Abstract
The Mercer Clay bed in central Pennsylvania has produced feedstocks for the refractory industry in the USA and has also been investigated as a source of alumina and lithium. Developments in global markets for these commodities, as well as rare earth elements, have led to their classification as critical minerals in the USA, in turn renewing production opportunities for the Mercer Clay resource. The work reported here includes a review of the clay types and minerals involved, as well as past mineral processing and extractive metallurgy test work, and reports new research results from renewed investigation of the deposit as a polymetallic resource. This renewed work has found lithium contents that exceed 1,000 ppm, lying directly below the overlying Mercer coal, where the alumina content ranged from 32 to 34 wt%. Total rare earth concentrations were somewhat lower than have been found elsewhere in the region, and the highest contents were also found to be stratigraphically close to the coal. Further work is required to establish the mineral hosts for lithium and rare earths and to define the extent of enriched alumina, lithium, and rare earth concentrations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Policy and Market Drivers for Critical Mineral Research in the USA
Technological innovations in the defense, electronics, energy, medical, and telecommunications sectors have involved the development of new applications and significant worldwide market growth for certain non-ferrous metals. This in turn has driven new searches for mineral resources from which these metals can be produced.
In the USA, there are both policy and economic drivers behind the search for new mineral resources. In 2015, non-fuel minerals used by major industries added $2.5 Trillion to the US economy [1]. Among the non-fuel mineral commodities for which the USA was import-dependent were feedstocks for primary aluminum production (alumina produced from bauxite), as well as other metals required for technological applications, such as lithium and rare earth elements (REEs). More recently, as a result of the 20 December, 2017, Presidential Executive Order (1387), many of these commodities are now on the US Department of Interior’s Critical Minerals list [2].
One potential resource for these commodities, having received attention in the past as an alternative to imported bauxite, is the Mercer underclay (Mercer Clay), which is found directly below the Mercer coal zone in the Northern Appalachian region of the USA. Multiple organizations, including the US Bureau of Mines, the US Geological Survey, the Pennsylvania Geological Survey, and The Pennsylvania State University have conducted both geologic exploration research and investigations into the potential for alumina recovery, using both mineral processing and extractive metallurgy processes.
Largely subsequent to that body of work, elevated lithium contents were found in the Mercer Clay, and contents of selected rare earth elements (REEs) were also reported [3]. These results, including elevated lithium contents, have been reported for the portion of the deposit found in central Pennsylvania, especially Centre, Clearfield and Clinton Counties. In this region, previous mining operations supported a significant refractory manufacturing industry, facilitating both exploration work and industry interest.
Much of the work regarding alumina production from the Mercer Clay, as well as other high-alumina US clays, occurred prior to 1970. There have been technological advances in both mineral processing and extractive metallurgy since then, possibly offering improved process economics. Additionally, the potential for recovering other metals simultaneously with smelter-grade alumina can result in further economic improvements. This potential is especially notable regarding lithium, with previous work having found contents over 1,000 ppm, and REEs. Both lithium and REEs are considered critical mineral commodities in the USA.
Current market conditions, policy drivers, and technological advances are such that a re-examination of the Mercer Clay is merited, as a multiple commodity mineral resource. This paper will summarize previous work resulting from both geologic exploration and mineral processing and extractive metallurgy research on the subject. Also presented will be recent assay and mineralogical analyses of the material from central Pennsylvania.
1.2 Background on Research on the Mercer Clay as a Mineral Resource
The use of high-alumina Mercer Clay as feedstock for refractory production dates back decades. Some knowledge of the extent of the resource was gained through its production for this application. Concerns about the supply of imported bauxite during World War II led to examination of alternate sources of smelter feedstock in the USA [4]. This in turn led to a series of geologic, mineral processing, and extractive metallurgy research involving the Mercer Clay as a potential alternate alumina resource for aluminum production. The deposit has been discussed in that context in subsequent reviews of alternative sources of alumina [5, 6]. This body of work has produced information on mineralogy, as well as the behavior of these minerals in mineral processing and extractive metallurgy systems, to be discussed here.
National Bureau of Standards (NBS) Standard 97, from the Mercer Clay in Clearfield County, PA, contains 1,070 ppm lithium. This and other earlier data led to a sampling program by the USGS involving mines in the Mercer Clay in several locations in Pennsylvania, including Clearfield and Clinton Counties [3]. These activities, preliminary investigations of high-alumina clays in the USA as potential lithium resources, resulted in a set of published data on both lithium contents of the Mercer Clay and its mineral form in the clay [3, 7, 8].
The body of work on the Mercer Clay as a lithium resource also included data on concentrations of selected REEs, these being sufficiently high in some cases to merit further investigation. Subsequent work by the US Department of Energy (DOE), based on the prior results, has focused on underclays in the Northern Appalachian Region, and rare earth concentrations in the Mercer horizon have been among the data generated recently by DOE.
2 Geological Context
2.1 Overview
The study area lies entirely within the Appalachian Plateaus Physiographic Province of western and central Pennsylvania. Sites in Clearfield County and northwestern Centre County lie within the Pittsburgh Low Plateau Section of the Province, while sites in Clinton County and northern Centre County lie in the Deep Valleys Section. Most of the sites lie within the Medium-volatile bituminous coal fields of the Main Bituminous Field. The region was a structural, geomorphic, and depositional basin from post-Devonian to Pennsylvanian and Permian time. The basin, i.e., the Appalachian Basin [9] or Allegheny Synclinorium [10] or Pittsburgh-Huntingdon Basin [11], is a broad, elongate structural trough that trends in a northeast-southwest direction between the Cincinnati Arch and the Allegheny Front and plunges to the southwest [11].
