Abstract
Pollen–pistil interaction is one of the widely studied cell–cell communication events in angiosperms. Pollination of the receptive pistil with compatible pollen and the subsequent steps that determines the success of fertilization depend on the cooperative events between pollen and pistil. Before the onset of these interactive events between pollen and pistil, both the participants prepare themselves for the upcoming complex cellular events. Receptive surface of stigma is characterized by the accumulation of reactive oxygen species and expression of specific biomolecules and enzymes, such as non-specific esterases and peroxidases. Pollen grains produce nitric oxide that scavenge reactive oxygen species generated on stigma surface after pollination and just prior to pollen germination. Present work reports accumulation of glycoproteins, lipids and phospholipids at the base of stigmatic papillae in mature receptive stigma. A 31 kDa glycoprotein has been detected in stigma homogenate. Stigma exhibit enhanced expression of peroxidase isoforms relative to buffer soluble fractions of pollen, i.e. intact pollen, internal pollen and tryphine fractions. A 54 kDa protease is expressed in pollen grains as well as stigma. Biochemical analyses of these biomolecules in pollen and stigma relative to other vegetative (corolla of ray and disc floret, bracts, young leaves) and reproductive (anther wall and ovary) parts highlight the implications of these biomolecules in pollen–stigma interaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pollen–pistil interaction is a fundamental process in the reproductive biology of angiosperms. The physical contact between male gametophyte (pollen) and receptive surface of pistil (stigma) and later stylar tissue and pollen tube guidance to embryo sac (female gametophyte) involves a complex series of cellular and molecular interactions (Shakya and Bhatla 2018). The initial contact between pollen and stigma following pollination determines the fate of sexual reproduction in sporophytic self-incompatible plants. However, in plants exhibiting gametophytic self-incompatibility the recognition of compatible pollen takes place during later stage when the pollen tube is traversing through the style. The ornate surface of pollen and stigma, and biomolecules associated with them are crucial for their diverse characters and functions (Edlund et al. 2004). Among members of family Asterceae, Senecio squalidus has been the most investigated genus to understand the events related to pollen–pistil interactions (McInnis et al. 2006; Allen et al. 2011). In the recent past, significant findings related to pollen–stigma interaction in sunflower have also been reported (Shakya and Bhatla 2010; Sharma and Bhatla 2013a; b; Sharma 2019).
The receptive surface of stigma may contain extracellular proteins in the form of pellicle (in case of dry stigma) or a heterogeneous mixture of biomolecules in the form of exudates in case of wet stigma (Shakya and Bhatla 2018). Sunflower has semi-dry type of stigma in which exudates are present at the base of papillae (Shakya and Bhatla 2010). An accumulation of reactive oxygen species (ROS/H2O2) on the surface of stigma and its subsequent scavenging post pollination is correlated with nitric oxide (NO) production by pollen (McInnis et al. 2006; Sharma and Bhatla 2013a). With attainment of stigma maturity there is an increase in expression of ROS scavenging enzymes, such as peroxidases (POD) and superoxide dismutase (SOD) (Sharma and Bhatla 2013b).
Complexity of pollen–stigma interaction indicates toward involvement of a plethora of biomolecules which are yet to be elucidated. In the present work, histochemical differences in terms of distribution of polysaccharides (glycoproteins), proteins, phospholipids between the young non-papillate stage and mature papillate stage of stigma has been done to highlight the biochemical features of mature receptive stigma. An attempt has been made to analyze some of the biomolecules, such as glycoproteins, POD and proteolytic enzymes in stigma and pollen to investigate their differential distribution, and discuss their possible roles prior to pollen germination. Different fractions of pollen grains, namely tryphine (pollen coat), internal pollen and intact pollen have been analyzed to ascertain the spatial significance of these biomolecules in context of post pollination interaction between pollen and stigma.
Materials and methods
Plant material
Sunflower seeds (variety Morden) were procured from National Seeds Corporation, Delhi. Crops were raised in the garden of Department of Botany, University of Delhi. Pollen grains were harvested immediately after anthesis. Stigma and pistil were harvested 1 day after anthesis, and other floral parts, such as corolla of disc floret, anther wall and ovary were harvested from the disc florets from which stigma was taken whereas corolla of ray floret, bracts and young leaves were taken from the same capitulum. After harvesting all the samples were stored in liquid nitrogen for subsequent biochemical analyses.
