Abstract
The use of phosphorus by mankind is long established. From use in agriculture, foods, high tech electronics, and more recently in EV battery cathode production, one cannot escape its impact on today’s society. This paper will review and describe the circular journey of phosphorus through its value chain from the mining operation of phosphate ore through beneficiation into downstream chemicals production and finally into the EV battery cathode production space. This will include a review of phosphoric acid production from phosphate concentrate using wet process route and thermal route. The wet process route discussed will be via dissolution of phosphate concentrate by sulphuric acid. The thermal route will discuss thermal decomposition of phosphate concentrate using submerged arc furnace to produce yellow phosphorus followed by its combustion to produce phosphorus pentoxide and subsequent hydration to produce phosphoric acid. It will also discuss briefly other phosphate derivatives for specialty chemicals and recovery of phosphorus units from wastewater treatment plants, sewage sludge and incinerator ash. Finally, the paper will discuss the use of phosphorus in EV batteries in the form LFP cathode materials and its production. It will also briefly discuss the LFP battery recycle.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
According to Hilton [1,2,3,4], the defining principle of a phosphorus circular economy is conservation (zero waste) based on:
-
The default condition being that accessing “primary” resources is necessary only when “secondary (reusable)” resources are not sufficiently available.
-
Linear “Extractive” industries require a new, “circular” narrative, based on redefined “life cycles” for all resources.
-
All resources stay within the system boundary, even if no foreseeable solution to their current status as “waste” is available.
The above three items, as illustrated in Fig. 1, should form the basis of sustainable development goals (SDG) for the phosphorus extractive industry as a whole.
Defining principle of a phosphorus circular economy is conservation (Zero Waste) [4]
Van Kauwenbergh [5] developed a list of deposits across the globe that were considered of economic or potentially economic deposits. Fig. 2 illustrates Van Kauwenbergh’s [2] data set. The three groups of phosphates were classified as igneous, sedimentary and island type deposits.
Economic and potentially economic phosphate deposits of the world [5]
Figure 2 illustrates the location of the three most common forms of phosphate ore across the globe. The Island deposit type makes up a very small amount of the global phosphate ore market. The two main deposit types are igneous and sedimentary. Albeit that Fig. 2 is dated the content has not changed much over the last 13 years. The operators of major sedimentary mining operations have expanded their capacity by opening up new parcels of land for mining, as such there are very few new deposits being developed. The key sedimentary producers are located in Peru, Florida, Morocco, Saudi Arabia, Jordan, and China. That said, three sedimentary deposits currently being developed include Chaketma (Tunisia), Amaroo (Australia), AC Fertilizer Company (Algeria), and Paradise South (Australia).
Igneous phosphate deposits are more sparsely located round the globe. Key deposits include the Kola peninsula (Finland/Russia), Phalaborwa (South Africa), and Quebec.
Phosphoric acid production facilities are designed to accept either sedimentary phosphate rock concentrate or igneous phosphate rock concentrate. This is because the different rock types react differently in the wet phosphoric acid production process. Igneous phosphate is less reactive than the equivalent sedimentary rock.
The phosphorus journey begins with the extraction of primary phosphorus in the form of phosphate in the form of apatite [6] from the ground via open pit mining and in some rare cases underground mining methods. The easier, simpler to process ore bodies have long been depleted leaving the more complex ores for today’s metallurgists to deal with. The ores are processed in beneficiation plants to remove impurities before the phosphate rock concentrate is either sold on the open market or converted into phosphoric acid in downstream chemical production facilities via the wet acid process or thermal phosphorus routes. The facilities can produce a range of phosphorus rich chemicals from merchant grade acid through technical grade and food grade acids. These can in turn be converted into ammoniated fertilizers, compound fertilizers with varying ratio of nitrogen, phosphorus, and potassium (NPK) as well as used in foods and electronic industries.
The advent of the electric vehicle (EV) battery has given rise to a new use for purified phosphoric acid in the production of lithium ferro phosphorus (LFP) cathode material for EV battery cathode assemblies. The production of LFP relies on raw materials such as lithium carbonate, lithium hydroxides, iron salts, and purified phosphoric acid.
With the production of the various raw materials for LFP cathodes and the phosphorus associated with NPK based fertilizers, there are always waste streams from the ore beneficiation through downstream chemicals to cathode material manufacture. To minimize the buildup of waste materials from primary production operations, it has become necessary, if not essential, to look at recycling or repurposing the by or co product streams.
Recycling of phosphorus in various forms is vital to conserve resources, as well as reduce the residual phosphorus load on the environment predominantly as run off into river systems. Phosphorus can be recovered from sewage liquid streams, solid waste from sewage, incinerator ash, and EV batteries to name a few.
The objective of this paper is to review and describe the processing of phosphorus-based commodities from the mine pit all the way through phosphate beneficiation, phosphoric acid production, and its use in the manufacture of lithium iron phosphate cathode material as well as other materials in the phosphorus value chain. There have been technical review papers that cover portions of the phosphorus value chain but none to the authors knowledge that attempts to discuss the full value chain.
2 Mining and Beneficiation
The extraction and processing of phosphate ore today is carried out in remote dry (both hot and cold environs) areas of the globe with limited water, lower ore grades, and power constraints such as Morocco, Tunisia, Egypt, Saudi Arabia, Peru, Australia, Russia, and China.
The beneficiation of phosphate ore typically follows a dry crushing and screening operation where ore is prepared for the downstream wet beneficiation circuits. As will be discussed further in this paper, the use of dry sensor-based sorting has taken on new meaning with the drop in P2O5 grades in the ground by helping to reduce waste mass ahead of the wet processing circuit. The crushing is traditionally achieved using gyratory crushers, jaw crushers, and cone crushers for igneous phosphate ores. The crushers used in sedimentary phosphate ore processing are mineral sizers, jaw crushers and impact crushers.