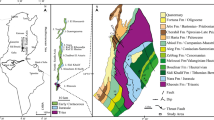
Coal-bearing rocks in Pennsylvania are of Pennsylvanian and Permian ages. In western Pennsylvania the coal-bearing strata are divided into the Pottsville, Allegheny, Conemaugh, Monongahela, and Dunkard Groups (Gp). The Pottsville Gp in which the Mercer clays are found is dominated by sandstone, contains discontinuous coals and claystone intervals, with a basal contact that is disconformable [12], and varies in thickness from 140’ to 200’ in northcentral Pennsylvania [13]. The group is divided into the Elliott Park and Curwensville Formations (Fm). The Curwensville Fm encompasses the Mercer coals and clays and spans up to the base of the Clarion Fm, the basal formation in the overlying Allegheny Gp. The areal extent of the coal measures, and the clay-producing district examined in this work, are shown in the map in Fig. 1.
2.2 Geology of the Mercer Member
The Pottsville Gp contains interbedded intervals of sandstone, shale, coal, and clay. At the base is the Pottsville sandstone, a light-gray trough-cross-bedded quartz-pebble sandstone and conglomerate that fines upwards through the formation [13, 14]. The sandstone contains high amounts of silica, kaolinite, and chert cement in areas where overlain by clay [15]. The Mercer Member is an interval of coal, shale, and claystone beds that range from a few inches to tens of feet in thickness [12]. The coals are described as thinly bedded, blocky vitrain bone coal with thin shaley intervals; coals range from 3 to 30″ thick and the most pronounced Lower Mercer coal overlies lenticular bodies of claystone in places [16] that vary laterally in thickness from 0 to 20’ [6]. The Mercer Clays range in character from dark gray silty clay shale to medium gray, non-fissile claystone [14]. These are characterized as flint, plastic, and nodule clays [15]. The claystone is crystalline and consists of minerals such as kaolinite, diaspore, boehmite, and gibbsite [15]. The flint clays are approximately 40% Al2O3, while nodule clays are ~ 70% Al2O3 and contain accessory minerals such as tourmaline and zircon. Plastic clays are lower in aluminum content and typically contain quartz and illite [15]. The areas of the Mercer Member that lack clay exhibit thin beds of coal underlain by graywacke sandstone that consists of chlorite and metamorphic rock fragments.
The Mercer clays contain organic material derived from fossilized roots and other plant remains. Invertebrate fossils from brackish-water and marine environments can be found within shales that overly some of the coals [13]. Other microbial fossils make up the bulk of the organic constituents in the clay [15]. The Mercer Clay is interpreted to have developed on paleotopographically high areas from weathering of the underlying southwest-dipping Mauch Chunk Formation [17]. High alumina clays formed on top of resistant sandstone shelves in a swampy, coastal environment where chemical leaching enriched the hard clay in aluminum. Williams and Bragonier [15] suggested that the presence of permeable sandstones beneath the clay may have enhanced the percolation of water through the clay, at least seasonally, and therefore created a more oxidizing environment as compared to adjacent locales where no underlying sandstone existed and a reducing environment was encountered. Alternatively, Bolger and Weitz [18] described Mercer clays as having formed as colloidal gels transported in sluggish streams into swamps where the gels crystallized into the clays, with subsequent diagenesis associated with post-lithification shrinkage cracking and crack filling by recrystallized clay minerals. Similarly, Erickson [19] interpreted Mercer clays as having formed from kaolinite and high alumina precipitates and gels that accumulated in an acidic reducing coastal swamp environment.
3 Mineralogy of the High-Alumina Mercer Clay
The nature and distribution of aluminum-bearing minerals in the Mercer Clay has been the subject of significant research with respect to its potential as an alternative to imported bauxite for US aluminum smelter feedstock. While a smaller body of research has been published regarding lithium and rare earth contents, this set of data also suggests potential for deposit as a resource for those elements.
3.1 Al-, Si-, and Fe-Bearing Minerals
Clays found below the Mercer coal in the region have had several conventions for classification, based on texture, alumina content, and behavior in the refractory production process. Broadly, they can be divided into soft clays and high-alumina or hard clays. Mineral content governs where a clay falls within these classifications. The first classification, offered by Foose [16], appears in Table 1.
Foose’s classification used terminology from the mining industry in the region. Subsequently, Erickson [19] offered updated terminology, which was presented for comparison with other definitions by Bragonier [20], as shown in Table 2. While the nomenclature in Table 1 is correlated with Al2O3 content that found in Table 2 is based in part on the fraction of diaspore nodules in the rock. Table 2 also correlates multiple sets of terminology including that of Weitz and Bolger [21] and the industry conventions behind the Foose classification. Hard clays, or high-alumina clays, range from “burnt” nodule clay to semi-flint clay. Non-refractory, or soft clays, contain less Al2O3. Quartz and illite are found in the plastic clays but are absent in the high-alumina clays [15].
The most abundant Al-bearing minerals found in high-alumina zones of the Mercer Clay in central Pennsylvania are kaolinite, diaspore, boehmite, and gibbsite [15]. Among these, kaolinite is the most common and is typically fine grained. As alumina content increases above that of stoichiometric kaolinite, diaspore becomes more common. Most of the diaspore is found as grains in nodules that can range from a fraction of a millimeter to several centimeters in diameter [22]. Nodules can become sufficiently abundant to compose the entire deposit, resulting in rock with 70% alumina [15]. Boehmite occurs in small grains, either in the Al-rich nodules or in the kaolinite-rich groundmass [22]. Gibbsite has also been found, as a minor constituent [19].