Preparation of pollen fractions
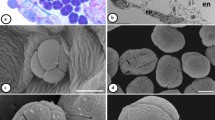
Experiments were carried out on intact pollen and two pollen fractions, namely tryphine and internal pollen, i.e. pollen grain without tryphine. Tryphine was isolated from the intact pollen grains, according to Doughty et al. (1993). Intact pollen grains (300 mg) were treated with 1 mL of cyclohexane in a microfuge tube and vortexed 2–3 times for 1 min. Following centrifugation at 5000g for 5 min at room temperature (RT), supernatant was collected and used as a source of tryphine. The pelleted pollen grains were used as ‘internal pollen fraction’. The supernatant was transferred to a dry beaker and the cyclohexane was evaporated by keeping the beaker in front of a blower at RT. After evaporation, the leftover matter containing tryphine was dissolved in a suitable buffer/solvent as per the specific experimental requirement. Before homogenizing the internal pollen fraction for various experiments, it was also air dried at RT to remove the traces of cyclohexane. Figure 1 depicts the schematic of pollen fractionation in to tryphine and internal pollen.
Schematic depiction of fractionation of pollen in to tryphine and internal pollen. a Scanning electron micrograph (SEM) of sunflower pollen. b Magnified view of pollen. c SEM of pollen after treatment with cyclohexane. d Magnified view of cyclohexane treated pollen (Shakya 2008)
Preparation for microscopy
Stigmas were excised from florets 1 day after anthesis, and fixed in formalin–acetic acid–alcohol (FAA; 10:5:50). Baker’s formalin-calcium mixture was used as a fixative for stabilizing the phospholipids and lipids. Following overnight fixation, samples were dehydrated through graded tertiary butyl alcohol (TBA) series (50, 70, 85, 95 and 100%) with changes every 1 h. Stigmas fixed in Baker’s formalin–calcium were first dehydrated in ethanol series (30%, 50% and 70%) with change every 1 h and then passed through graded TBA series. Stigmas were then infiltrated and embedded in paraffin wax. Stigmas fixed in Baker’s formalin-calcium fixative were treated with chloroform for 1 h before paraffin infiltration and embedding. Serial sections of stigmas (10 µm thick) were cut using a rotary microtome.
Histochemistry
Periodic Acid-Schiff’s (PAS) reaction was used for localizing all carbohydrates in the sections, according to Lyon (1991). Proteins were localized using mercuric bromophenol blue (BPB) stain and for localization of phospholipids orange G and aniline blue were used as described by Ruzin (1999). Sections subjected to PAS and BPB staining procedures were mounted in DPX whereas after staining for phospholipids they were mounted in euparol. Imaging of sections was undertaken using photomicroscope (Axioskop from Zeiss, Germany). Photographs were taken using AxioCam digital camera and analyzed using Axiovision software (Zeiss, Germany).
Preparation of tissue homogenates from stigma and other plant parts
Each tissue (1 g) was ground to fine powder in liquid nitrogen and homogenized using grinding medium [0.1 M Tris, pH 7.5, 0.4 M Sucrose, 10 mM potassium chloride (KCl), 1 mM magnesium sulphate (MgSO4), 1 mM ethylenediamine tetraacetic acid (EDTA), 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1% 2-mercaptoethanol]. The homogenate was filtered through four layers of muslin cloth and centrifuged at 10,000g for 30 min at 4 °C. Protein was acetone precipitated and pellet obtained after centrifugation for 20 min at 5000g at 4 °C was dissolved in a minimum volume of grinding medium. The dissolved protein was again centrifuged for 10 min at 5000g at 4 °C. The supernatant was used for in situ localization of glycoproteins, POD and proteases.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE analysis of polypeptides from total soluble proteins (TSP) was carried out according to Laemmli (1970). Proteins were resolved by vertical slab gel electrophoresis at a constant current of 25 mA. Gels were stained for protein bands with a solution of Coomassie Brilliant Blue R-250 (0.2% w/v) in methanol: acetic acid: water [4 (v/v):1 (v/v): 5 (v/v)].
