Abstract
Chemo-mechanical modeling of lithium-ion batteries is essential to achieve mechanical stability of the electrode. Tremendous efforts have been devoted to address underlying mechanisms of mechanical degradation caused by diffusion-induced stress and its effects on battery performance. Although the binder is an electrochemically inactive component of the electrode, it can play a crucial role in the development of diffusion-induced stress of the active particle network by regulating the electronic conduction pathways. Therefore, it is important to include binders in modeling of lithium-ion batteries for comprehensive understanding of the chemo-mechanical behavior of the composite electrode. In this review, we summarize the existing modeling techniques and their practical applicability to investigate the chemo-mechanical response of the particle–binder systems. First, we highlight the general models describing chemo-mechanical behavior of diffusion-induced stress in lithium-ion batteries. Next, we underline the single particle, multi-particle and pseudo-two-dimensional representative volume element models that include the binders. Finally, we provide suggestions for the computational approach to bridge the gap between the simulations and their applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemo-mechanical Model
As global demand for electric vehicles (EVs) has been rapidly increased in the past few years, research on energy storage devices, especially lithium-ion batteries, has gained considerable interest. Upon the heightened demand for longer cycle life, faster charging, and larger charge capacity, the battery industry is forced to adapt new ways to satisfy those demands. One way to enhance the battery performance is to avoid structural failure of the battery electrodes. The structural deterioration of the battery materials directly regulates several capacity degrading mechanisms [1,2,3]. The mechanical failures cause electrical isolation, and then the particles become inactive and can no longer store ions. In this way, the capacity of the electrode decreases. Therefore, the full utilization of the battery capacity is highly dependent on the mechanical failures [4].
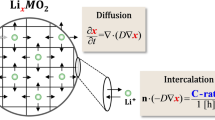
The electrode consists of the aggregation of active material micro-particles that store lithium-ions, as schematically presented in Fig. 1. When the active particle is lithiated/delithiated, the particles expand/shrink, as schematically shown in Fig. 1a. Since it takes time for the lithium-ions to diffuse from the surface of the particle to the core, the distribution of lithium-ions is non-uniform along the radial direction, which causes diffusion-induced stress inside the particles. Since it is difficult to experimentally quantify the stress levels, several researchers have developed chemo-mechanical models to calculate the diffusion-induced stresses [5,6,7,8,9,10]. For this purpose, researchers have used the Christensen and Newman model [5]. Later, this model was modified to study the effects of fast charging [11, 12], dendrite growth [13], particle morphologies [14,15,16,17], failure of solid–electrolyte interface (SEI) [18,19,20], external pressure [21, 22], and diffusion-dependent material properties [23, 24]. Some researchers have simulated the progressive mechanical failure induced by the lithiation/delithiation process [25,26,27,28,29,30,31,32].
Challenges in Chemo-mechanical Modeling
The electrode material is heterogeneous in nature [33]. There are two main material phases: active material and inactive material, as shown in Fig. 2a, b. The primary material phase is the active material, which is responsible to store lithium-ions. The typical active materials for the anode are graphite [34] and silicon [35]. The typical materials for the cathode are LiMn2O4 (LMO) [36], LiCoO2 (LCO) [37], LiFePO4 (LFP), LiNi0.5Mn0.2Co0.3O2 (NMC-523) [38], LiNi0.6Mn0.2Co0.2O2 (NMC-622) [39], and LiNi0.3Mn0.3Co0.3O2 (NMC-333) [40]. The inactive material, which consists of binders and conductive additives, is the secondary phase of the electrode. Although the secondary phase does not store lithium-ions, it provides the mechanical and electrical stability of the electrode material. The conductive additives provide the conduction pathways of electrons between the current collector and the active particles. The binders provide the necessary cohesion between the active particles. The typical materials of the conductive additives are carbon black (CB), acetylene black (AB), and carbon nanotubes (CNTs), and those of the binders are polyvinylidene fluoride (PVDF), carboxyl methyl cellulose (CMC), styrene butadiene rubber (SBR), and sodium–alginate (SA).

© 2017, Elsevier B.V., Wang et al. [75]; copyright© 2007, Elsevier B.V., Harris et al. [41]; copyright© 2010, Materials Research Society, Chen et al. [42]; copyright© 2013, The Electrochemical Society, Xu et al. [43]; copyright© 2019, Elsevier Ltd., respectively
a Distribution of PVDF in graphite anode electrode of lithium-ion battery. b SEM image showing the conductive bridge like structure between graphite particles. c Fracture inside the active particle. d Failure of the binder material. e Debonding at the interface between the particle and binder. Figures in (a–e) are reproduced with permission from Jaiser et al. [74]; copyright
Due to excessive stress, the composite electrode can undergo mechanical failure in different ways of particle fracture [41], binder failure [42], and interfacial debonding [43]. Figure 2c–e shows the scanning electron microscope (SEM) images of these failures observed in experimental studies. Since mechanical failure can occur both inner and inter particles, to precisely determine the effect of mechanical failure on battery performance, inactive materials such as binders should be included in simulation models. However, it is difficult to model the particle–binder system due to the complex morphologies and dynamic material response of the active and inactive materials. In this regard, we summarize the current modeling techniques to determine the chemo-mechanical response of the particle–binder model systems.
Scope of the Review
In this review, we aim to cover three objectives. First, we provide a comprehensive overview of the current modeling techniques to determine the chemo-mechanical behavior of lithium-ion batteries at continuum scales: single particle, multi-particles, and representative volume element models. Second, we outline the main findings of the models for the investigation of mechanical behavior and associated mechanical failures such as particle binder interface debonding. Last, we highlight the critical points and main challenges in designing the chemo-mechanical models for particle–binder systems.