High-alumina clays in the region have been produced for the regional refractory industry [16]. The use of these clays for refractory production has been governed in part by the iron content: higher iron contents have rendered some of the material unsuitable as feedstock for refractory production. Iron carbonates can comprise up to 30% of high iron content claystones [22]. As a result, high-iron, high-alumina Mercer Clay, not suitable for refractory production, has been a focus of research as an alternate source of smelter-grade alumina (to be discussed subsequently). Iron carbonates occur as “individual grains in veinlets and as radiating crystals in rosette-like masses that resemble the nodular aggregates of aluminum hydroxides found in high-alumina claystones” [22]. Pyrite occurs as stringers and small crystals and may be associated with organics or tourmaline within the clay [20]. Iron oxides can be present as a “brownish coating around grains and nodules, and as opaque masses” [19].
3.2 Accessory Minerals
Several minerals have been identified in the Mercer Clay that contains critical mineral commodities (USGS classification [2]). These include tourmaline, which may include lithium, and zircon, a possible host of zirconium and hafnium. Additionally, analyses by the US Geological Survey on samples from the mines in the Mercer Clay district in central Pennsylvania included lanthanum and neodymium contents as high as 150 ppm each [3]. More recently, a Mercer Clay total lanthanide content exceeding 500 pm has been found in Clearfield County, PA [23], this being reported as part of a DOE-sponsored exploration program for REEs in the US coal measures.
3.3 Lithium Distribution
The primary mineralogical host of lithium in Pennsylvanian high-alumina clays is unknown. Lithium can be hosted in at least three different ways in these rocks, each of which indicates a different lithium source — it can be (1) structurally bound in clay minerals at the time of formation (precipitation from inland sea waters and/or oxidation of organic-rich peat bogs); (2) adsorbed onto the surfaces of clay minerals (weathering and hydrologic circulation following sedimentary deposition); or (3) concentrated in detrital minerals such as tourmaline (derived from erosion of igneous or metamorphic rocks in paleomountains that once existed in southeast) [7].
First, in the case of structurally bound clay minerals, kaolinite is the primary constituent of PA high-alumina clays, and lithium may participate in a coupled substitution with magnesium whereby magnesium substitutes for aluminum and lithium occupy a vacancy to accommodate the charge imbalance (Li+ + Mg2+ = > Al3+) [24]. It has also been suggested that structural lithium is hosted in minor cookeite (a chlorite group mineral) in the Mercer clays [8]. Second, lithium is known to be concentrated in late-stage magmatic fluids that form pegmatites, from which it can later be leached and potentially adsorbed onto clays or accumulated in evaporites [25]. Although there was no large-scale magmatic activity in this region during or subsequent to the Pennsylvanian Period, igneous rocks are present in the source terrain to the southeast. Finally, magmatic and metamorphic tourmalines can be recycled from older rocks and incorporated into sedimentary materials. Tourmaline has been recognized as a minor but pervasive accessory mineral in Mercer clays from central Pennsylvania [20, 22]. A fourth possibility would involve the presence of evaporitic brines enriched in lithium that may transfer that enrichment to clays either directly via precipitation of smectite or indirectly by adsorption onto clay surfaces [26].
Both tourmaline and zircon in the Mercer Clay were reported as accessory minerals by Weitz [22], and Bragonier [20] reported an average grain size for these minerals of 250 µm, being well-rounded and “not diagenetic or post-diagenetic.” Bragonier also suggested that these minerals with this grain size may have been concentrated through specialized sedimentary processes during deposition of clay particles or that “they were deposited under normal sedimentary processes with a larger detrital fraction that has undergone subsequent removal,” with petrographic evidence favoring the second possibility.
While lithium-bearing clay minerals and tourmaline could both be sources of lithium, their association with other more abundant minerals in the Mercer Clay can be instructive, notably from the standpoint of mineral processing and extractive metallurgy process design. The work of Tourtelot and Meier [7] and Tourtelot and Brenner-Tourtelot [3] reported lithium contents for Pennsylvanian clays in the region, including those from the Mercer horizon. In addition to a lithium assay, each result included a sample description, using nomenclature not unlike those found in Tables 1 and 2. Descriptive terms found in those works include “shale,” “plastic clay,” “flint clay,” “diaspore” (outer ring of nodules), and “diaspore (central part of nodule).” Of these descriptive categories, the highest lithium contents found were associated with flint clay, as shown in Table 3. This data set represents all of the samples that produced lithium assays of 1,000 ppm or greater (sixteen analytical results). Of these, fifteen are described as flint clay, and one is described as the “outer ring” of a diaspore nodule. Of the former, two are also described as adhering to the outer ring of a diaspore nodule, and one is described as flint clay “containing diaspore.”
Data with analyses of the material within diaspore nodules themselves are much more limited in the Tourtelot (USGS) series of publications [3, 7, 8] and are presented here in Table 4. Lower concentrations are found here as compared with the flint clay associations found in Table 3.
Possibilities for the mineral phase in which lithium is present include tourmaline, which has been found by multiple authors, as well as a clay phase (cookeite, from [8]). In the case of the former, tourmaline has been reported as dispersed at random throughout the groundmass [20], with no mention of their presence in diaspore nodules. As diaspore nodules are the result of non-clay mineral formation at the expense of clay, lower concentrations of clay-hosted lithium might also be expected in these nodules, as compared with a surrounding kaolinite groundmass (i.e., the flint clay portion of the rock).