Electrophoretic detection of glycoproteins
Carbohydrates with free 1, 2-glycol groups were detected electrophoretically in TSP using Schiff’s reagent, according to Dubray and Bezard (1982). Aliquots equivalent to 70 µg protein were resolved on 12.5% linear SDS PAGE at 25 mA for 5 h at 22 °C. After electrophoresis gel was soaked overnight in a solution containing 25% isopropyl alcohol and 10% acetic acid at RT. After transferring the gel to 7.5% acetic acid for 30 min, it was incubated in 0.2% periodic acid for 1 h at 4 °C. Gel was stained immediately by incubating in Schiff’s reagent (Molecular Biology Grade. SRL, Mumbai) at 4 °C for 1 h. Destaining of the gel was done by washing it with 7.5% acetic acid at RT. Pink coloured bands developed indicating glycoproteins.
Estimation of POD activity
POD activity was estimated spectrophotometrically following Alba et al. (1998). Tissue (1 g) was homogenized in 3 mL of 50 mM sodium acetate (pH 4.0) and filtered through 3-layered muslin cloth. The filtrate was centrifuged at 10,000g for 20 min at 4 °C and the supernatant was used as enzyme source. Aliquot equivalent to 100 µg of TSP was mixed with 2.4 mL of freshly prepared substrate solution (prepared by dissolving 0.6 mM o-dianisidine and 8.8 mM hydrogen peroxide in 50 mM sodium phosphate, pH 6.0). The reaction was monitored by following the absorbance changes at 460 nm during 1–8 min against substrate blank containing 2.4 mL of substrate solution mixed with 100 µl of 50 mM sodium acetate (pH 4.0). The extinction coefficient was assumed to be 11.3 mM.
Zymography for POD activity
Zymographic detection of POD isoforms was done using the protocol of Alba et al. (1998). Aliquots of each TSP equivalent to 100 µg protein were mixed with reducing Laemmli sample buffer and loaded on a 15–20% gradient gel. Electrophoresis was performed at 25 mA for 5 h at 7 °C. After electrophoresis, gel was incubated in 0.2 M sodium acetate buffer (pH 5.0) containing 1.3 mM benzidine and 1.3 mM H2O2 until brown bands showing POD isoforms appear.
Zymography for cytosolic proteases
In gel cytosolic protease activity was detected in stigma, pistil, corolla of the disc floret, anther wall, corolla of ray floret, ovary of ray floret, bracts and young leaves. For detection of proteolytic activity 0.1% gelatin was used as substrate as described by Heussen and Dowdle (1980). Aliquots from TSP equivalent to 100 μg of protein were mixed with non-reducing Laemmli sample buffer solution and incubated at 40 °C for 20 min before loading on the 7–14% SDS gel. Electrophoresis was performed at 25 mA for 5 h at 7 °C. After electrophoresis protein bands were renatured by incubating the gel for 40 min in 50 mM Tris buffer (pH 7.5) containing 2.5% Triton X-100. The gel was transferred to a developing buffer (50 mM Tris, pH 7.5 containing 0.1% 2-mercaptoethanol) in a water bath shaker for 2.5 h at 37 °C. Bands representing protease activity appeared as transparent zones after staining with 0.1% amido black dissolved in methanol: acetic acid: water [1.5 (v/v): 0.5 (v/v): 9.5 (v/v)].
Results
Accumulation of carbohydrates, proteins, lipids and phospholipids in stigma
Carbohydrates were localized in the papillate (mature) and non-papillate (young) regions of the stigma using PAS reaction. Carbohydrates accumulate in the intercellular spaces of transmitting tissues in both young non-papillate (Fig. 2a) and mature papillate stigmas (Fig. 2b). Cells lining the secretory canal (SC) also secrete carbohydrates which are released at the base of papillae during receptive period. Proteins are localized in abundance in papillate regions of mature stigma (Fig. 2d) as compared to young non-papillate region (Fig. 2c). Proteins stain intensely in papillae, transmitting tissue and vasculature. In the non-papillate region, phospholipids have been localized in the epidermis, transmitting tissue and vasculature (Fig. 2e). In the mature papillate stigma phospholipids have been localized mainly at the base of papillae (Fig. 2f).