Mathematical Models for Particle–Binder Systems
The chemo-mechanical model is constructed by coupling the lithium diffusion in active material phases and the mechanical deformations. The deformation is associated with both the lithium-induced expansion/contractions and the mechanical constraint provided by the binder material. The governing equations that are generally used to model the chemo-mechanical response of the particle–binder systems are summarized in Table 1. The stress-dependent lithium diffusion can be solved by Eq. (1) [44, 45]. For single particle, the constant lithium flux can be assumed, and the flux is determined by the total active surface, which is not covered by the binder, charge rate, and volume of the particle [34]. Otherwise, the flux is defined by Eq. (2), based on the cell overpotential given by Eq. (3) [36, 39]. To solve for the mechanical deformations, Eq. (4) is utilized with assumption of elastic strain [46]. The elastic strain is calculated by subtracting the diffusion-induced strain form the total strain of the active material, which is defined in Eq. (5) [46]. For the binder material, since there is no diffusion-induced deformation, the total strain is the same as the elastic stain, as given in Eq. (6) [34, 46]. The interfacial debonding at the particle–binder interface is solved by Eqs. (7) and (8). The damage index of Eq. (9) is used to quantify the total level of mechanical failure at the interface [47,48,49].
Single Particle Model
A simple model for the particle–binder systems is made by using single active particle attached to binder domains. The single particle–binder model can be categorized by (i) partially covered by binders and (ii) fully covered by binders. The articles reviewed in this section are summarized in Table 2.
Partially Covered by Binders
Since the binder does not conduct lithium-ions, the lithium flux is applied to the particle surface that is not covered by the binder, as schematically presented by Iqbal et al. [44] in Fig. 3a. In the case of partial binder coverage, the lithium concentration distribution and associated stress development are asymmetric, as shown by Iqbal and Lee [34] in Fig. 3b, c, respectively. The stress development is mainly affected by two mechanisms: (i) due to the binder constraint and (ii) due to the diffusion-induced stress. The first mechanism is dominant in small particles under low charge rates. For the combination of small sizes and low charge rates, large stress is generated at the interface between particle and binder, leading to high probability of mechanical failure at the particle–binder interface. The second mechanism is dominant in large particles under high charge rates. Therefore, for the combination of large sizes and high charge rates, large stress is generated inside the particle, leading to high probability of particle fracture. The corresponding particle size and charge rate map generated by Iqbal and Lee [34] is shown in Fig. 3d. Iqbal et al. [44] calculated the limiting state of charges, based on three types of failures of particle fracture, binder failure, and interfacial debonding, as shown in Fig. 3e.

© 2022, Elsevier Ltd. and Figures in (b–d) are reproduced with permission from Iqbal et al. [34]; copyright© 2018, The Electrochemical Society
a Schematics of a representative case of single particle–binder based system and possible mechanical failures induced by lithiation. The distribution of (a) lithium concentrations and (c) first principal stress in a representative case of single particle attached to a binder. (d) Particle size verses charge rate failure map. (e) The critical state of charges (\({\mathrm{SOC}}_{\mathrm{crt}}\)) before the mechanical failures. Figures in (a, e) are reproduced with permission from Iqbal et al. [44]; copyright
Similarly, Cai and Guo [50] calculated the J-integral for predefined cracks inside the active particle to find the probability of crack propagation. Researchers also have calculated strain energies and von-Mises stresses to investigate the fracture probability. For example, Higa and Srinivansan [35] explored the mechanical response of a silicon active particle that is partially covered by PVDF binder under lithiation. In the study, an axisymmetric cylindrical model system was employed to perform numerical simulations. The strain energies and von-Mises equivalent stress were computed with various sizes and binder stiffness values. In the model, the lithium concentrations were not solved in the binder domain since the binder was considered as an electrochemically inactive phase of the anode. It was concluded that the surrounding material increases the stress levels and provides additional modes of mechanical failures, as compared to the isolated active particle systems. In addition, smaller particle experienced reduced stress levels due to the uniform distribution of the lithium concentrations. Here, it should be noted that the model system was developed as a cylindrical active particle sandwiched between two cylindrical inactive binder domains. In this way, the shear stress was significantly reduced at the interface between particle and binder. As a result, the findings of this articles vary from the other studies of partially covered particle–binder systems. It was also found that increasing the binder stiffness increases the stress level inside the particle as well as the binder.
Moving one step further, researchers also have determined the mechanical response of particle–binder systems with multiple particle–binder connections. For example, Iqbal et al. [51, 52] found that increasing the number of particle–binder connections increases the chances of mechanical failures at the particle–binder interface since the mechanical constraint increases.
Fully Covered by Binder
Assuming that the binder is ionically conductive, researchers used a model of the fully covering binder with a uniform thickness [53, 54] or the embedded particles inside the binder matrix [55]. For example, Takahashi et al. [45] developed a chemo-mechanical model to determine the mechanical response of wet and dry particle–binder samples with a spherical graphite active particle that is completely covered by a PVDF binder layer. They used the measured properties of graphite/PVDF systems obtained by stress–strain experimental tests. They found that the Young's modulus and tensile strength of the wet samples decrease significantly, compared to those of the dry samples. Similarly, Wang et al. [56] developed the coupled stress-diffusion framework to predict the mechanical response of the composite particle–binder system, undergoing continuous charging/discharging cycles at different rates. For the model, two spherical silicon active particles completely covered by CMC binder were considered. To investigate the inelastic shape changes, the binder was modeled as Neo–Hookean hyperelastic material. Permanent shape changes were observed in the silicon active particles due to the inelastic deformations of the binder. The plastic deformation caused by the contact of the binder became dominant when high cycling rates were considered.
