaliamoorthy S, Ranyaphi RA, Das J, Gupta S, Athili J, Yumnam JY, Chanu LI (2011) In vitro micropropagation of three rare, endangered, and endemic rhododendron species of Northeast India. In Vitro Cell Dev Biol Plant 47:674–681. https://doi.org/10.1007/s11627-011-9377-0
Mao AA, Vijayan D, Singha RKN, Pradhan S (2018) In vitro propagation of Rhododendron wattii Cowan—a critically endangered and endemic plant from India. In Vitro Cell Dev Biol Plant 54:45–53. https://doi.org/10.1007/s11627-017-9869-7
Martin K (2002) Rapid propagation of Holostema ada-kodien Schult. a rare medicinal plant, through axillary bud multiplication and indirect organogenesis. Plant Cell Rep 21:112–117. https://doi.org/10.1007/s00299-002-0483-7
Martin KP (2003) Rapid axillary bud proliferation and ex vitro rooting of Eupatorium triplinerve. Biol Plant 47:589–591. https://doi.org/10.1023/B:BIOP.0000041067.23890.58
Mohammad N, Vaishnav V, Mishra J, Mahesh S, Kumar P, Ansari SA (2016) Genetic fidelity testing in micropropagated plantlets of Albizia procera(Roxb.) Benth using RAPD and ISSR markers. Indian For 142:558–562
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–477. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. Vitro Cell Dev Biol Plant 34:267–275. https://doi.org/10.1007/BF02822732
Nayar MP, Sastry ARK (1987) Red data book of Indian plants, vol 1. Botanical Survey of India, Calcutta
Rajender K, Thirupathi M, Raju D, Jagmohan RK (2012) Micropropagation of Crotalaria laburnifolia, an ethnomedicinally important plant herbal species. J Phytol 4:14–16
Rani V, Raina SN (2000) Genetic fidelity of organized meristem derived micropropagated plants: a critical reappraisal. Vitro Cell Dev Biol Plant 36:319–330. https://doi.org/10.1007/s11627-000-0059-6
Sanjappa M (2001) Leguminosae. In: Singh NP, Singh DK (eds) Floristic diversity and conservation strategies in India, vol IV. Angiosperms (Selected Groups) and Ethnobotany. Botanical Survey of India, Calcutta, pp 1847–1902
Singh RK, Raghuvanshi SS (1989) Plantlet regeneration from nodal segment and shoot tip derived explants of lentil. Lens Newsl 16:33–35
Singh RK, Singh S, Anandhan S, Quiroz-Figueroa FR, Ruiz-May E (2019) An efficient protocol for in vitro propagation of the wild legume Cicer microphyllum, a crop wild relative of chick pea (Cicer arietinum) In Vitro Cell Dev Biol Plant 55:9. https://doi.org/10.1007/s11627-018-09958.y
Sreekumar S, Seeni S, Pushpangadan P (2000) Micropropagation of Hemidesmus indicus for cultivation and production of 2-hydroxy 4- methoxy benzaldehyde. Plant Cell Tissue Organ Cult 62:211–218. https://doi.org/10.1023/A:1006486817203
Teeluck JM, Kaudeer BF, Ramful M, Boodhram I, Sanmukhiya MR, Soulange JG (2016) Genetic fidelity of in vitro propagated breadfruit (Artocarpus altilis) using inter simple sequence repeat markers. Int J Agric Biol 18:911–916. https://doi.org/10.17957/IJAB/15.0185
Ugraiah A, Sreelatha VR, Krishna Reddy PV, Rajasekhar K, Sandhya Rani S, Karuppusamy S (2011) In vitro shoot multiplication and conservation of Caralluma bhupenderiana Sarkaria – an endangered medicinal plant from South India. Afr J Biotech 10:9328–9336. https://doi.org/10.5897/AJB10.2132
Williams DJ, McHughen A (1986) Plant regeneration of the legume Lens culinaris Medik (lentil) in vitro. Plant Cell Tissue Organ Cult 7:49–152. https://doi.org/10.1007/BF00043039
Wotavova-Novotna K, Vejsadova H, Kindlmann P (2007) Effects of sugars and growth regulators on in vitro growth of Dactylorhiza species. Biol Plant 5:198–200. https://doi.org/10.1007/s10535-007-0040-x
Acknowledgements
This research was part of the Lead Garden Project, funded by the Ministry of Environment, Forest and Climate Change (MoEF & CC), New Delhi, India. The authors are grateful to the Director, BSI, Kolkata for the constant encouragement and support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seventhilingam, K., Selvam, H. & Kalaivanan, B.V. Micropropagation and clonal fidelity assessment of acclimatized plantlets of Crotalaria longipes Wight & Arn. using ISSR markers. Vegetos 34, 325–331 (2021). https://doi.org/10.1007/s42535-021-00203-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-021-00203-3