Expression of glycoproteins is more pronounced in pollen
The tryphine fraction contains very little protein as compared to internal pollen fraction (Fig. 3a). Tryphine contains almost one-tenth of protein present in internal pollen. However, stigma contains almost twenty five times of proteins (25 mg g−1 fr. wt) present in intact pollen (Fig. 3b). The polypeptide profile reveals majority of polypeptides with molecular weight in the range of 20–70 kDa. The expression of three polypeptides 36, 42 and 86 kDa has been found to be specific to the internal pollen fraction. Tryphine did not show expression of any specific polypeptide. The 25 kDa polypeptide is over expressed in stigma as compared to pistil (Fig. 3c) indicating its abundance in stigma whereas the expression of 40 and 43 kDa polypeptides is slightly more in pistil indicating their abundance in style. The expression of 25 kDa is common between pollen fractions and pistil. Only two polypeptides 25 and 43 kDa have been found to be common between stigma and pollen.
a Total protein content in different fraction of pollen grains. b Comparison of TSP present in pollen and stigma. c SDS-PAGE profile. d Glycoprotein isoforms presents in tryphine (lane 1), internal pollen (lane 2), intact pollen (lane 3), stigma (lane 4), pistil (lane 5), corolla of disc floret (lane 6), corolla of ray floret (lane 7), bracts (lane 8) and young leaves (lane 9). M molecular weight marker
Glycoprotein profile showed expression of four glycoproteins with molecular weight in 12.5–33 kDa range. Pollen grains expressed all the four glycoproteins with molecular weight 12.5, 26.5, 31 and 33 kDa. These are expressed in both the fraction of pollen grains (Fig. 3d, Lane 1, 2). The expression of 31 kDa is high as compared to 12.5 and 26 kDa whereas 33 kDa is least expressed. Stigma and pistil, however, showed expression of 31 kDa glycoprotein only (Fig. 3d, Lane 4, 5). Both stigma and pistil samples were analyzed to investigate if there is some style specific glycoprotein expressed in pistil. In fact, the expression of 31 kDa glycoprotein has been shown to be common to pollen and stigma. Interestingly, glycoproteins have not been detected among other floral (corolla of disc floret and bracts) and vegetative (young leaves) parts except in corolla of ray floret where the same 31 kDa band appears (Fig. 3d, Lane-7).
Pollen and stigma differ in their POD isoform expression pattern
Stigma showed significant POD activity as compared to different pollen fractions. Among pollen fractions, tryphine showed almost twice the POD activity as in internal pollen. Specific POD activity in stigma has been found to be almost six times that of tryphine (Fig. 4a). Zymogram for POD showed expression of seven peroxidase isoforms in stigma (high expression of one isoform). Among pollen fractions, three isoforms expressed in internal pollen fraction whereas only one isoform expressed in intact pollen grains (Fig. 4b). Expression of only one isoform has been found to be common among stigma and pollen, with its higher expression in the internal pollen as compared to that in stigma. Interestingly, zymographic analysis did not detect any POD activity in tryphine. Non detection of in gel POD activity in tryphine may be due to use of different substrate (benzidine) as used for spectrophotometry method (o-dianisidine).
Cytosolic protease activity is more pronounced in stigma
Zymographic analysis of protease activity has shown the presence of a 54 kDa protease in stigma, pistil, corolla of disc floret, anther wall, corolla of ray floret and ovary (Fig. 5a, lanes 1–8). However, the expression of 54 kDa protease is more in stigma as compared to pistil and other floral (corolla of disc floret, corolla of ray floret) parts. Two additional proteases have also been expressed, one each in anther wall (Fig. 5a, lane 4) and ovary (Fig. 5a, lane 6). In ovary, an additional 92 kDa protease has also been detected whereas in anther wall two protease bands in the molecular weight range 55–54 kDa have been observed. It is important to note that the additional protease isoforms have been expressed only in reproductive tissues. In vegetative parts, namely bracts (Fig. 5a, lane 7) and young leaves (Fig. 5a, lane 8), a 55 kDa protease has been expressed. The activity of 54 kDa protease has been shown to be more or less similar in the pH range 6.0–8.5 (Fig. 5b). More basic (> 8.5) and acidic (< 6.0) conditions lead to reduction in the activity of 54 kDa protease. Protease activity could not be detected in different pollen fractions (data not shown).