In contrast to Higa and Srinivansan [35], Santimetaneedol et al. [57] experimentally proved that PVDF starts to deform inelastically under high strain-rates. Later, they implemented elastic–viscoplastic binder model to determine the chemo-mechanical response of the particle–binder composite model system. However, they employed an axisymmetric model, representing a spherical silicon active particle surrounded by PVDF binder. In addition, the coupling between the lithium diffusion and stress was not considered. Similarly, Xu and Zhao [58] developed the chemo-mechanical model by introducing a strong coupling between the lithium-diffusion and large-elasto-plastic deformations of the active material. For this purpose, elastic-perfectly-plastic silicon active particle was considered. The model system was constructed as a spherical active particle fully embedded inside the binder matrix.
Debonding at the Particle–Binder Interface
High level of stresses at the particle–binder interface can lead to interfacial debonding, as summarized in Fig. 4. Lee et al. [59] introduced one way coupling between the chemo-mechanical and cohesive zone models to simulate the debonding phenomenon. In the study, a spherical graphite active particle that is partially attached to a PVDF binder was used as a model system. Due to large stresses at the edge of the interface, the damage initiated from the edge and propagated towards the center of the interface. Later on, this model was extended to investigate the effects of the charging rate and particle size on the debonding by Iqbal et al. [48], as shown in Fig. 4c. It was found that as the charge rate and particle size increase, debonding at the interface tend to decrease, as seen in Fig. 4f. The result is opposite to the finding of an isolated particle model: as the size or charge rate increase, the possibility of particle fracture increases [60]. It was also found that at low state of charge, the stress levels at the interface were not high enough to initiate the debonding. However, at high state of charges, the stress increased at the interface and debonding initiated, suddenly propagating throughout the interface. This model was upgraded by introducing the full coupling between the chemo-mechanical and cohesive zone models by Iqbal et al. [49] and used to determine the interfacial debonding for lithiation and delithiation cases, as presented in Fig. 4a–c. The fully coupled damage model was further utilized to investigate the effects of binder characteristics on the debonding mechanisms [47]. Similarly, Zhang et al. [61] investigated the interfacial failure between the silicon micropillars and binder coating.

© 2020, The Electrochemical Society and Figures in (c, f) are reproduced with permission from Iqbal et al. [48]; copyright© 2020, Elsevier B.V
a Schematic of the debonding phenomenon at the particle–binder system. b The trend of lithiation induced accumulated damage. c Representative contours of the distribution of damage index at the particle–binder interface. Effects of interface debonding on d lithium diffusion and e stress generation. f Debonding failure map based on particle size and charge rate. Figures in (a, b, d, e) are reproduced with permission from Iqbal et al. [49]; copyright
Multiple Particle–Binder Model
The modeling technique given in the preceding section is a good start to understand the interplay of lithium-diffusion and stress generation in particle–binder systems and to predict the effects of several complex geometry and material features on the chemo-mechanical response. To extend the study at the micro-scales of the particle level to the cell scale, multiple particle–binder models have been developed with either half- or full-cell model. The multiple particle–binder modeling is divided into two categories: (i) computer generated geometries and (ii) reconstructed geometries based on the tomographic data option from ex-situ experiments. The articles about multiple particle–binder models are summarized in Table 3.
Computer Generated Particle–Binder Network
By a computer, the particle–binder systems can be generated with simplified geometries like two-dimensional (2D) circular particles [38] or the three-dimensional (3D) spherical [62] or elliptical particles. The particles can be isolated, or they are connected to each other to include the particle–particle interactions. For example, considering the stress-dependent overpotential, Ali et al. [36] simulated the chemo-mechanical response of multiple particle–binder system in order to investigate the effects of particle size, particle location and the charge rate. The electrode microstructure was modeled by placing randomly sized and isolated active particles that are fully enclosed by a binder layer of uniform thickness. Later on, using a full cell model with lithium metal anode and a cathode consisting of particle and binder network, Ali et al. [39] found inhomogeneous lithium concentration and stress distribution inside the electrode, as shown in Fig. 5a. The computational geometry of the cathode is generated by randomly placing the NMC-622 particles while the binder was modeled as a shell type, which covered the active particles with a uniform thickness. The binders were systematically connected to include the surrounding interactions. The nonuniform lithium concentrations distribution was found across the electrode with larger concentration in smaller particles. Moreover, larger stresses were observed at the particle–particle contacts. Similarly, Rahani and Shenoy [63] simulated the chemo-mechanical behavior of a porous composite particle–binder anode. The PVDF binder was considered to deform elasto-plastically during the lithiation of the graphite active particle. To model the computational geometry, various sized circular active particles were randomly distributed inside the electrode. Later, the binder was included in a form of either layers surrounding the active particles or bridge structures connecting the particles. Large stresses were observed in regions where the particles contact with other particles. Using two active particles, Wu and Guo [64] investigated the effects of the particle–binder and particle–particle contacts on the mechanical response. Later, researchers have used the statistics like particle size distributions from tomographic data to generated 3D representative volume element (RVE) to simulate the chemo-mechanical response [43, 65], as shown a representative case in Fig. 5b.