The wet processing circuit consists of grinding, classification, magnetic separation, flotation, dewatering, filtration, and drying. When processing igneous phosphate ores, semi autogenous grinding (SAG) mills, rod mills, and ball mills are typically used. With higher throughputs, use is made of SAG mills in combination with ball mils. For smaller throughputs, use is made of rod mill and ball mill combination. For sedimentary phosphate ore comminution, use is made of rod mills and ball mills either in combination or on their own. Use is also made of rotary drum scrubbers for partial size reduction of the softer ores.
Figure 3 illustrates a typical process flow for treating sedimentary phosphate ores. The milled slurry is classified using hydro cyclones or hydraulic classifiers. For igneous phosphate ore processing, the classifier overflow is next passed over a low intensity magnetic separation (LIMS) step to remove iron rich magnetic materials prior to flotation. The sedimentary phosphate classifier overflow is sent to a second set of classifiers for removal of ultra fine slimes prior to flotation.
Figure 4 illustrates a typical process flow for treating igneous phosphate ores. The flotation of igneous phosphate ore requires the stagewise addition of reagents to condition the slurry for pH conditioning, iron depression, silica depression, and a phosphate collection. The flotation of sedimentary phosphate ore can be carried out to either float the phosphate as a concentrate or as tails while floating the gangue minerals. The flotation of phosphates makes use of mechanical and non-agitated flotation cells and columns.
The phosphate concentrate from the flotation circuit is dewatered in thickeners to increase the solids concentration prior to being pumped to a filtration circuit. The selection of filters can be either vacuum belt or pressure plate and frame filters. Typically, in phosphate beneficiation, the preferred filter is the vacuum belt filter.
Once the phosphate concentrate has been dewatered to a suitable moisture content, the material is sent to a dryer circuit where the excess moisture is removed. The dryers typically employed are rotary drum dryers or flash dryers.
The residues from both igneous and sedimentary phosphate beneficiation are collected in thickeners where the slurries are flocculated to aid in settling. The thickener underflow streams are either pumped directly to wet stack tailings storage facilities or they can be dewatered using vacuum belt or pressure filters before the filter cake is conveyed to engineered dry stack storage pads.
2.1 Waterwise Technologies
To a large extent, phosphate beneficiation plants are in areas where there is limited water or brackish hard water. A key development to come out of this predicament is the drive towards using saline/hard water in wet beneficiation with quality water (potable water) used to rinse the chlorides from the final phosphate concentrate. At present, the cationic amine flotation route for silica removal is most amenable to saline conditions. In recent years, flotation chemical suppliers have sought to improve the ability of anionic fatty acid type collectors to cope with chlorides and hard water conditions.
A less pleasant issue is the litany of tailings dam breaches over the last few years around the globe. This has forced operators and equipment suppliers to develop methods to better manage the recovery of water from tailings. The tailings are traditional thickened and then pumped to residue storage facilities with decant water return systems. The newer approach is to dewater residue using large filter presses and in some instances decanter centrifuges where the solids are conveyed onto stacks or into old mine workings.
2.2 Dry Processing and Preconcentration
In times of water scarcity and dwindling ore grades, preconcentration and dry processing is becoming a norm. This has been achieved using sensor based sorting and other technologies such as tribo-electrical separation. Technology providers such as Steinert and Tomra have developed sensor-based sorting technologies over the last decade initially for magnetic materials, and then for sulphide minerals, and finally the ability to separate silica from phosphate.
Figure 5 illustrates a typical sensor-based ore sorter used in the minerals industry. To date, the largest sensor-based sorting operation in phosphate is located at the Ma’aden Wa’ad Al Shamal Phosphate Company plant in Saudi Arabia.
Tomra sensor-based ore sorter [7]
Another area of interest is the processing of phosphate ores (high silica sedimentary ore and igneous ore) is the use of high-pressure grinding rolls (HPGR) comminution technology. The technology is energy efficient relative to a conventional crushing and grinding circuit. The use of the HPGR technology is used to limit the amount of ultra fines generated in the comminution stage by reducing the amount of breakage required in size reduction to liberate the P2O5 from entrained gangue.
2.3 Low Grade Ore Applications
The extraction of phosphate ore from sedimentary deposits is usually achieved from run of mine ore grades with phosphate content (expressed as P2O5) in the low to mid-twenties (P2O5). The reject streams from this material still contain P2O5 values (15 to 24%).
Technology has been developed over the last decade that can recover this lost P2O5 from the reject streams in the form of either mono calcium phosphate (MCP), di calcium phosphate (DCP), or phosphoric acid using either hydrochloric acid (HCl) or sulphuric acid (H2SO4) digestion. The HCl digestion route, developed by EcoPhos (part of Prayon Technology) can treat ores with impurities (Al + Fe) not normally accepted for use in the wet phosphoric acid production process. The impurities are removed as chloride salts as a byproduct of the manufacture of MCP and DCP. The choices of either the HCl or H2SO4 process rely on the availability of locally produced or supplied HCl (ex-chloralkaline plants) or H2SO4 (ex-base metal refineries).
The MCP/DCP can be used as animal feed supplements or as a high purity P2O5 feedstock to produce phosphoric acid (H3PO4). Where there is no access to HCl, Prayon has developed a sulphuric acid based leaching process to treat the low-grade ore using weak H2SO4 as well as limestone to either produce MCP or DCP.
The DCP can be used as a feedstock for phosphoric acid production instead of phosphate concentrate. The DCP as a phosphoric acid raw material will have low impurity levels and consequently, the gypsum by-product will be cleaner compared with using phosphate concentrate as raw material.