a Zymographic detection of cytosolic protease activity in different parts of the sunflower (Lane 1: stigma, lane 2: pistil, lane 3: corolla of disc floret, lane 4: anther wall, lane 5: corolla of ray floret, lane 6: ovary of ray floret, lane 7: bracts, lane 8: young leaves). b Effect of pH on protease activity in stigma
Discussion
The receptive surface of stigma contains various extracellular components, such as lipids, proteins, glycoproteins, carbohydrates, amino acids and phenols (Shivanna 2003). The stigma of sunflower characterized as semi-dry type does not show apparent stigmatic secretions during receptive period (Shakya and Bhatla 2010) as compared to wet type, such as Lilium and Nicotiana (Heslop-Harrison and Shivanna 1977). At the base of the stigmatic papillae exudates are present which are secreted by the secretory cells lining the stylar canal and transmitting tissue which initially gets accumulated in intercellular spaces forming extracellular matrix (ECM). These secretions are released at the base of the papillae through discontinuities present in cuticle (Heslop-Harrison 2000). A comparative histochemical study carried out in present work has shown accumulation of carbohydrates, proteins and lipids in ECM of transmitting tissue and cells lining the cavity of stylar canal. These secretions at the base of the stigmatic papillae have been shown to rich in neutral lipids (Shakya and Bhatla 2010).
Glycoproteins have been shown to play an essential role during the pollen–stigma adhesion process. Several glycoproteins have been reported to be present in pollen grains (Aelst and Went 1992; Kimura et al. 2002; Suarez-Cervera et al. 2005). Consistent with the reports of the presence of a 31 kDa glycoprotein in the pollen grain of palm (Elaeis guineensis; Kimura et al. 2002), a similar glycoprotein has been reported from sunflower pollen grains. However, present work has also reported three additional glycoproteins (12.5, 26.5 and 33 kDa). Glycoproteins, namely S locus glycoprotein (SLG), S locus-related glycoprotein-1 (SLR1) and S locus-related glycoprotein-2 (SLR2), have been reported from stigma of Brassica oleracea (Luu et al. 1999; Takayama et al. 2000). SLG is an extremely polymorphic protein and has been investigated extensively for its role in self-incompatibility. It encodes a 55 kDa glycoprotein which is secreted into the cell wall of stigmatic papillae (Kandasamy et al. 1989). SLR1 glycoprotein has also been localized on the cell wall of stigmatic papillae and affects pollen adhesion in intraspecific, interspecific and intergeneric pollinations (Umbach et al. 1990; Luu et al. 1999). Present work has reported a 31 kDa glycoprotein in stigma of sunflower. However, its location in stigma is yet to be elucidated. SLR1 and SLG have been reported to interact with the pollen coat proteins (PCPs) in vitro (Doughty et al. 1993). A 7-kDa pollen coating-borne peptide in Brassica napus has been found to interact with S-locus glycoproteins and S-locus-related glycoprotein (Hiscock et al. 1995). Similarly, in Brassica oleracea an interaction between a non-glycosylated pollen coat protein (7 kDa) and S-locus-specific and perhaps S-locus-related glycoproteins, has also been observed (Takayama et al. 2000).
High level of POD activity is a marker for stigma receptivity. All types of stigma surface show high POD activity indicating their importance in stigma for participation in interaction with pollen. Contrary to esterase activity that shows no appreciable change with stigma development POD levels have been reported to be at peak during receptive stage (McInnis et al. 2006). In Senecio squalidus another member of Asteraceae, five POD isoforms have been reported in the stigmas. Consistent with previous reports quantitative differences have been observed in the expression of POD isoforms in stigma and pistil of sunflower (Bredemeijer and Blaas 1975). However, unlike previous reports, no qualitative changes in peroxidase isoforms have been observed in stigma and pistil (data not shown). Furthermore, the peroxidase activity of sunflower stigma is similar to Nicotiana tabacum i.e. expression of POD isoforms is more in stigma than in pistil (Bredmeijer and Blaas 1975). Stigma-specific peroxidase (SSP) having a molecular weight of 35 kDa, has been reported in the papillae of Senecio squalidus (McInnis et al. 2006). It has been localized in the cytoplasmic regions of the stigmatic papillae and on the surface of these cells, probably as one of the components of proteinaceous pellicle, which is a characteristic feature associated with dry type of stigmas.