© 2021, John Wiley & Sons Ltd. And Figures in (b) are adopted from Liu et al. [65] under Creative Commons Attribution License
a Computer generated two-dimensional particle–binder network and inhomogeneous lithium-concentration distributions and associated stress generation at the cathode. b Computer generated three-dimensional representative volumetry element based on the realistic electrode statistics, and the results of stress-assisted lithium-concentration distributions and concentration-dependent stress generation. Figures in (a) are reproduced with permission from Ali et al. [39]; copyright
Reconstructed Geometries from Tomographic Data
To use actual geometries for numerical simulations, a few researchers have used the X-ray tomography to reconstruct the 3D particle–binder computational geometries. The overview of the reconstruction is highlighted in Fig. 6. Trembacki et al. [40] reconstructed the computational meshes from the tomography-based image stacks of the composite particle–binder electrode network. However, due to the difficulty in distinguishing between the active and nonactive material phases, the composite binder is separately placed inside the reconstructed particle network. Without considering the binder, von-Mises stresses were doubled in the particle–binder systems due to the particle–particle contact. When a thin coating layer of binder is considered, the mean stresses decreased up to 4.5 times. Moreover, when the binder is placed between the particles contact locations, the electrical conductivity is predicted more accurately, compared to the binder coating. Similarly, Wu et al. [66] developed a two-dimensional microstructural resolved model based on realistic electrode geometries. With the irregular shaped particle–binder systems, the distribution of stress and lithium concentrations were highly nonuniform. Higher shear stresses were observed at the local concave curvatures, compared to the smooth particle surfaces and on the surfaces near the rigid current collector. The PVDF binder was considered to deform with the viscoelastic behavior.
a Reconstruction of the multiparticle and carbon/binder network. b Reconstruction of computational mesh of particle–binder network from X-ray CT tomographic data and the simulation results of the lithiation-induced damage in active particles. Figures in (a, b) are adopted from Jiang et al. [76] and Boyce et al. [67] under Creative Commons Attribution License, respectively
Mechanical Failure in Multiple Particle Systems
Moving further, researchers also have investigated the mechanical failures in multiple particle–binder systems. For example, Xu et al. [43] constructed the microstructural resolved model from the tomographic data to analyze the heterogeneous damage in composite cathode particles embedded inside the binder matrix. The degree of particle fracture and interfacial debonding was significantly higher in particle–binder systems closed to the separator due to deeper charging cycles, compared to the systems closed to the current collectors. Furthermore, impedance was increased when interfacial debonding is considered in the simulations, as shown in Fig. 7. Similarly, Liu et al. [65] developed a fully coupled electro-chemo-mechanical model at a cell level to examine the effects of the binder on the stress and charge heterogeneities, as shown in Fig. 5b. A 3D RVE consisting of multiple particles was generated, based on the statistical features extracted from tomographic data. Later, reconstructed active particles were fully embedded inside the binder matrix to simulate the interactions between particles and binders. The gradual growth in the interface debonding was also simulated to investigate its effect on the increase in impedance at the electrode. Furthermore, Boyce et al. [67] simulated the particle fracture in reconstructed computational geometries as shown in Fig. 6b.
Results of the a debonding at the particle–binder interface, b impedance growth due to interface damage and c change in voltage response due to interface failure. Figures are reproduced with permission from Xu et al. [43]; copyright
Perspectives and Advances
Multiscale Approach to Model Particle–Binder Systems
In battery electrodes, complex time-dependent and concentration-dependent mechanisms occur due to large deformation and complex degradation of the active materials under charging/discharging process. To better predict the effects of inactive binder domain on the overall battery performance, more advanced modeling schemes should be considered. In this regard, multiscale approach offers better opportunities in bridging the gaps between multiple length and time scales [68, 69]. The mechanical failures of the particle–binder interface at the atomistic scale should be linked to continuum models like microscopic and mesoscopic models to predict the impedance increase and capacity fade.
Interaction Between SEI Formation and Binders
The formation and degradation of the solid-electrolyte interface at the anode is one of the major mechanisms of capacity deterioration in lithium-ion batteries [18]. However, there is a huge research gap between the formation and deterioration of SEI and the presence of inactive material phase such as binder. For example, how the SEI formation is affected by the binder domain? Does SEI form only at the electrode–electrolyte interface or does it also form at the particle–binder interface? To answer such questions more detailed theoretical studies are needed.
Use of Artificial Intelligence to Optimize Material Properties
Currently, most of the modeling techniques only provide theoretical aspects of the studies with the lack of bridging between the experimental and numerical data. This is because it is difficult to analyze quantitatively battery response by experiment due to the complex and coupled nature of the physical phenomena in battery systems. In addition, it takes a lot of time to simulate coupled multi-physics of battery numerically. For a proper linking of experimental and theoretical studies, machine learning models can be utilized to optimize the material properties and predict the battery performance.
Particle–Binder Model Systems Based on Realistic Geometries
Most of the current computational particle–binder models use computer generated simple geometries; for example, spherical, cylindrical, and elliptical active particles are attached to the binder. Although many techniques have been developed to build the actual active materials network at the electrodes, sharp geometric discontinuities hinder applying the computer model to the study of the chemo-mechanical behavior. Therefore, more in-depth studies are needed to show how the geometric discontinuities play a role in the failure of particle–binder interface. For this, the physical and chemical changes at various geometrical discontinuities should be observed by in-situ, and then the observation should be verified by theoretical computation techniques. The coupling work of experimental observation and computational varification will be helpful in finding the optimal binder structures for lithium-ion batteries.
Modeling of Disruption of Electronic Conduction Pathways
Apart from providing the mechanical stability to the network of active materials, the binder is responsible to ensure the conduction of electrons between the current collector and active particles. Since the binder is not conductive material, conductive additives such as carbon black are added to make a carbon–binder matrix. In this way, the binder distribution inside the electrode provides the essential electronic conduction pathways. Due to the failure of the interface between active particles and binders, the electronic conduction network deteriorates. As a result, the impedance or contact resistance increases in the electrode. Therefore, in the simulation study about mechanical failure, it is necessary to consider the deterioration of electronic conduction pathways due to the interfacial failure.