2.4 Waste Rock and Process Residues
With today’s ever-increasing SDG need to recycle as much material that is extracted from the ground to prevent wastage, there is an expectation that both tailings and waste rock from mining activities can be recycled or repurposed rather than just storing tailings or creating waste rock dumps. The use of waste rock from igneous phosphate mining activities is looked upon favourably as the waste rock is usually very amenable for use in the construction and building industries. It can be used in numerous applications from ballast, concrete aggregate, erosion barriers, gabions, and asphalt chip and more. The tailings from igneous phosphate beneficiation can be used in the manufacture of geopolymers as well as being used for plaster sand, brick making, and as a sand replacement. It is worth noting that the main constituents in igneous phosphate tailings are typically calcium alumina silicates which are the basic building blocks of any cementitious material.
The use of waste rock from sedimentary phosphate deposits takes the form all building material and subbase backfill material for construction purposes. The beneficiated tailings as a finer sand product can be classified dewatered and used in the building industry as sand for concrete ascend for cement making and plaster sand. The material can also be used in the production of shaped bricks and cinder ash type blocks.
Where this material cannot be used in alternative applications, the best option for the material is for it to be used as back full for existing strip-mining operations as part of a continuous rehabilitation programme to return the land to its original format and appearance.
3 Phosphoric Acid
Phosphoric acid (PA) is used as a major raw material for fertilizers, chemicals, and LFP battery cathode material. The basic premise of phosphoric acid as raw material for each of these downstream applications is the concentration of the acid and required purity. Required purity levels for battery and electronic application would require the highest purity compared to fertilizers.
3.1 Phosphoric Acid Production
Phosphoric acid is produced two processes: wet process and thermal process. The majority of the of the phosphoric acid is produced by the wet process of which approximately 90% is converted to fertilizers [8]. There are two main processes in the wet process route, and these are dihydrate (DH) and hemihydrate (HH) processes. The thermal process constitutes a smaller portion of phosphoric acid production and uses thermal energy in a furnace to release phosphorus as gas, which would be combusted and condensed to produce phosphoric acid.
3.2 Wet Process Route
The wet process typically uses sulphuric acid to release the phosphorus from the rock to produce phosphoric acid. A typical flow scheme is shown in Fig. 6.
The process is divided into 3 main sections: reaction, filtration, and evaporation (or concentration). The output from the process is phosphoric acid (H3PO4) and waste material in the form of phosphogypsum (PG). The PG formed is either the dihydrate form (CaSO4.2H2O) or the hemihydrate form (CaSO4.0.5H2O). As such, the common technologies in the production of wet process phosphoric acid are the dihydrate technology [9] or the hemihydrate technology [10]. It is the conditions in the reactor (e.g. temperature, water content) that govern the type of the PG produced [11].
3.2.1 Reaction
The reaction section consists of agitated reaction vessel, reaction slurry cooling and off-gas scrubbing as shown in Fig. 7. Simply put, in the reaction, vessel phosphate concentrate reacts with sulphuric acid to produce phosphoric acid. The simplified reaction equation is as shown in Eq. 1 [8]:
The reaction vessels are designed by technology providers and can take the form of [9]:
-
Single concrete compartmentalized reactor
-
Single concrete annular reactor
-
Series of reaction tanks
Critical to the reactor design are the residence time, temperature, water, and solids content, sulphuric acid feed as also free sulphate content within the reaction slurry [11]. These factors play a crucial role for the formation of either the dihydrate or hemihydrate PG. The reaction slurry is agitated to ensure dispersion and distribution of reactants. The temperature typically controlled by vacuum (or flash) cooling, where the reactor slurry is pumped at high rates to a flash chamber that operates under vacuum conditions. Under vacuum conditions, the boiling point of water is reduced and enables the slurry to be cooled by evaporation of water. The vacuum is provided by either a vacuum pump or steam ejectors.
Gases evolved in the evaporation process is extracted from the reactor by a fume extraction fan to a scrubbing system, prior to releasing into the atmosphere. The gases are typically hydrogen fluoride (HF) and carbon dioxide (CO2). HF is a by-product of the main reaction as shown in Eq. 1 above. The HF complexes with active silica, which is either present in sufficient quantities in the rock feed or added to the reaction, to form silicon tetrafluoride (SiF4). The SiF4 compound is readily removed by the circulating water in the scrubber liquor where it forms fluosilicic acid (FSA), which is neutralized using lime prior to disposal. The CO2 is a by-product of carbonate-bearing minerals like calcite and dolomite. CO2 is released when these minerals react with sulphuric acid. The scrubbing system typically consists of pre-scrubbing column as well as scrubbing vessels (vertical columns or horizontal cross flow type) [11]. Fig. 8 illustrates a typical spray tower scrubber.
3.2.2 Filtration
The reaction slurry is pumped to the filtration circuit where the phosphoric acid is separated from the PG. This is by the flow reactor slurry onto a filter cloth subjected to vacuum on the underside. The vacuum removes the liquid phase, thus retaining the cake on the cloth. The common filters used in the phosphoric acid industry are either the tilting pan filter or the horizontal vacuum belt filter. The former takes up less real estate.
Fig. 9 illustrates the filtration process that is carried out in four stages [11] with countercurrent filter cakes washes:
-
1.
Extraction of the filter product acid that will be pumped to storage tanks. The product acid concentration ranges from approximately 28 to30% for DH process and 40–45% for HH process. Percentage is expressed as P2O5 not H3PO4.
-
2.
First washing stage where the cake is washed with liquor from the second stage. The filter liquor from this stage is returned to the reaction tank as return acid. It will contain weak phosphoric acid. The concentration of the acid will depend on the technology employed for the process.