POD activity has been reported in the pollen grains of several plant species (Poddubnaya-Arnoldi et al. 1961; Bredemeijer 1982). Concomitant with the previous reports, POD activity in the buffer soluble fractions of intact pollen, internal pollen and tryphine fractions was found to be negligible. The pollen grains of Oenothera organensis also do not exhibit any peroxidase activity (Lewis et al. 1967). It has been proposed that the function of pollen peroxidases may be related to the phenolic compounds present in the stigma and stigmatic exudates in case of wet type of stigmas (Bredemeijer 1982; 1984). Phenolic compounds, such as chlorogenic acid, caffeic acid and cinnamic acid, present in the stigmatic exudates, act as hydrogen donors for the POD activity. Moreover, phenolics present either on the stigma surface or in the stigmatic exudates, are thought to be involved in the stimulation or inhibition of IAA oxidase activity thereby influencing the growth processes.
Initial stages of pollen–stigma interaction, such as pollen adhesion, hydration and germination on stigma surface involve complex cellular dialogue between them. All these events require active participation of the various enzymatic complexes including proteases (Graff de et al. 2001; Swanson et al. 2004). Luu et al. (1997) have demonstrated that the proteolytic digestion of proteinaceous pellicle reversibly decreases pollen adhesion at its later stages, thereby suggesting the formation of complexes between pollen coat/walls proteins and pellicle, which facilitate hydration of compatible pollen. It has also been suggested that these treatments might result in the damage of proteins and enzymes present on the stigma surface, which are supposed to activate the pollen cutinase necessary for the degradation of cuticle (Radlowski 2005). Analyses pertaining to proteolytic activity have been done on the reproductive structures of plants, such as stigma (Verrisimo et al. 1996), style (Heimgartner et al. 1990; Ramalho-Santos et al. 1997), ovary (Chen and Foolad 1997) and pollen grains (Radlowski et al. 1994a, b, 1996; Bagarozzi et al. 1996, 1998; Grobe et al. 1999). Present work has reported the presence proteases in the floral parts such as, stigma, pistil, pollen grains, corolla of disc floret, bracts, ovary and anther wall. In addition, protease activity has also been reported in young leaves. Contrary to the reports of Verissimo et al. (1996), who have reported two proteases (46 and 48 kDa) from stigma of Cynara cardunculus, sunflower stigma has a single 54 kDa protease. This 54 kDa protease is also expressed in the pollen grains of sunflower, though the expression is very less as compared to stigma (data not shown). The nature of the sunflower protease is yet to be elucidated. Radlowski (1996) has reported a 60 kDa aspartic protease in maize pollen. In contrast to the reports of a 30 kDa protease in non-pollinated ovaries of Pisum sativum, two proteases having molecular weight 54 and 92 kDa have been reported from ovaries of sunflower.
Conclusions
In this study, accumulation of biomolecules associated with receptivity of stigma, such as carbohydrates (glycoproteins), proteins, lipids and phospholipids has been shown at the base of the stigmatic papillae of sunflower. The accumulation of such heterogenous mixture at the base of papillae is indicative of their putative involvement in pollen acceptance on surface of stigma, a characteristic feature of species exhibiting sporophytic self-incompatibility. Overexpression of glycoprotein isoforms in pollen fractions, specifically 12.5, 26 and 33 kDa (isoforms specific to pollen) in pollen coat may be related to the initial recognition event that occurs at pollen–stigma interface (attachment foot) where mixing/recognition between candidate molecules takes pace. High POD activity in stigma relative to pollen is correlated with its receptivity. This is in accordance with high esterase activity reported in stigma of sunflower earlier. Furthermore, expression of two specific POD isoforms in pollen coat can be implicated in initial recognition event. So far, structural and biochemical investigations on pollen–stigma interaction in sunflower have reported findings pertaining to spatial and temporal distribution of lipidic constituents and associated enzymes, stigma receptivity markers, flavonoids and proteolytic enzymes. In future, molecular approaches are expected to further investigate the key players involved in this initial recognition process that takes place on stigma surface preceding penetration by pollen tube.