Morphology and Structure of Carbon–Binder Domain
In most of theoretical studies, the binder morphology is simplified as a solid layer with uniform thickness. However, the binder feature is more complex in real batteries: the binder exists in form of porous polymer fibers and carbon-binder network with the addition of carbon black particles. The carbon black particles usually exist from 30 to 40 nm in size, or they agglomerate to form larger secondary particles. Thus, carbon black significantly alters the internal structure of the polymeric binder by forming linear fibrous or cluster network. In addition, by absorbing the electrolyte, the mechanical binding can be deteriorated, affecting the electrical conductivity and mechanical adhesion. Therefore, in-depth experimental and theoretical understandings are required to accurately model the chemo-mechanical response of the particle–binder systems. With a help of advanced understanding, manufacturers will be able to produce mechanically robust electrode microstructures with low electronic resistivities.
Conclusions
In this review, theoretical models that are developed to understand the chemo-mechanical response in batteries with inclusion of the binders are summarized. The binder provides an important role in regulating not only the mechanical stability but also chemo-mechanical response of the active particles. In the simulation, computer-generated simple shape can be used for the binder modeling. To improve the level of prediction, it is necessary to use realistic composite electrode geometries in simulations. However, it is challenging to obtain realistic geometries and to numerically solve the chemo-mechanical response for the complicated geometries. In addition, the binder is typically modeled as an elastic material. however, as a polymer material, the binder deforms nonlinearly and exists in form of porous fibrous structure, further increasing the complexity of modeling. Therefore, continuous efforts should be paid to develop advanced techniques for the simulation of the particle–binder systems and to extend the micro-scale model to the cell model to relate mechanical issue to cell-level battery performance.
References
T. Li, X.-Z. Yuan, L. Zhang, D. Song, K. Shi, C. Bock, Degradation mechanisms and mitigation strategies of nickel-rich NMC-based lithium-ion batteries. Electrochem. Energy Rev. 3, 43–80 (2020). https://doi.org/10.1007/s41918-019-00053-3
J. Duan, X. Tang, H. Dai, Y. Yang, W. Wu, X. Wei, Y. Huang, Building safe lithium-ion batteries for electric vehicles: a review. Electrochem. Energy Rev. 3, 1–42 (2020). https://doi.org/10.1007/s41918-019-00060-4
F. Zhang, Q.-A. Huang, Z. Tang, A. Li, Q. Shao, L. Zhang, X. Li, J. Zhang, A review of mechanics-related material damages in all-solid-state batteries: mechanisms, performance impacts and mitigation strategies. Nano Energy 70, 104545 (2020). https://doi.org/10.1016/j.nanoen.2020.104545
J.R. Szczech, S. Jin, Nanostructured silicon for high capacity lithium battery anodes. Energy Env. Sci. 4, 56–72 (2011). https://doi.org/10.1039/C0EE00281J
J. Christensen, J. Newman, Stress generation and fracture in lithium insertion materials. J. Solid State Electrochem. 10, 293–319 (2006). https://doi.org/10.1007/s10008-006-0095-1
W.H. Woodford, Y.-M. Chiang, W.C. Carter, “Electrochemical shock” of intercalation electrodes: a fracture mechanics analysis. J. Electrochem. Soc. 157, A1052–A1059 (2010). https://doi.org/10.1149/1.3464773
M. Pharr, Z. Suo, J.J. Vlassak, Variation of stress with charging rate due to strain-rate sensitivity of silicon electrodes of Li-ion batteries. J. Power Sources. 270, 569–575 (2014). https://doi.org/10.1016/j.jpowsour.2014.07.153
Z.S. Ma, Z.C. Xie, Y. Wang, P.P. Zhang, Y. Pan, Y.C. Zhou, C. Lu, Failure modes of hollow core–shell structural active materials during the lithiation–delithiation process. J. Power Sources. 290, 114–122 (2015). https://doi.org/10.1016/j.jpowsour.2015.05.008
A.A. Tahmasbi, M.H. Eikerling, Statistical physics-based model of mechanical degradation in lithium ion batteries. Electrochim. Acta 283, 75–87 (2018). https://doi.org/10.1016/j.electacta.2018.06.119
C.-F. Chen, P. Barai, K. Smith, P.P. Mukherjee, Scaling relations for intercalation induced damage in electrodes. Electrochim. Acta 204, 31–49 (2016). https://doi.org/10.1016/j.electacta.2016.03.106
L. Weng, J. Zhou, R. Cai, Analytical model of Li-ion diffusion-induced stress in nanowire and negative Poisson’s ratio electrode under different operations. Int. J. Mech. Sci. 141, 245–261 (2018). https://doi.org/10.1016/j.ijmecsci.2018.04.013
L. Ji, Z. Guo, S. Du, L. Chen, Stress induced by diffusion, curvature, and reversible electrochemical reaction in bilayer lithium-ion battery electrode plates. Int. J. Mech. Sci. 134, 599–609 (2017). https://doi.org/10.1016/j.ijmecsci.2017.10.048