3.4 REE Distribution
While some elevated concentrations have been reported for selected REEs in the Mercer Clay previously, there is considerably less known about its REE than its Li content and mineralogy.
Recent assay results developed under contract to the US Department of Energy have found elevated total REE concentrations in several Lower Allegheny and Pottsville Group underclays in central Pennsylvania. Total rare earth concentrations exceeding 500 ppm have been found in both the Mercer and Lower Kittanning horizons [23].
There can be variations in both concentration and individual element value across the suite of REEs found in a deposit. One useful means of presenting the distribution of individual REEs is through normalizing the concentration of each element, in order of increasing atomic number, to its chondrite concentration. This presentation, a chondrite-normalized distribution, can also provide an expedient means of comparing the relative concentrations of REEs, individually, of different potential ores.
In Fig. 2, the chondrite normalized distribution of lanthanides in a central Pennsylvania (Clinton County) Mercer Clay [23] is shown, normalized to the CI chondrite values of Anders and Grevesse [27] and displayed on a log scale. In addition to the Mercer Clay analysis, an analysis of Mountain Pass rare earth ore is presented. This data set has been calculated from the ore grade and its relative contributions from each element, as found in Krishnamurthy and Gupta [28]. The total rare earth element content for the Mountain Pass ore analysis was 7.7% (reported as oxide). The Mountain Pass deposit has been a significant rare earth producer in the USA [29].
While the total rare earth element content of the Mountain Pass ore analysis used for Fig. 1 was approximately two orders of magnitude higher than that of the Mercer Clay sample (536 ppm, sample PA-006, in [23]), a comparison of the two plots shows that the Mercer Clay sample is actually enriched in the heavier rare elements (holmium through lutetium) and the dysprosium contents are roughly comparable. These data suggest that the Mercer Clay merits consideration as a source of heavy REEs, notably where they could be co-produced with other mineral commodities such as alumina and lithium.
Information regarding the minerals that host REEs in the Mercer Clay remains to be developed, and this information will be essential in process design for their recovery from this potential resource. As has been mentioned in the context of the overlying Lower Kittanning Underclay [30], REEs in the Mercer Clay may be present in detrital or authigenic minerals or adsorbed onto clay surfaces.
3.5 Summary: The Mercer Underclay as a Polymetallic Resource
Significantly, the elevated contents of all of the elements of interest (Al, Li, and TREE) suggest the potential of the Mercer Clay as a polymetallic resource, covering multiple commodities on the USGS Critical Minerals list. Monetizing this opportunity, however, will require the extraction of these elements from the clay and their rendering in forms that are compatible with the aluminum, lithium, and REE value chains. Requirements for the extracted mineral commodities would include market-compatible products (smelter-grade alumina, lithium carbonate, separated rare earth compounds), at purities that meet market standards.
4 Metallurgical Research: The Mercer and Other High-Alumina Clays
4.1 Overview
The concept of geometallurgy relates geology to metallurgical outcomes, where geological, mining, and processing data are co-analyzed “to generate useful information and knowledge to optimize resource profitability” [31]. Toward this end, it is advantageous to begin an examination of potential processing options, within the context of the body of knowledge that has been developed to date regarding the mineral characteristics of the Mercer Clay. While gaps remain in the knowledge of the mineralogy regarding both lithium and REEs in the deposit, the following are relevant to mineral processing and extractive metallurgy applications:
-
Among the high-alumina varieties of the Mercer Clay (flint clays and nodule clays found in Tables 1 and 2), there is a fine-grained groundmass of kaolinite, with an increasing predominance of diaspore nodules as alumina content increases. The alumina content increases as the proportion of nodules in the volume of the rock increases. Nodules can range in diameter from a fraction of a millimeter to several centimeters.
-
Iron-bearing constituents vary in both mineralogy and predominance. Iron-bearing minerals can include carbonates, oxides, and pyrite, and iron contents (as Fe2O3) can range in excess of 20% [19]. Masses of iron carbonates can resemble the Al-rich nodular aggregates found in high-alumina claystones.
-
Tourmaline and zircon grains with average diameters of 250 µm have been reported in the Mercer Clay.
-
The lithium present in the Mercer Clay appears to be associated with the flint clay type. Minerals hosting lithium may include tourmaline and cookeite, the latter being a clay mineral. Limited information suggests a diminished lithium presence within diaspore nodules.
-
While the rare earth minerals in the material have yet to be established, recent work regarding the Mercer Clay has shown enrichment in heavy REEs as compared with published information for the Mountain Pass ore.
Available processes for the recovery of alumina, lithium, and REEs from the Mercer Clay include both mineral processing and extractive metallurgy techniques. Previous research regarding clay products in Pennsylvania has involved both, including that specifically focused on the Mercer Clay. There is also a body of prior research available regarding application of mineral processing to similar mineral assemblages.
4.2 Mineral Processing
Research on the application of mineral processing technologies to the Mercer Clay has involved the reduction of the iron content. While in the past this has been a goal to improve marketability in the refractory industry, it is also relevant to the production of alumina, lithium, and REEs. The enrichment and separation of minerals that are rich in these elements, and the removal of iron- and silica-bearing minerals, would be useful outcomes.
There are also similarities in the mineral separation challenges involving the Mercer Clay and those found in the beneficiation of kaolin products, the latter finding application in the production of paper, ceramics, fiberglass, and paint [32]. Clay feedstocks for kaolin production have similarities with the Mercer flint clays in that the dominant mineral component is kaolinite. In the case of kaolin products, less abundant Fe-, Ti-, and Si-bearing minerals are removed to meet market requirements [32, 33].