-
3.
Second washing stage where the cake is washed using liquor from the third washing stage.
-
4.
Third washing stage uses process water to wash the final PG to extract phosphoric acid prior to PG storage.
The filter performance is governed by solids content in the slurry feed, temperature, viscosity, and the size and shape of crystals [11]. Based on these factors, operations will adjust the speed of the filter, vacuum applied, and cake wash water quantity. The performance of the filters will dictate the phosphoric acid recoveries, which would otherwise be lost to the PG.
The PG from the filters is considered waste and stored. PG is stored in huge piles known as stacks. For a 1 million tpa merchant-grade phosphoric acid solution (54% P2O5) process, approximately 2.5 million tpa PG is produced. It is transported via conveyors or slurried and pumped away for storage. Dry stacking uses the former method, while wet stacking uses the latter. In wet stacking, recovered acidic water could be brought back into the process as PG slurry water or in some instances, it could be used to recovery lost phosphoric acid.
Stacking and managing is costly and is estimated to be US$ 25/ton PG [12]. The cost components are:
-
Value of land,
-
Environmental issues including reporting and compliance,
-
Construction,
-
Process and heavy equipment,
-
Operations and maintenance and monitoring,
-
Utilities requirements,
-
Eventual cost of closing the stack,
-
Remediation of surrounding land after closure.
While the business-as-usual (BAU) model is tempting and a well-trodden path, companies are increasingly exploring avenues to reap benefits of this product. Nearly all gypsum is used in three prime applications: building construction, cement (where it is used as a setting retarder), and agriculture (mostly for soil conditioner and fertilizer). Another common application is wet or powdered plaster. Minor applications include dentistry and surgical/medical, e.g. plaster casts.
The reaction and filter circuits are interconnected in that the performance of one affects the other. Therefore, when the phosphoric acid plant is being operated, personnel will have to analyse the system before making changes and an important consideration being water balance.
3.2.3 Evaporation
The filter product acid has to be concentrated via evaporation for downstream uses. The evaporation process uses single stage forced circulation process and consists of:
-
Heat exchanger
-
Flash chamber
-
Fluosilicic acid (FSA) recovery
-
Barometric condenser
-
Vacuum pump
-
Acid circulation pump.
In the evaporation section as illustrated in Fig. 10, filter product acid is concentrated to approximately 54% acid expressed as P2O5. The acid is concentrated by way of flash chamber which operates under vacuum, which lowers the boiling point of water. The loss of water concentrates the acid. Vacuum is applied using a vacuum pump and maintained by the barometric condenser.
The evaporation process temperature is maintained by low pressure steam (LP steam). The LP steam source is usually an adjacent sulphuric acid plant that produces high pressure steam as part of heat recovery. The LP steam heats the recirculating acid in the heat exchanger before the flash chamber.
The evaporation process releases fluorine as hydrogen fluoride (HF) or silicon tetrafluoride (SiF4). These fluorine-laden gases are captured in the FSA unit as FSA. The recovered FSA is either neutralized and disposed or sold as calcium fluoride. FSA can also be sold for downstream conversion to aluminium fluoride or cryolite for the aluminium industry.
The concentrated acid is pumped to storage tanks for downstream processing into fertilizer or chemicals. In some instances, the acid may be clarified to removed solids prior to being sold to the market or long distance transportation by rail or road. The clarifier underflow, typically called sludge, will either be returned to the reactor that will allow phosphate units to be recovered or used to produce downstream fertilizer such as triple super phosphate (TSP) of monoammonium phosphate (MAP). Note that if the downstream fertilizer plant is adjacent to the phosphoric acid unit, sludge removal is not required as it will aid in the formation of granulated fertilizers. The sludge composition is dependent the phosphate rock quality fed to the phosphoric acid plant and will comprise of an array of compounds. Typical compounds are gypsum, sodium, and potassium fluorosilicates, calcium fluoride, and complex aluminium and iron phosphate compounds.
The sludge and PG streams from the phosphoric acid process are sources of 17 rare earth elements (REE). These are the 15 lanthanides as well as yttrium and scandium. It has been reported [13] that the phosphate rock has REE concentration ranging from 0.01 to 0.1% and that the concentration is dependent on rock origin as well as phosphate (P2O5) content of the phosphate rock. The REE can potentially be recovered using ion exchange, solvent extraction, and decanter centrifuge. While the technical feasibility for the recovery/extraction of REE has been studied and proven, the economic feasibility has not yet been achieved [14].
3.3 Thermal Process Route
The thermal process route uses thermal energy to release phosphorus and subsequently for the production of phosphoric acid. The units are divided into two major facilities: yellow phosphorus (YP) plant and thermal phosphoric acid (TPA) plant. Fig. 11 illustrates the YP plant furnace section of the thermal phosphorus production facility.
Schematic showing typical arrangement of a delta type furnace [8]
3.3.1 Yellow Phosphorus Plant
Phosphate rock, silica, and metallurgical coke are delivered to the YP plant where they will be unloaded and conveyed to their respective covered storage locations. The phosphate rock, silica, and metallurgical coke will be conveyed to their respective sizing and screening units within a raw materials preparation area. The raw materials preparation area will be equipped with a dust collection system. The screened materials will be conveyed and stored in storage bins.
The sized phosphate rock, silica, and metallurgical coke will then be conveyed to the top of the electric submerged arc furnace within the YP area. The feed materials will be fed via vertical chutes into the furnace. The feed rate of the raw materials will be proportioned and mixed with silica and metallurgical coke using accurate flow measurements [8]. The material feed proportions will primarily be based on P2O5 to carbon ratio as well as silica (Si) to CaO ratio. However, contents of other species (e.g. sulphur) should also be considered, which could affect silica and carbon (coke) feed rate relative to phosphate rock.