References
Aelst ACV, Went JLV (1992) Ultrastructural and immuno-localization of pectins and glycoproteins in Arabidopsis thaliana pollen grains. Protoplasma 168:14–19
Alba CM, de Forchetti SM, Quesada MA, Valpuesta V, Tigier HA (1998) Localization and general properties of developing peach seed coat and endosperm peroxidase isoenzyme. Plant Growth Regul 17:7–11
Allen AM, Thorogood CJ, Hegarty MJ, Lexer C, Hiscock SJ (2011) Pollen–pistil interactions and self-incompatibility in the Asteraceae: new insights from studies of Senecio squalidus (Oxford ragwort). Ann bot 108:687–698
Bagarozzi DA, Pike R, Potempa J, Travis J (1996) Purification and characterization of a novel endopeptidase in ragweed (Ambrosia artemisiifolia) pollen. J Biol Chem 271:26227–26232
Bagarozzi DA, Potempa J, Travis J (1998) Purification and characterization of an arginine-specific peptidase from ragweed (Ambrosia artemisiifolia) pollen. Am J Respir Cell Mol Biol 18:363–369
Bredemeijer GMM, Blaas J (1975) A possible role of a stylar peroxidase gradient in the rejection of incompatible growing pollen tubes. Acta Bot Neerl 24:37–48
Bredemeijer GMM (1982) Pollen peroxidases. J Palynol 18:1–11
Bredemeijer GMM (1984) The role of peroxidases in pistil–pollen interactions. Theor Appl Genet 68:193–206
Chen F, Foolad MR (1997) Molecular organization of a gene in barley which encodes a protein similar to aspartic protease and its specific expression in nucellar cells during degeneration. Plant Mol Biol 35:821–831
de Graff BHJ, Derksen JWM, Mariani C (2001) Pollen and pistil in the progamic phase. Sex Plant Reprod 14:41–55
Doughty J, Hdderson F, Mc Cubbin A, Dickinson H (1993) Interaction between a coating borne peptide of the Brassica pollen grain and stigmatic S (self-incompatibility)-locus-specific glycoprotein. Proc Natl Acad Sci USA 90:467–471
Dubray G, Bezard G (1982) A highly sensitive periodic acid-silver stain for 1, 2-dio groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem 119:325–329
Edlund AF, Swanson R, Preuss D (2004) Pollen and stigma structure and function: the role of diversity in pollination. Plant Cell 16(Suppl):S84–S97
Grobe K, Becker WM, Schlaak M, Petersen A (1999) Grass group I allergens (β-expansins) are novel, papain-related proteinases. Eur J Biochem 263:33–40
Heslop-Harrison Y (2000) Control gates and micro-ecology: the pollen–stigma interaction in perspective. Ann Bot 85:5–13
Heslop-Harrison Y, Shivanna KR (1977) The receptive surface of the angiosperm stigma. Ann Bot 41:1233–1258
Heimgartner U, Pietrzak M, Geertsen R, Brodelius P, da Silva Figueiredo AC, Pais MSS (1990) Purification and partial characterization of milk clotting proteases from flowers of Cynara cardunculus. Phytochemistry 29:1405–1410
Heussen C, Dowdle EB (1980) Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem 102:196–202
Hiscock SJ, Doughty J, Willis AC, Dickinson HG (1995) A 7-kDa pollen coating-borne peptide from Brassica napus interacts with S-locus glycoproteins and S-locus-related glycoprotein. Planta 196:367–374
Kandasamy MK, Paolillo DJ, Faraday CD, Nasrallah JB, Nasrallah ME (1989) The S-locus specific glycoproteins of Brassica accumulate in the cell wall of developing stigma papillae. Dev Biol 134:462–472
Kimura Y, Maeda M, Kimura M, Lai OM, Tan SH, Hon SM, Chew FT (2002) Purification and characterization of a 31-kDa palm pollen glycoprotein (Ela g Bd 31 K), which is recognized by IgE from palm pollinosis patients. Biosci Biotechnol Biochem 66:820–827
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lewis D, Burrage S, Walls D (1967) Immunological reactions of single pollen grains, electrophoresis and enzymology of pollen protein exudates. J Exp Bot 18:371–378
Luu DT, Heizmann P, Dumas C (1997) Pollen–stigma adhesion in kale is not dependent on the self- (in) compatibility genotype. Plant Physiol 115:1221–1230
Luu DT, Marty-Mazars D, Trick M, Dumas C, Heizmann P (1999) Pollen–stigma adhesion in Brassica spp. involves SLG and SLR1 glycoproteins. Plant Cell 11:251–262
Lyon H (1991) Theory and strategy in histochemistry: a guide to the selection and understanding of techniques. Springer-Verlag, Berlin