Mineral processing research involving the Mercer Clay has included laboratory flotation and electrostatic (triboelectric) separation testing. Additional mineral processing tests involving high-alumina Missouri clays have included work with cyclone classification and tabling. Mineral processing technologies applied in the production of kaolin products have included, in addition to particle size reduction, cyclone classification, flotation, and magnetic separation [32, 34]. The objective in all cases has been to separate non-aluminous minerals from a kaolinite-rich feedstock.
Early beneficiation work regarding the Mercer Clay was driven by the need to minimize the iron content of the material as shipped for refractory production. Smith [35] reported results of flotation tests, using a Fagergren Laboratory Flotation Cell, using Mercer Clay samples that had been ground to 75% passing 74 µm. Under a range of pH conditions and reagent additions to the process, iron rejection to the process tailings achieved values in excess of 35%. However, other work in that series [36] examined the flotation behavior of another sample from central Pennsylvania, resulting in “no appreciable beneficiation” (i.e., iron removal). That work suggested that the nature of the distribution of iron species (oxides “intimately associated with clay and diaspore) rendered their liberation “difficult.” That work used particle size distributions ranging from 89 to 99% passing 74 µm.
Beneficiation of kaolins through flotation has been a commercial practice in the USA for decades, including the introduction of ultrafiltration [34], which allowed for the technology to be applied to finer particle sizes (less than 50 µm).
More recent work on flotation has been applied to “coal-series kaolins” in China. These are found in the coal measures of China [37], as the Mercer Clay is in Pennsylvania. Flotation tests on very finely ground (− 10 µm) coal-series kaolin, using a multi-stage circuit (rougher/2-stage cleaner/scavenger) circuit resulted in an increase in the Al2O3 content of the material from 21.7 (feed) to 34.8% (product), at an Al2O3 recovery exceeding 74% [38].
The nodular varieties of the Mercer Clay contain both kaolinite and diaspore. In addition to the removal of non-aluminous minerals, the separation of kaolinite from diaspore can yield a diaspore product with a significantly increased Al2O3 content and a kaolinite product. The latter makes up the groundmass of flint clay, where results to date suggest a lithium association. Work continues worldwide in the search for means to produce aluminum smelter feedstocks from unconventional ores, and recent reviews by Gibson et al. [39] and Zhang et al. [40] cover the separation of diaspore from clay minerals (including kaolinite) through flotation. Example results reviewed include those of Jiang et al. [41], where flotation tests on − 74 µm mixtures of diaspore, kaolinite, and illite produced a result with a diaspore recovery of over 95% at a kaolinite recovery in the 30% range (illite recovery would not be a concern in the case of the nodular Mercer Clay). These and the other results covered in the reviews suggest that the emerging body of work on flotation for the separation of diaspore from kaolinite could result in useful application to the Mercer Clay, resulting in a diaspore product suitable for processing into alumina, and a kaolinite-rich product that could also host elevated lithium contents. However, as was the case of the early work on flotation, knowledge of the mineral liberation with grinding, involving the Mercer Clay, will be required.
In addition to flotation, there is a body of laboratory physical separations results that is relevant to the potential for beneficiating the Mercer Clay. Laboratory electrostatic separation work was reported by Skelly [42], the purpose being to separate free silica. Some reduction in free silica was shown for a central Pennsylvania flint clay product from Clearfield County (8–10%). That result was achieved using a 212 by 53 µm particle size. Little separation was produced using two other clay products in that work.
High intensity magnetic separation is used in kaolin beneficiation [43], where processes operate on very fine feed particles. The potential for this type of process for the removal of paramagnetic (non-aluminous) minerals from the Mercer Clay will be influenced by the degree of liberation of these minerals (i.e., the particle size distribution). The availability of industrial high intensity magnetic separators, introduced subsequent to most of the research done on the Mercer Clay, merits further research on the subject.
The US Bureau of Mines conducted laboratory physical separations testing on high-alumina clays from Missouri, with characteristic not unlike those of the Mercer Clay. That set of work involved examination of the removal of both quartz and pyrite from ground clay materials. One set of results, by Powell et al. [44], involved wet-ground pyrite-rich clay (composed of kaolinite, pyrite, and quartz), with a 300 micron top size. Laboratory concentration testing was done using a 30 mm hydrocyclone system. The result of the grinding of pyritic clay samples was such that the majority of the product (85 + %) was finer than 37 µm, while significant amounts of pyrite remained in the coarser fractions. This is shown in Fig. 3. Laboratory cyclone tests on this material resulted in a slight increase of the Al2O3 content of the (overflow) product over that of the feed. Perhaps more significantly, the pyrite content of the (underflow) tailing stream was enriched from 2.9% in the feed to 10.5%. Previous work has suggested that tourmaline and zircon are found in grains in the 250 µm size range in the Mercer Clay. If these minerals were to concentrate in the coarser fractions of a grinding product, in a fashion similar to pyrite in the Powell et al. work, hydrocyclone testing could yield a means to concentrate these heavy minerals in a stream separate from the alumina-rich minerals found in the deposit. More work on both mineralogical characterization and liberation behavior of the Mercer Clay will be required in order to further pursue this possibility.
Distribution of pyrite and Al2O3 in a ground pyrite-rich clay sample, data from Powell et al. [44]
4.3 Extractive Metallurgy
As much of the early work on the Mercer Clay involved its potential as a source of domestically produced aluminum smelter feed, laboratory work has focused on the recovery of smelter-grade alumina, and some of the results have progressed to the point of flowsheet design.