The electric furnace is a submerged electric arc furnace. The furnace will be equipped with cylindrical, pre-baked carbon-graphite electrodes that are vertically hung electrodes via suspension cables or water-cooled electrode clamps. The reaction, which is endothermic, between phosphate rock, silica, and coke (carbon source) is as shown in Eq. 2 [8] below at a reaction temperature greater than 1100 °C. Optimum reaction temperature is between 1400 and 1600 °C. Temperatures over 1700 °C will not only result in side-reactions but also increases energy consumption.
The furnace will produce molten slag and ferro phosphorus alloy as by-products, which will be tapped, cooled, and conveyed for storage. The gas stream, which will be rich in phosphorus with carbon monoxide and hydrogen, will leave the furnace via an off-gas duct through an electrostatic precipitator to condensers. The electrostatic precipitators serve to remove dust from the off gas. The phosphorus gas in the condensers will condense to liquid phase by water sprays.
The liquid phosphorus and the spray water from the condensers will be gravity fed into a collection sump. Although most of the dust and particulates in the off-gas stream will have been captured by the electrostatic precipitators, some carryover of these contaminants will be expected in the condenser outlet stream. In the collection sump, the liquid phosphorus, which is denser than the water, will separate out and settle at the bottom. The particulates, however, will be distributed between the water and phosphorus layers.
The water will be separated from the yellow phosphorus and transferred to holding tanks to separate out the solids (sludge or mud) from the water. The water will then be recycled to the spray condensers. Neutralization may be required depending on the pH of the water stream. The liquid yellow phosphorus will be pumped to purification tanks to separate out the solids. The clean yellow phosphorus will be pumped to storage, while the sludge will be transferred to sludge (mud phosphorus) tanks. Low pressure (LP) steam will be supplied to the tanks and piping system to maintain the temperature of the YP at approximately 80 °C.
The clean yellow phosphorus will be pumped from the storage tanks TPA. Fig. 12 illustrates the thermal grade phosphoric acid plant.
Typical flowsheet of a TGA plant [8]
3.3.2 Thermal Phosphoric Acid Plant
The liquid YP will be pumped to a combustion tower and atomised via a nozzle and is combusted (oxidized) to P2O5 gas in the presence of dried air. Compressed air will be used for atomisation. The dried air for combustion will be blown directly into the combustion chamber. The burning capacity is influenced by the degree of atomisation and quantity of excess air. The flame temperature will be in the range of 1800–2000 °C. The combustion reaction equation is as shown in Eq. 3 [8] below.
The P2O5 gas is condensed in a hydration (or condensing) tower by contact with recirculating phosphoric acid to produce thermal grade phosphoric acid (TGA). The P2O5 gas enters the hydration tower from the top via a gas duct and will be condensed by downward facing recirculating acid. The acid will be delivered by spray nozzles located at the top of the tower. The contact between the sprayed acid and P2O5 gas will result in the absorption of P2O5 into the recirculating acid stream. The absorption process will concentrate the acid; however, a diluted phosphoric acid stream will also be directed to the top of the tower from downstream scrubbing system, and this provides a means to control the concentration and maintain the target concentration of 85.5% H3PO4 (equivalent to 61.9% P2O5). The acid, TGA, will be collected at the bottom of the tower. A portion of the TGA will be pumped to storage tank, while the remainder will be pumped to a set of plate heat exchangers to be cooled using cooling water. The cooled acid will be returned to the hydration tower. The reaction for hydration is represented by Eq. 4 [8] below.
The tail gas steam from the hydration tower will contain phosphoric acid mist, water vapour, and non-condensable gases (e.g. nitrogen). To improve system efficiency and recovery of phosphoric acid, a scrubbing system consisting of venturi wet scrubbing and wire-mesh contactors (demisters) will be typically used. The diluted phosphoric acid stream collected within the scrubbing system will be pumped to the hydration tower as mentioned earlier.
The oxidation reaction within the combustion tower is exothermic. For every tonne of YP combusted, approximately 24,290 kJ is released [8]. This heat energy will be recovered in a waste heat boiler to produce steam that could be used to preheat combustion air and demineralized water as well as maintain YP temperature.
To meet specifications of the downstream users for high purity acid that could be used for specialty chemicals, battery materials, and electronics, it is critical that the TPA meets the required industry quality. Purification of TGA to TPA will involve de-arsenification process, which will also reduce other heavy metals. Fig. 13 illustrates a typical de-arsenification flowsheet. The arsenic and other metals will precipitate as sulphides. Sodium sulphide (2% solution) is typically used as the arsenic removing agent [8].
Typical de-arsenification process flow [8]
The imported sodium sulphide (Na2S) solution and TGA will be pumped from their respective storage tanks and mixed in an inline static mixer and fed into an agitated reactor. The reactor will be operated and maintained at ~80 °C via steam sparging into the vessel. The reaction between TGA and Na2S is as shown in Eq. 5 [8].
The H2S will then react with arsenic (As3+) and other heavy metals (e.g. lead) to form sulphides that will precipitate (arsenic sulphide and lead sulphide) as shown in Eqs. 6 and 7 [8]. Similarly, other heavy metals will precipitate as sulphides in the reactor.