McInnis SM, Emery DC, Porter R, Desikan R, Hancock JT, Hiscock SJ (2006) The role of stigma peroxidases in flowering plants: insights from further characterization of a stigma-specific peroxidase (SSP) from Senecio squalifus (Asteraceae). J Exp Bot 57:1846–1853
Poddubnaya-Arnoldi VA, Zinger NV, Petrovskaja TP, Polunina NN (1961) Histochemical study of the pollen grains and pollen tubes in the angiosperms. Rec Adv Bot 1:682–685
Radlowski M (2005) Proteolytic enzymes from generative organs of flowering plants (Angiospermae). J Appl Genet 46:247–257
Radlowski M, Kalinowski A, Królikowski Z, Bartkowiak S (1994a) Protease activity from maize pollen. Phytochemistry 35:853–856
Radlowski M, Kalinowski A, Siedlewska A, Adamczyk J, Królikowski Z, Bartkowiak S (1994b) The regulating activity of native protease in maize pollen grains. Flower Newsl 17:49–52
Radlowski M, Kalinowski A, Adamczyk J, Królikowski Z, Bartkowiak S (1996) Proteolytic activity in the maize pollen wall. Physiol Plant 98:172–178
Ramalho-Santos M, Pissarra J, Verissimo P, Pereira S, Salema R, Pires E, Faro CJ (1997) Cardosin A, an abundant aspartic proteinase, accumulates in protein storage vacuoles in the stigmatic papillae of Cynara cardunculus L. Planta 203:204–212
Ruzin SE (1999) Plant microtechnique and microscopy. Oxford University Press, New York
Shakya R (2008) Structural and biochemical analysis of pollen–stigma interaction in sunflower. Ph.D. thesis, Department of Botany, University of Delhi, India
Shakya R, Bhatla SC (2010) A comparative analysis of the distribution and composition of lipidic constituents and associated enzymes in pollen and stigma of sunflower. Sex Plant Reprod 23:163–172
Shakya R, Bhatla SC (2018) Pollination, fertilization and seed development. In: Bhatla SC, Lal MA (eds) Plant physiology, development and metabolism. Springer, Singapore, pp 821–856
Sharma B, Bhatla SC (2013a) Accumulation and scavenging of reactive oxygen species and nitric oxide correlate with stigma maturation and pollen–stigma interaction in sunflower. Acta physiol plant 35:2777–2787
Sharma B, Bhatla SC (2013b) Structural analysis of stigma development in relation with pollen–stigma interaction in sunflower. Flora 208:420–429
Sharma B (2019) An analyses of flavonoids present in the inflorescence of sunflower. Braz J Bot 42:421–429
Shivanna KR (2003) Pollen biology and biotechnology. Oxford Press, New Delhi
Suarez-Cervera M, Asturias JA, Vega-Maray A, Castells T, Lopez-Iglesias C, Ibarolla I, Arilla MC, Gabarayeva N, Seoane-Camba J (2005) The role of allergenic proteins Pla a 1 and Pla a 2 in the germination of Platanus acerifolia pollen grains. Sex Plant Reprod 18:101–112
Swanson R, Edlund AF, Preuss D (2004) Species specificity in pollen–pistil interactions. Annu Rev Genet 38:793–818
Takayama S, Shiba H, Iwano M, Asano K, Hara M (2000) Isolation and characterization of pollen coat proteins of Brassica campestris that interact with S locus-related glycoprotein 1 involved in pollen–stigma adhesion. Proc Natl Acad Sci USA 97:3765–3770
Umbach AL, Lalonde BA, Kandasamy MK, Nasrallah JB, Nasrallah ME (1990) Immunodetection of protein glycoforms encoded by 2 independent genes of the self-incompatibility multigene family of Brassica. Plant Physiol 93:739–747
Verissimo P, Faro C, Moir AJG, Lin Y, Tang J, Pires E (1996) Purification, characterization and partial amino acid sequencing of two new aspartic proteinases from fresh flowers of Cynara cardunculus L. Eur J Biochem 235:762–768
Acknowledgements
The author thanks Council of Scientific and Industrial Research for financial support and is grateful to Professor S C Bhatla, Department of Botany, University of Delhi for providing laboratory facilities for this work.
Funding
This work was funded by Council of Scientific and Industrial Research, India (Grant number F.NO.2-56/2002(II)E.U.II).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shakya, R. Stigma receptivity with pollen in sunflower accompanies novel histochemical and biochemical changes in both male and female reproductive structures. Vegetos 33, 376–384 (2020). https://doi.org/10.1007/s42535-020-00118-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42535-020-00118-5