W. Mu, X. Liu, Z. Wen, L. Liu, Numerical simulation of the factors affecting the growth of lithium dendrites. J. Energy Storage 26, 100921 (2019). https://doi.org/10.1016/j.est.2019.100921
C. Lim, B. Yan, L. Yin, L. Zhu, Simulation of diffusion-induced stress using reconstructed electrodes particle structures generated by micro/nano-CT. Electrochim. Acta 75, 279–287 (2012). https://doi.org/10.1016/j.electacta.2012.04.120
W. Mai, M. Yang, S. Soghrati, A particle-resolved 3D finite element model to study the effect of cathode microstructure on the behavior of lithium ion batteries. Electrochim. Acta 294, 192–209 (2019). https://doi.org/10.1016/j.electacta.2018.10.072
X. Zhang, W. Shyy, A. Marie Sastry, Numerical simulation of intercalation-induced stress in Li-ion battery electrode particles, J. Electrochem. Soc. 154, A910 (2007). https://doi.org/10.1149/1.2759840
C. Xu, L. Weng, B. Chen, J. Zhou, R. Cai, An analytical model for the fracture behavior in hollow cylindrical anodes. Int. J. Mech. Sci. 157–158, 87–97 (2019). https://doi.org/10.1016/j.ijmecsci.2019.04.035
Y. Ali, N. Iqbal, S. Lee, Role of SEI layer growth in fracture probability in lithium-ion battery electrodes. Int. J. Energy Res. 45, 5293–5308 (2021). https://doi.org/10.1002/er.6150
A.M. Colclasure, K.A. Smith, R.J. Kee, Modeling detailed chemistry and transport for solid-electrolyte-interface (SEI) films in Li–ion batteries. Electrochim. Acta 58, 33–43 (2011). https://doi.org/10.1016/j.electacta.2011.08.067
L. Liu, P. Guan, Phase-field modeling of solid electrolyte interphase (SEI) evolution: considering cracking and dissolution during battery cycling. ECS Trans. 89, 101–111 (2019). https://doi.org/10.1149/08901.0101ecst
P. Stein, Y. Zhao, B.-X. Xu, Effects of surface tension and electrochemical reactions in Li-ion battery electrode nanoparticles. J. Power Sources. 332, 154–169 (2016). https://doi.org/10.1016/j.jpowsour.2016.09.085
X. Zhang, H.-S. Chen, D. Fang, Effects of surface stress on lithium-ion diffusion kinetics in nanosphere electrodes of lithium-ion batteries. Int. J. Mech. Sci. 169, 105323 (2020). https://doi.org/10.1016/j.ijmecsci.2019.105323
K. Zhang, Y. Li, B. Zheng, Effects of concentration-dependent elastic modulus on Li-ions diffusion and diffusion-induced stresses in spherical composition-gradient electrodes. J. Appl. Phys. 118, 105102 (2015). https://doi.org/10.1063/1.4930571
R. Deshpande, Y. Qi, Y.-T. Cheng, Effects of concentration-dependent elastic modulus on diffusion-induced stresses for battery applications. J. Electrochem. Soc. 157, A967–A971 (2010). https://doi.org/10.1149/1.3454762
M. Klinsmann, D. Rosato, M. Kamlah, R.M. McMeeking, Modeling crack growth during Li extraction and insertion within the second half cycle. J. Power Sources. 331, 32–42 (2016). https://doi.org/10.1016/j.jpowsour.2016.08.142
M. Klinsmann, D. Rosato, M. Kamlah, R.M. McMeeking, Modeling crack growth during Li extraction in storage particles using a fracture phase field approach. J. Electrochem. Soc. 163, A102–A118 (2016). https://doi.org/10.1149/2.0281602jes
M. Klinsmann, D. Rosato, M. Kamlah, R.M. McMeeking, Modeling crack growth during Li insertion in storage particles using a fracture phase field approach. J. Mech. Phys. Solids. 92, 313–344 (2016). https://doi.org/10.1016/j.jmps.2016.04.004
R. Xu, K. Zhao, Corrosive fracture of electrodes in Li-ion batteries. J. Mech. Phys. Solids. 121, 258–280 (2018). https://doi.org/10.1016/j.jmps.2018.07.021
H. Haftbaradaran, A. Maddahian, F. Mossaiby, A fracture mechanics study of the phase separating planar electrodes: phase field modeling and analytical results. J. Power Sources. 350, 127–139 (2017). https://doi.org/10.1016/j.jpowsour.2017.03.073
A. Mesgarnejad, A. Karma, Phase field modeling of chemomechanical fracture of intercalation electrodes: role of charging rate and dimensionality. J. Mech. Phys. Solids 132, 103696 (2019). https://doi.org/10.1016/j.jmps.2019.103696
X. Zhu, Y. Chen, H. Chen, W. Luan, The diffusion induced stress and cracking behaviour of primary particle for Li-ion battery electrode. Int. J. Mech. Sci. 178, 105608 (2020). https://doi.org/10.1016/j.ijmecsci.2020.105608
Y. Zhang, C. Zhao, Z. Guo, Simulation of crack behavior of secondary particles in Li-ion battery electrodes during lithiation/de-lithiation cycles. Int. J. Mech. Sci. 155, 178–186 (2019). https://doi.org/10.1016/j.ijmecsci.2019.02.042
S. Müller, P. Pietsch, B.-E. Brandt, P. Baade, V. De Andrade, F. De Carlo, V. Wood, Quantification and modeling of mechanical degradation in lithium-ion batteries based on nanoscale imaging, Nat. Commun. 9 (2018). https://doi.org/10.1038/s41467-018-04477-1.