The use of hydrometallurgy to produce alumina from clays includes leaching the Al-bearing species into solution, as well precipitating it from solution at an acceptable purity. Given that the Mercer Clay includes Si-bearing kaolinite, and Fe-bearing minerals with concentrations that can exceed 20%, the challenge involves means by which these elements can be kept out of the product.
Research by Conley et al. [4] resulted in the design of a process that involved sintering of the Mercer Clay with limestone and soda ash, followed by leaching and a set of steps involving desilication and precipitation and calcination of an alumina product. The desilication step was complicated, involving lime addition and autoclaving.
Fetterman [45] developed an alternative process whereby the Mercer Clay would be roasted with ammonium sulfate, followed by leaching with 3% H2SO4. Alum would be precipitated, re-dissolved with water, and precipitated again, and a further purification step involving Primene would remove additional iron from the precipitate.
Chao and Sun [46, 47] conducted thermodynamic and laboratory investigations involving the sulfation, at elevated temperatures, of Al-bearing minerals found in the Mercer Clay. These included kaolinite and diaspore, and the process would involve production of aluminum sulfate in a baking or roasting environment, followed by cooling and leaching of the aluminum-bearing material into solution. Under the conditions involved, Si-bearing species are not sulfated and remain in the solid leach tailings. Results from their work also produced selective sulfation of Al-bearing species over Fe-bearing species.
The work of Chao and Sun resulted in a process with advantages with respect to smelter-grade alumina production from the Mercer Clay and other similar feedstocks, with the potential to selectively solubilize Al-bearing species in the deposit over the major elemental impurities (silicon and iron). This can significantly reduce the treatment of the leach solution and other purification steps required to produce smelter-grade alumina.
Sulfation at elevated temperatures has been shown in the laboratory to be a means of selectively producing a leachable aluminum compound from Mercer Clay minerals, selectively over both Si- and Fe-bearing species. Sulfation for this application may be referred to as “baking” or “sulfation roasting,” depending on the temperature involved — the authors consider sulfation at temperatures below the boiling point of concentrated H2SO4 to be “baking” and above that temperature to be “roasting.”
From the standpoint of polymetallic recovery, sulfuric acid baking, followed by leaching, is a long-established practice for the commercial production of REEs [48]. Additionally, one clay-associated lithium production project under development has included sulfation roasting in its extraction system [49]. In both cases, as is the case with extraction of Al-bearing species, sulfation at elevated temperatures has the potential to convert insoluble minerals into lithium and rare earth compounds that can be leached.
The body of work reported here on extractive metallurgy involving the Mercer Clay was applied to the material without prior beneficiation. Other work, cited in Sect. 4.2, suggests that further mineral processing research may provide means for beneficiating the material ahead of an extractive metallurgy step. As Hazen and Robertson state in the 2019 SME Mineral Processing and Extractive Metallurgy Handbook [50], “Exhaust the options for physical separation before accepting the result that you must treat the whole ore. A difficult physical separation is almost always cheaper than a chemical separation.” Technology has evolved significantly since much of the cited mineral processing work was reported, and further mineral processing research on the Mercer Clay may produce results that can significantly reduce extractive metallurgy costs, especially where the removal of iron species ahead of the process can be accomplished, and where products with enriched lithium and Al2O3 contents can be produced through a kaolinite-diaspore separation.
The goals of the work reported here have been to present a unified review of the research results that have been reported for the Mercer Clay, relevant to the potential resource of three critical mineral commodities (alumina, lithium, rare earths), and to report preliminary results from current work. The new research is being undertaken to address unanswered questions involving the mineralogy of the Mercer Clay. These questions must be answered to guide further mining, mineral processing, and extractive metallurgy research and include the following:
-
1.
What are the lithium and rare earth-bearing minerals found in the deposit, and are there useful mineral associations?
-
2.
Are there stratigraphic associations for critical mineral enrichments?
-
3.
What are the characteristic grain sizes of these minerals?
-
4.
How do these minerals behave in grinding systems as compared with the major mineral components (i.e., liberation behavior)?
-
5.
What is the liberation behavior of iron-bearing minerals?
-
6.
Is there liberation behavior involving kaolinite and diaspore that can facilitate a kaolinite-diaspore separation by flotation to produce a diaspore product and a (potentially lithium-rich) kaolinite product?
-
7.
Would any byproducts be suitable for applications where soft clay is used?
In addition to providing mineral processing-related information, the answer to the first question is required to begin thermodynamic analyses that could yield common operating conditions for the sulfation of all the species of interest (Al-, Li-, and REE-bearing minerals). The answer to the second question can influence mine design, in turn impacting production economics. Results to be presented here will address the first and second questions.
5 Experimental
5.1 Description of Samples
Previous work on the Mercer Clay, reported here, was conducted from the 1940s through the 1980s. Much of the geochemical data generated during that time involved samples from active clay mines. Due to cessation of operations and site reclamation in the intervening decades, access to exposures of the material is among the challenges of reassessing the resource. An additional challenge is finding locations where previous work has established, notably, elevated alumina and lithium contents.
A description of the Morgan Run clay-producing district in Clearfield County appears in Foose [16]. Two exposures in that district were sampled for chemical analysis for this work. In both cases, samples were taken above and below the Mercer coal; the intervals sampled below the coal are considered here to be the Mercer Clay. While sampling extended as much as 150 cm below the coal, in neither case was the underlying bedrock formation encountered in the exposure. As such, the complete interval of the Mercer Clay was not sampled.