The sulphide precipitates from the reactor will then be separated by filtration from the acid via plate and frame filters. Activated carbon could be used as filter aid. It will be mixed in a carbon slurry tank with de-arsenicated acid (TPA) from the TPA storage tank. This 25% activated carbon slurry is pumped into the reactor prior to transferring the reactor contents to the plate and frame filter. The activated carbon serves to capture any residual sulphides as well as organic species. The clear acid from the filter (filtrate) will then be transferred to a H2S stripping column that will serve to remove residual H2S in the acid. The stripping will be via filtered air blowing upwards. The filtered air will contact the acid flowing downwards, with the contact between acid and air maximized with packings. The stripping column will be fitted with a demister pad to capture and recover acid mist. The H2S-stripped acid will flow into de-arsenicated acid (TPA) storage tanks. The filter cake from the plate and frame filter will be collected, stored, and disposed as hazardous material.
The gas streams from reactor, filter, stripping column, TPA storage tanks will be ducted to a scrubbing system where the H2S will be reacted with recirculating sodium hydroxide (NaOH). The scrubbing reaction will enable recovery of Na2S according to Eq. 8 [8]. The recovered Na2S will be transferred to the Na2S storage tank and recycled.
The production of yellow phosphorus generates large amounts of calcium silicate slag as a by-product. Typically, the slag is granulated during the tapping process. The granulated slag is dewatered and stored on the smelter site. Currently, research indicates that YP slag which is a calcium silicate can be used in the manufacture of clinker cement, cinder blocks, and sand products.
The clean CO-rich gas coming from the furnace off gas cleaning circuit can be used as a source of energy for local heating. Heat from the non-condensable gases may be recovered to:
-
Pre-heat the furnace feed materials to reduce moisture,
-
Use in the raw material briquetting plant to briquette feed material prior to the furnace
-
Fuel for the local boiler
-
Combusted prior to release into the atmosphere.
4 Other Phosphate Derivatives
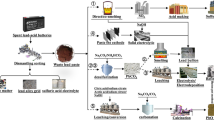
The downstream uses of both wet process and thermal process are as depicted in Fig. 14 below and have been described in previous sections. The value chain of phosphorus cannot be complete without a short description of other minor industrial applications via the production of phosphorus trichloride and phosphorus pentasulphide. These two phosphate rock derivatives have small and specialized markets. It must be stated that all the producers of these two compounds have integrated downstream facilities.
Phosphorus trichloride is made by the reaction of yellow phosphorus with chlorine. It has no direct uses but is a precursor for downstream applications as shown in Fig. 14.
Phosphorus pentasulphide is manufactured by reacting yellow phosphorus and molten sulphur in a reactor in an inert gas atmosphere. Vapours of phosphorus pentasulfide are condensed to the liquid form and then fed to a flaker, prior to packaging or ground into a powder. Its application is also shown in Fig. 14.
5 The Phosphate Value Chain and Circular Economy
The phosphate value chain depicted in Fig. 14 shows the possible routes for the flow of phosphorus from the ground to the end user. The flow will be inevitably incorporate many intermediate processes and handling that can lead to wastage that may or may not be recoverable, hence inefficiencies. Hermann et al. [15] describe that the objective of a circular economy is to enable redesigning, reusing, and recycling resources, materials and products, which will enable continuous use of phosphorus units, while avoiding waste.
The role of circular economy is never ending and involves continuous investigation by phosphorus consumers throughout the value chain to look for ways of improving efficiencies and recovery of phosphorus units.
The phosphoric acid industry has been practicing circular methods, especially with various ways of improving P2O5 recoveries in processing. In wet process phosphoric acid, producers have investigated ways of improving efficiencies via revamps and retrofits to squeeze extra units. Typical DH and HH units usually have efficiencies of 95% and 93% respectively, but this could be increased to 99% with retrofits [10]. This efficiency improvements will mean more phosphorus units recovered rather than disposed in the waste. The improved recoveries will also translate to cleaner phosphogypsum that can find uses, rather than disposed in gypsum stacks. Note that high P2O5 in gypsum stack typically means higher acidity, which will require complex and cost-prohibitive processing.
Further down the value chain, soluble fertilizers or liquid fertilizers are seeing increased demand. This is due to better and more efficient uptake by plants. This easily translates to less runoff to water bodies and subsequent eutrophication, which is a well-documented downside of traditional granular fertilizers.
Efforts are also being made to recover phosphorus units from other waste material. This includes wastewater, sewage sludge, and incinerator ash. Monat et al. [16] describe the process of recovering phosphorus units from wastewater for instance utilizes water treatment plants fitted with electrodialysis units and reverse osmosis. The benefits here include recovery of clean water and recovery of phosphorus units that can potentially be fed to the phosphoric acid plant as raw material.
Meyer et al. [17] have investigated the recovery of phosphorus units from sewage sludge to be used fertilizer either as direct recovery from the sewage plant or recovery of phosphorus units from sewage incinerator ash. Lorick et al. [18] note that the challenges here are:
-
The plant availability, which is lower than that of the traditional mineral fertilizer
-
Geo-spatial limitations with regard to logistics of the sewage treatment facility and agricultural lands.
Phosphoric acid is not just important as a raw material for the production of plant nutrients, which is subsequently consumed by humans for their nutrition, but plays a critical role in energy transition and the climate. The demand for phosphorus in the battery industry has seen a surge recently with each producer looking for means of improving battery performance. One such material is the lithium iron phosphate (LFP) used in battery cathodes. One of its precursors is phosphoric acid.
6 Introduction of Lithium Iron Phosphate
Lithium iron phosphate (LFP) batteries are one of the earliest types of lithium-ion battery. LFP cathode material has theoretical capacity of 170 mAh/g, and relatively low energy density limited by the voltage (3.4V) comparing with energy density of the ternary lithium battery. However, the technology advance, such as carbon coating, metal doping, and cell-to-pack (CTP) technology, has improved its conductivities and energy density, which has now made LFP a very competitive option used in electric vehicles (eVs) battery sector and energy storage systems (ESS) battery sector.