N. Iqbal, S. Lee, Mechanical failure analysis of graphite anode particles with PVDF binders in Li-Ion batteries. J. Electrochem. Soc. 165, A1961–A1970 (2018). https://doi.org/10.1149/2.0111810jes
K. Higa, V. Srinivasan, Stress and strain in silicon electrode models. J. Electrochem. Soc. 162, A1111–A1122 (2015). https://doi.org/10.1149/2.0091507jes
Y. Ali, N. Iqbal, S. Lee, Simultaneous effect of particle size and location on stress development in the electrodes of lithium-ion batteries. Int. J. Energy Res. 44, 12145–12157 (2020). https://doi.org/10.1002/er.5795
H. Mendoza, S.A. Roberts, V.E. Brunini, A.M. Grillet, Mechanical and electrochemical response of a LiCoO2 cathode using reconstructed microstructures. Electrochim. Acta 190, 1–15 (2016). https://doi.org/10.1016/j.electacta.2015.12.224
R. Fang, H. Ge, Z. Wang, Z. Li, J. Zhang, A two-dimensional heterogeneous model of lithium-ion battery and application on designing electrode with non-uniform porosity. J. Electrochem. Soc. 167, 130513 (2020). https://doi.org/10.1149/1945-7111/abb83a
Y. Ali, N. Iqbal, S. Lee, Inhomogeneous stress development at the multiparticle electrode of lithium-ion batteries. Int. J. Energy Res. 45, 14788–14803 (2021). https://doi.org/10.1002/er.6754
B.L. Trembacki, D.R. Noble, V.E. Brunini, M.E. Ferraro, S.A. Roberts, Mesoscale effective property simulations incorporating conductive binder. J. Electrochem. Soc. 164, E3613–E3626 (2017). https://doi.org/10.1149/2.0601711jes
S.J. Harris, R.D. Deshpande, Y. Qi, I. Dutta, Y.-T. Cheng, Mesopores inside electrode particles can change the Li-ion transport mechanism and diffusion-induced stress. J. Mater. Res. 25, 1433–1440 (2010). https://doi.org/10.1557/JMR.2010.0183
J. Chen, J. Liu, Y. Qi, T. Sun, X. Li, Unveiling the roles of binder in the mechanical integrity of electrodes for lithium-ion batteries. J. Electrochem. Soc. 160, A1502–A1509 (2013). https://doi.org/10.1149/2.088309jes
R. Xu, Y. Yang, F. Yin, P. Liu, P. Cloetens, Y. Liu, F. Lin, K. Zhao, Heterogeneous damage in Li-ion batteries: experimental analysis and theoretical modeling. J. Mech. Phys. Solids. 129, 160–183 (2019). https://doi.org/10.1016/j.jmps.2019.05.003
N. Iqbal, I.U. Haq, S. Lee, Chemo-mechanical model predicted critical SOCs for the mechanical stability of electrode materials in lithium-ion batteries. Int. J. Mech. Sci. 216, 107034 (2022). https://doi.org/10.1016/j.ijmecsci.2021.107034
K. Takahashi, K. Higa, S. Mair, M. Chintapalli, N. Balsara, V. Srinivasan, Mechanical degradation of graphite/PVDF composite electrodes: a model-experimental study. J. Electrochem. Soc. 163, A385–A395 (2016). https://doi.org/10.1149/2.0271603jes
N. Iqbal, S. Lee, Stress-regulated pulse charging protocols via coupled electrochemical-mechanical model for the mechanical stability of electrode materials in lithium-ion batteries. J. Power Sources. 536, 231376 (2022). https://doi.org/10.1016/j.jpowsour.2022.231376
N. Iqbal, Y. Ali, I.U. Haq, S. Lee, Progressive interface debonding in composite electrodes of Li-ion batteries via mixed-mode cohesive zone model: effects of binder characteristics. Compos. Struct. (2021). https://doi.org/10.1016/j.compstruct.2020.113173.
N. Iqbal, Y. Ali, S. Lee, Analysis of mechanical failure at the interface between graphite particles and polyvinylidene fluoride binder in lithium-ion batteries. J. Power Sources. 457, 228019 (2020). https://doi.org/10.1016/j.jpowsour.2020.228019
N. Iqbal, Y. Ali, S. Lee, Debonding mechanisms at the particle–binder interface in the Li-ion battery electrode. J. Electrochem. Soc. 167, 060515 (2020). https://doi.org/10.1149/1945-7111/ab8479
X. Cai, Z. Guo, Coupled mechano-diffusion J-integral in active particles under the influence of binder. Eng. Fract. Mech. 231, 107031 (2020). https://doi.org/10.1016/j.engfracmech.2020.107031
N. Iqbal, Y. Ali, S. Lee, Mechanical degradation analysis of a single electrode particle with multiple binder connections: a comparative study. Int. J. Mech. Sci. 188, 105943 (2020). https://doi.org/10.1016/j.ijmecsci.2020.105943
N. Iqbal, Y. Ali, S. Lee, Chemo-mechanical response of composite electrode systems with multiple binder connections. Electrochim. Acta 364, 137312 (2020). https://doi.org/10.1016/j.electacta.2020.137312
G. Singh, T.K. Bhandakkar, Analytical investigation of binder’s role on the diffusion induced stresses in lithium ion battery through a representative system of spherical isolated electrode particle enclosed by binder. J. Electrochem. Soc. 164, A608–A621 (2017). https://doi.org/10.1149/2.0361704jes
G. Singh, T.K. Bhandakkar, Semianalytical study of the effect of realistic boundary conditions on diffusion induced stresses in cylindrical Lithium ion electrode-binder system. Int. J. Mech. Sci. (2019). https://doi.org/10.1016/j.ijmecsci.2019.105141.