Descriptions of the samples, including stratigraphic positions, are shown in Tables 5 and 6. The Morgan Run 1 section primarily consists of soft gray clay with two thin coal layers. The uppermost coal layer is overlain by shale, which in turn is overlain unconformably by a lenticular sandstone bed. The bottom of the section is characterized by a harder grey clay. The Morgan Run 2 section is likewise characterized by soft gray clay, with only one thin coal layer at the site. Millimeter-sized cavities are common in the clays near the bottom of the section, and some of the cavities are lined with what appear to be reddish oxides.
The stratigraphic succession of the samples is presented in Fig. 4.
5.2 Sample Preparation and Analysis
Both the search for elevated lithium and rare earth contents and identification of the stratigraphic positions of enrichments required elemental and mineralogical analyses. Following grinding, samples were digested and analyzed through two routes. Analyses for major oxides used a Thermo 7400 Inductively Coupled Plasma Emission Spectrometry (ICP-AES) system, following a lithium metaborate fusion and digestion. Lithium and rare earth contents were determined using a Thermo Fischer Scientific X Series 2 Inductively Coupled Plasma Mass Spectrometer (ICP-MS) with Collision Cell Technology, after peroxide fluxing to oxidize organics and multi-acid sample digestion. As lithium contents were required analyses, lithium metaborate digestion was not used for the ICP-MS analyses.
Mineralogical analyses for samples were generated using X-Ray diffraction. Finely powdered bulk clay samples were loaded onto silicon wafers, and diffraction spectra were collected using a Malvern Panalytical X’pert3 MPD with a Cu Kα X-ray source. The collected spectra were processed using JADE peak identification software.
Elemental analyses gained through spectrometric techniques were used to reconstitute stratigraphic distributions of key elements through the columns, as presented in the “Results and Discussion” below.
6 Results and Discussion
6.1 Analytical Results
The contents of key elements, Al, Li, and total REEs are of primary importance here, as they govern the production costs of the associated critical mineral commodities. From a geometallurgical perspective, additional parameters of interest are the stratigraphic positions of any enrichments, which may influence mining strategies and costs, and the relative contents of key gangue minerals and elements that can influence extractive metallurgy production costs, notably iron. Analytical results for major elements, and lithium and rare earths, appear in Tables 7, 8, 9, and 10.
The clays are composed primarily of SiO2 (42–57%) and Al2O3 (25–36%), with lesser amounts of K2O (< 1–5%), Fe2O3 (< 1–5%), and TiO2 (1–3%). The average SiO2:Al2O3 ratio is 1.5:1. Total volatiles by loss on ignition range from 9 to 15%. Lithium concentrations in the clays are highly variable, ranging from 96 to 1,300 PPM. REE abundances are broadly similar to those in post-Archean Australian shale (PAAS; [51]), with moderate enrichment (up to 2 × PAAS) in the heavy REEs. Total rare earth elements (TREE) range from 84 to 348 ppm. The TREE contents of these samples are lower than previous results for the Mercer Clay, as seen in Fig. 2. However, that result was from material sampled in Clinton County (see Fig. 1), while the results presented in Tables 7, 8, 9, and 10 are from samples gathered in Clearfield County. Another explanation could be that the acid digestion technique used for the ICP-MS samples, while necessitated by the need to analyze for lithium, did not completely dissolve REEs found in refractory minerals such as zircon.
XRD results showed that kaolinite and illite are common to all samples. Some samples, including (but not exclusively) the high-Li samples, contain chlorite, and one sample was found to contain montmorillonite. Some samples additionally contain quartz and/or orthoclase. Tourmaline and rare earth-bearing minerals were not detected. Although no tourmaline was identified, it is possible that the trace amounts of may not be picked up in the XRD spectra. While tourmaline had been found previously in the Mercer Clay, the USGS work [3, 7] had suggested that lithium in the Mercer clay is present in a chlorite-group mineral (cookeite). The results for the materials examined in this work suggest that the latter is the case. This is significant as lithium present in tourmaline, which is considered a “heavy mineral” might be better suited to recovery by mineral processing methods than lithium present in a clay mineral. However, these results are preliminary, and more work regarding the mineral host of lithium is required.
No diaspore was identified, consistent with the lack of nodules in the analyzed samples. Underlying nodular clays were observed visually in the field at nearby locations, but were not present in the sampled profiles.
In Table 2, analyses from previous works had shown that samples with 1,000 PPM lithium had largely been found in clays that fit the “flint clay” description. From the classification scheme of Foose [16], flint clay and semi-flint clay occupy a range of Al2O3 contents from 35 to 38 wt%. The analytical results in Tables 7 and 8, corresponding to lithium contents exceeding 1,000 PPM, range in Al2O3 analysis from 32 to 34 wt%.
The iron contents of the samples tested for this work are also of note. Much of the previous work on mineral processing and extractive metallurgy applied to the Mercer clay (see Sects. 2 and 3), was devoted to dealing with iron present in the clay. Higher iron-content claystones have been found in the Mercer clay, in the 30 wt% Fe2O3 range [22]. However, compared with the previous work, the iron contents found here were relatively low. All samples were less than 5 wt% Fe2O3, and all but one were in the 1–3 wt% Fe2O3 range, suggesting less cost and complexity for removing iron from process streams in industrial mineral processing or extractive metallurgy processes applied to these potential feedstocks.
6.2 Stratigraphic Distributions of Elements Associated with Critical Mineral Commodities
The degree to which the rocks are enriched in the elements associated with these commodities will affect mining and production economics. Enrichments of the elements of interest may not be found in the same stratigraphic interval, but the stratigraphic proximity of the enrichments of individual elements can influence economics; where they are closer to each other in the sequence, they can be removed with less co-production of lower value rock intervals.