With the increased demand of EV battery and ESS, lithium-iron-phosphate (LFP) material is rapidly gaining popularity due to its cost and safety advantages versus the ternary cathode material.
Wood Mackenzie [19] forecasts LFP will be the most used cathode materials in passenger car and commercial vehicle batteries by 2050, accounting for 45.7% of total cathode demand, because of their low cost, low toxicity, and well-defined performance.
The production of LFP requires lithium source, iron source, and phosphorous source. The high-level block flow diagram outlining the process is depicted in Fig. 15 below. Depending on the LFP production technologies applied, some of the popular feed material sources can be varied and listed in Table 1.
6.1 Pathway from Phosphate Ore to LFP Production
Phosphorous source is a critical component in the production of LFP. The surging demand for LFP has resulted in a corresponding increase in the demand for phosphate. Phosphate mining companies are showing increasing interest in developing a vertically integrated value chain from phosphate ore mining to the production of LFP. Along the value chain from phosphate ore to LFP production, the following opportunities are available for ore mining companies to consider in their integration efforts.
At an entry level of the value chain to integrate to LFP production, mining groups can investigate the supply of high purity phosphoric acid that can be used to produce LFP. Most of LFP manufacturers require food grade phosphoric acid. The choice between a thermal or wet process route to produce high-purity phosphoric acid depends on the characteristics of the phosphate ore. Traditionally, the phosphoric acid produced by thermal route has higher purity. With the technology advance used in the purification, acids produced by wet process route are also well used in the LFP production. These two processes have been described earlier in this manuscript.
Further down to the supply chain, there is option to produce precursor material, such as iron phosphate, for sale to downstream LFP manufacturers who utilize the solid-phase method in their LFP production process. Depending on the specific LFP route chosen, the product of the precursor can be different. Understanding the market requirement from the downstream LFP manufacturers is critical.
Finally, it is to produce the final lithiated LFP product. The iron, phosphorus, and lithium sources are homogenously mixed, milled, spray dried, and thermally calcinated to produce the final LFP. The LFP production is subject to stringent process control and quality control to achieve the satisfied product specifications in terms of stoichiometric ratio chemistry, particle size distribution, particle morphology, and electrochemical attributes. It is essential that the product quality is consistent and meeting the downstream customers requirement.
In recent years, there has been growing interest in lithium-ferro-manganese phosphate (LFMp). particularly in the Chinese cathode material market. Introducing manganese into the formula of LiFexMn1-xPO4 offers the advantages of enhancing the energy storage capacity of batteries and prolonging battery life without sacrificing the cost efficiency and safety features of LFP.
6.2 LFP Recycle
The growing concerns as the waste batteries that are retired from the EV and ESS sectors would eventually contribute to environmental pollution.
LFP battery recycles have been extensively investigated in recent years. LFP batteries contain fewer valuable metals, which means applying conventional pyrometallurgical recycle method, such as smelting, is not economically feasible. Lithium cannot be recovered as it alloys with aluminium in the slag during the smelting. It might even cause an additional environmental burden [20]. The LFP battery recycle technologies can be categorized into three groups, including hydrometallurgical method, solid regeneration, and cascading usage.
6.2.1 Hydrometallurgical Recovery
Using hydrometallurgical method to recover the lithium and iron phosphate from spent LFP batteries has been extensively investigated. This route involved leaching the valuable elements from cathode material into solution using acid/alkaline reagent, followed by impurities removal and precipitation to produce lithium salts and iron phosphate, which are then used for LFP re-manufacture. The drawbacks of this method include the complexity of the process as well as high consumption of the reagents, which lead to higher capital cost and operating cost. However, it is reported that using hydrometallurgical method to recycle and remanufacture LFP could reduce the environmental burden by more than 30% compared with that using raw material [21].
6.2.2 Solid State Regeneration
The LFP material collected from the spent batteries is mixed with lithium resource, carbon resource, and processed via ball milling and sintering to produce LFP. This method is relatively simple and does not need significant amount of acid or alkaline reagent. However, the impurities cannot be sufficiently removed in the process. This method only suitable for the LFP powders that have low content of the impurity. The LFP material produced using solid state regeneration has inferior electrochemical properties, which limited development in industrial scale.
6.2.3 Cascading Usage
LFP battery used in EV sectors will be decommissioned when the battery capacity is below 80% of the original capacity [22]. This method is to repurpose the spent LFP battery into a lower energy requirement application, such as backup power for telecommunication tower [22], power tools, and PV power station [23]. The retired battery pack is assessed, disassembled, cleaned, calibrated, and reassembled, for the use in less power demanding applications [24]. It provides an environmental-friendly and cost-effective measure to prolong the life cycle of LFP batteries. However, safety concerns regarding to utilizing the repurposed batteries and difficulties to obtain large feedstock of the retired batteries with consistent battery quality, as well as difficulties to disassemble the EV-packs are the key barriers for the commercial development.
The biggest challenges to recycle LFP materials are its economic feasibility. Unlike lithium nickel cobalt manganese (NMC) material, which contain high value metal such as Ni, Co, and Li, LFP cathode material consists relatively abundant and cheap Fe and P. Developing green and low-cost recycling technology for battery recycle will be the key focus. Secondly, the lack of recyclable spent LFP batteries will limit the development of the battery recycle industry [25]. Last but not the least is the fire safety in the battery recycle facilities needs to be particularly addressed during engineering design and operation, where the spent battery, leaching reagent, aluminium foil scraps storage as well as carbon, etc. all expose risks of fire or even explosion.