J.M. Foster, X. Huang, M. Jiang, S.J. Chapman, B. Protas, G. Richardson, Causes of binder damage in porous battery electrodes and strategies to prevent it. J. Power Sources 350, 140–151 (2017). https://doi.org/10.1016/j.jpowsour.2017.03.035
H. Wang, S.P.V. Nadimpalli, V.B. Shenoy, Inelastic shape changes of silicon particles and stress evolution at binder/particle interface in a composite electrode during lithiation/delithiation cycling. Extreme Mech. Lett. 9, 430–438 (2016). https://doi.org/10.1016/j.eml.2016.03.020
A. Santimetaneedol, R. Tripuraneni, S.A. Chester, S.P.V. Nadimpalli, Time-dependent deformation behavior of polyvinylidene fluoride binder: implications on the mechanics of composite electrodes. J. Power Sources. 332, 118–128 (2016). https://doi.org/10.1016/j.jpowsour.2016.09.102
R. Xu, K. Zhao, Mechanical interactions regulated kinetics and morphology of composite electrodes in Li-ion batteries. Extreme Mech. Lett. 8, 13–21 (2016). https://doi.org/10.1016/j.eml.2015.10.004
S. Lee, J. Yang, W. Lu, Debonding at the interface between active particles and PVDF binder in Li-ion batteries. Extreme Mech. Lett. 6, 37–44 (2016). https://doi.org/10.1016/j.eml.2015.11.005
K. Zhao, M. Pharr, J.J. Vlassak, Z. Suo, Fracture of electrodes in lithium-ion batteries caused by fast charging. J. Appl. Phys. 108, 073517 (2010). https://doi.org/10.1063/1.3492617
X. Zhang, W.-L. Song, H.-S. Chen, D. Fang, Role of the binder in the mechanical integrity of micro-sized crystalline silicon anodes for Li-Ion batteries. J. Power Sources. 465, 228290 (2020). https://doi.org/10.1016/j.jpowsour.2020.228290
B. Wu, W. Lu, A battery model that fully couples mechanics and electrochemistry at both particle and electrode levels by incorporation of particle interaction. J. Power Sources. 360, 360–372 (2017). https://doi.org/10.1016/j.jpowsour.2017.05.115
E.K. Rahani, V.B. Shenoy, Role of plastic deformation of binder on stress evolution during charging and discharging in lithium-ion battery negative electrodes. J. Electrochem. Soc. 160, A1153–A1162 (2013). https://doi.org/10.1149/2.046308jes
Y. Wu, Z.-S. Guo, Modeling Li-ion concentration distribution and stress of porous electrode particles considering binder and direct particle contact. J. Energy Storage. 44, 103315 (2021). https://doi.org/10.1016/j.est.2021.103315
P. Liu, R. Xu, Y. Liu, F. Lin, K. Zhao, Computational modeling of heterogeneity of stress, charge, and cyclic damage in composite electrodes of Li-ion batteries. J. Electrochem. Soc. 167, 040527 (2020). https://doi.org/10.1149/1945-7111/ab78fa
W. Wu, X. Xiao, M. Wang, X. Huang, A microstructural resolved model for the stress analysis of lithium-ion batteries. J. Electrochem. Soc. 161, A803–A813 (2014). https://doi.org/10.1149/2.082405jes
A.M. Boyce, E. Martínez-Pañeda, A. Wade, Y.S. Zhang, J.J. Bailey, T.M.M. Heenan, D.J.L. Brett, P.R. Shearing, Cracking predictions of lithium-ion battery electrodes by X-ray computed tomography and modelling. J. Power Sources. 526, 231119 (2022). https://doi.org/10.1016/j.jpowsour.2022.231119
J. Fish, G.J. Wagner, S. Keten, Mesoscopic and multiscale modelling in materials. Nat. Mater. 20, 774–786 (2021). https://doi.org/10.1038/s41563-020-00913-0
X. Liu, L. Zhang, H. Yu, J. Wang, J. Li, K. Yang, Y. Zhao, H. Wang, B. Wu, N.P. Brandon, S. Yang, Bridging multiscale characterization technologies and digital modeling to evaluate lithium battery full lifecycle. Adv. Energy Mater. (2022). https://doi.org/10.1002/aenm.202200889.
A. Awarke, S. Lauer, M. Wittler, S. Pischinger, Quantifying the effects of strains on the conductivity and porosity of LiFePO4 based Li-ion composite cathodes using a multi-scale approach. Comput. Mater. Sci. 50, 871–879 (2011). https://doi.org/10.1016/j.commatsci.2010.10.024
A. Chauhan, E. Asylbekov, S. Kespe, H. Nirschl, Influence of carbon binder domain on the performance of lithium-ion batteries: impact of size and fractal dimension. Electrochem. Sci. Adv. (2022). https://doi.org/10.1002/elsa.202100151
S.A. Roberts, H. Mendoza, V.E. Brunini, B.L. Trembacki, D.R. Noble, A.M. Grillet, Insights into lithium-ion battery degradation and safety mechanisms from mesoscale simulations using experimentally reconstructed mesostructures. J. Electrochem. Energy Convers. Storage. 13, 031005 (2016). https://doi.org/10.1115/1.4034410
R. Xu, L. Scalco de Vasconcelos, K. Zhao, Computational analysis of chemomechanical behaviors of composite electrodes in Li-ion batteries. J. Mater. Res. 31, 2715–2727 (2016). https://doi.org/10.1557/jmr.2016.302
S. Jaiser, J. Kumberg, J. Klaver, J.L. Urai, W. Schabel, J. Schmatz, P. Scharfer, Microstructure formation of lithium-ion battery electrodes during drying–an ex-situ study using cryogenic broad ion beam slope-cutting and scanning electron microscopy (Cryo-BIB-SEM). J. Power Sources. 345, 97–107 (2017). https://doi.org/10.1016/j.jpowsour.2017.01.117
H. Wang, T. Umeno, K. Mizuma, M. Yoshio, Highly conductive bridges between graphite spheres to improve the cycle performance of a graphite anode in lithium-ion batteries. J. Power Sources. 175, 886–890 (2008). https://doi.org/10.1016/j.jpowsour.2007.09.103
Z. Jiang, J. Li, Y. Yang, L. Mu, C. Wei, X. Yu, P. Pianetta, K. Zhao, P. Cloetens, F. Lin, Y. Liu, Machine-learning-revealed statistics of the particle–carbon/binder detachment in lithium-ion battery cathodes. Nat. Commun. 11, 2310 (2020). https://doi.org/10.1038/s41467-020-16233-5
Acknowledgements
This research was supported by National Research Foundation of Korea grants, funded by the Ministry of Science and ICT (Nos. 2018R1A5A7023490 and 2022R1A2C1003003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iqbal, N., Choi, J., Lee, C. et al. A Review on Modeling of Chemo-mechanical Behavior of Particle–Binder Systems in Lithium-Ion Batteries. Multiscale Sci. Eng. 4, 79–93 (2022). https://doi.org/10.1007/s42493-022-00082-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42493-022-00082-z