7 Conclusion
Phosphorus is indeed a very important element and has many applications ranging from fertilizers, specialty chemicals and components in batteries. Regardless of its application, the phosphorus journey begins at the mine. This paper has attempted to describe this phosphorus journey from the mine to its processing to intermediate products as well as selected final products as well as recycling of an essential commodity — the LFP batteries.
Data Availability
We do not analyse or generate any datasets, because our work proceeds within a theoretical and experiential approach. One can obtain the relevant materials from the references below.
References
Hilton J (2022) Revaluing NORM residues: the circular economy dividend. Paper presented at Norm X Symposia 2022, Utrecht Accessed 11 Feb 2023
Hilton, J (2020) Leadership, innovation, partnership – setting the scene. Paper presented at International Conference on the Management of NORM in Industry, Virtual Event
Hilton J (2010) Phosphogypsum (PG): uses and current handling practices worldwide Aleff group. Paper presented at 25th Annual Regional Mining & Phosphate Conference, Florida Accessed 11 Feb 2023
Hilton, J 2021, ‘Revalorisation of residues in the scope of the circular economy insights from the fertiliser industry.’, paper presented at National Workshop on the Holistic Approach to NORM Management, Accessed 11 Feb 2023.
Van Kauwenbergh SJ (2010) World phosphate rock reserves and resources, www.IFDC.org. International Fertilizer Development Center, Alabama https://pdf.usaid.gov/pdf_docs/PNADW835.pdf
Cordell D (2010) The story of phosphorus, PhD Thesis. Linkoping University, UTS, Sydney and Linkoping University, Sweden
Tomra n.d., COM XRT 2.0, www.tomra.com. https://www.tomra.com/en/solutions/mining/products/com-xrt. Accessed 17 Feb 2023
Gilmour R (2013) Phosphoric Acid. CRC Press
UNIDO & IFDC (1998) Fertilizer manual. Kluwer Academic Publishers, The Netherlands
Gobbitt JM (2012) Yara hemihydrate (HH) and hemidihydrate (HDH) processes for phosphoric acid production. Procedia Eng 46:143–153
Becker P (1989) Phosphates and phosphoric acid: raw materials, technology, and economics of the wet process, vol 6, 2nd edn. M. Dekker, New York
Wing J (2016) The phosphogypsum era: utilizing the phosphate industry’s most abundant co-product. In: AIChE Clearwater Phosphate Conference, Florida
Al-Thyabat S, Zhang P (2015) REE extraction from phosphoric acid, phosphoric acid sludge, and phosphogypsum. Miner Process Extr Metall 124(3):143–150
Jang GG, Ladshaw A, Keum JK, Thompson JA, Zhang P, Tsouris C (2023) Continuous recovery of phosphoric acid and rare-earths containing particles from phosphoric acid sludge using a decanter centrifuge. Chem Eng J 458:141418. https://pdf.usaid.gov/pdf_docs/PNADW835.pdf
Hermann L et al (2018) Phosphorus processing—potentials for higher efficiency. Sustainability 10(5):1482. https://doi.org/10.3390/su10051482
Monat L, Zhang W, Jarošíková A, Haung H, Bernstein R, Nir O (2022) Circular process for phosphoric acid plant wastewater facilitated by selective electrodialysis. ACS Sustain Chem Eng 10(35):11567–11576
Meyer R, Luthin A, Traverso M (2022) Circular economy of phosphorus – challenges and findings in performing comparable LCA-studies of phosphorus-recycling. In Albrecht S, Fischer M, Scagnetti C, Barkmeyer M, Braune A (eds) E3S Web of Conferences, vol 349, p 01008
Lorick D, Harder R, Svanström M (2021) A circular economy for phosphorus in Sweden—is it possible? Sustainability 13(7):3733
Shang K (2021) Global cathode and precursor market - 2021 outlook to 2050. Wood Mackenzie - Commodity Market Report. https://my.woodmac.com/document/555115. Accessed 10 Feb 2023
Quan J, Zhao S, Song D, Wang T, He W, Li G (2022) Comparative life cycle assessment of LFP and NCM batteries including the secondary use and different recycling technologies. Sci Total Environ 819:153105 Accessed 22 Feb 2022
Chen Q, Lai X, Hou Y, Gu H, Lu L, Liu X, Ren D, Guo Y, Zheng Y (2023) Investigating the environmental impacts of different direct material recycling and battery remanufacturing technologies on two types of retired lithium-ion batteries from electric vehicles in China. Sep Purif Technol 308:122966
Ryszko U, Rusek P, Kołodyńska D (2023) Quality of phosphate rocks from various deposits used in wet phosphoric acid and P-fertilizer production. Materials 16(2):793
Lorick D (2019) Assessing the possibility of a circular economy for phosphorus in Sweden, Master’s Thesis in Industrial Ecology. Chalmers University of Technology, Sweden
Li X, Zhang L, Liu Y, Pan A, Liao Q, Yang X (2019) A fast classification method of retired electric vehicle battery modules and their energy storage application in photovoltaic generation. Int J Energy Res 44(3):2337–2344
Qi C, Wang S, Zhu X, Zhang T, Gou Y, Xie Z, Jin Y, Wang Y, Song L, Zhang M (2022) Environmental-friendly low-cost direct regeneration of cathode material from spent LiFePO4. J Alloys Compd 924:166612
Acknowledgements
Data from this manuscript had been presented in the Symposium in Honour of Patrick R. Taylor at the MINEXCHANGE 2023 SME Annual Conference & Expo, held in Denver, CO, February 26–March 1, 2023.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wingate, E., Prasad, R. & Liu, Y. Phosphorus — a Circular Journey from the Ground to the Recycling Line. Mining, Metallurgy & Exploration 40, 1469–1485 (2023). https://doi.org/10.1007/s42461-023-00839-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-023-00839-6