The Pennsylvania Period strata in the study area are relatively flat, and production of both the Mercer coal and the underlying Mercer clay has, in the past, been accomplished through both underground and surface mining. With respect to production of critical mineral commodities from the Mercer Clay, economic enrichments of Al and Li have been documented in the literature. Further, there is a significant carbon products manufacturing industry in the region, offering a potential outlet for any Mercer coal that might be produced. As such, coal may be added to the suite of potentially salable products.
The lithium contents of the sampled intervals, along with Al2O3 analyses, are shown in Figs. 5 and 6. In both cases, the Al2O3 content remains in a relatively narrow range with depth beneath the coal (31–34 wt% Al2O3). However, in both cases, the highest lithium content zone (1,000 + PPM Li) is directly under the coal. Tourtelot and Meier [7] found lithium enrichments near the top of what they called the “Mercer flint clay bed,” in Clearfield County. The relationship between the bottom of the coal and their sampling interval was not provided in that work.
While the Al2O3 content below the coal shows little variation through the sampled intervals here, as seen in Sect. 3.1, Mercer clay analyses in the region can exceed 60 wt% Al2O3 where diapore is present. As the sandstone that underlies the Mercer clay was not encountered in the exposures sampled, the full vertical extent was not examined, and zones with higher Al2O3 contents may exist beneath the bottom of the intervals that were sampled for this work.
The Fe2O3 and TREE contents of the sampled intervals for both sites are shown in Figs. 7 and 8. While the Fe2O3 analyses for all samples are relatively low, there is some enrichment directly under the coal in both cases.
Total rare earth contents are in both cases enriched in proximity to the coal. While these contents are lower than have been found elsewhere in the Mercer Clay, these data broadly suggest that within the intervals sampled, mining of the coal and immediately underlying rock would also result in production of the materials with the highest rare earth assays.
7 Summary and Conclusions
The goals of this paper have been to present a review of the Mercer Clay as polymetallic critical mineral resources and report preliminary results from renewed investigations, required to guide further mining, mineral processing, and extractive metallurgy research.
At different times over the last eight decades, the Mercer Clay has been examined as a source of alumina and for its lithium content, and more recently, elevated rare earth contents have been found. Its composition has driven commercial mining as feedstock for the production of refractory products. The economic significance of the deposit within the study area for this work has, in the past, provided exposures of the material for geoscience, mineral processing, and extractive metallurgy research. This body of previous work has been summarized here, and more recent policy developments related to critical minerals in the USA have led to a resumption of work under these topics. Current work is focused on the Mercer Clay as a polymetallic resource (multiple critical mineral commodities).
Work reported here has been guided by a geometallurgy approach (the overall goal being economic production potential). The objective of the work reported here has been to provide for answers to two questions have produced the results presented in this paper:
-
1.
What are the lithium and rare earth-bearing minerals found in the deposit, and are there useful mineral associations ?
-
2.
Are there stratigraphic associations for critical mineral enrichments?
Lithium contents approaching those of previous work (1,000 + PPM) were found as a result of this work. While a review of previous work has suggested that both tourmaline and clay minerals could be the lithium source, tourmaline was not detected by XRD analyses.
Lithium enrichments were found directly under the Mercer coal and were associated with Al2O3 contents on the low side of the “semi-flint clay” and “flint clay” range, as defined by Foose [16]. The latter association, with a characteristic Al2O3 content range, is similar to the associations found in previous work regarding the highest lithium contents in the Mercer clay, as presented here in Table 3.
Similarly, XRD analyses did not detect rare earth-bearing minerals, and TREE contents measured by ICP-MS were lower than have been found elsewhere in the Mercer Clay.
The most significant stratigraphic association involving lithium, found here, is that the highest lithium contents were in both cases directly under the Mercer coal. The highest rare earth contents found in the sampled intervals were also near the coal. This could allow for co-production of both lithium- and REE-bearing ores with the coal.
Kaolinite and diaspore are well established as dominant minerals in the high alumina Mercer clay through the study area. Mineral processing and extractive metallurgy research has been reviewed here with respect to beneficiation of high-alumina clays, as well as extractive metallurgy research specific to alumina production from the Mercer Clay. However the mineral forms of lithium and REEs remain to be identified prior to examination of processing options.
While the XRD technique did not detect tourmaline or REE minerals, microscopy may be required to conclusively establish their presence or lack thereof. The presence of both lithium and REEs in heavy minerals, such as tourmaline and monazite, can improve the prospects for the use of mineral processing techniques for their recovery from clays.
In addition to conclusively establishing the mineral forms of both lithium and REEs in the Mercer Clay, further work is required to both establish the full extent of the deposit as a resource and to begin identification of mineral processing and extractive metallurgy options for the recovery of salable critical mineral commodities.
With respect to the geographic extent of the Mercer Clay, much of the previous work was focused on the study area presented in Fig. 1, as this region has been a significant past producer of the clay for the regional refractory industry. However, the extent of the Mercer horizon is larger than the central Pennsylvania study area, and a Mercer clay analysis exceeding 35 wt% Al2O3 has been reported in southwestern Pennsylvania (Somerset County [52]). Extending the exploration beyond the study area may result in the establishment of a significantly larger resource.
Conclusions are as follows:
-
1.
Lithium contents in excess of 1,000 PPM were found at the sites sampled. In both cases the highest lithium contents were found directly under the Mercer coal zone.
-
2.
The samples with highest lithium contents also had a characteristic range of Al2O3 contents ranging from 32 to 34 wt%.
-
3.
Total rare earth elemental contents were lower than that previously reported from the Mercer Clay in Clinton County. Further exploration will better define trends in rare earth content through the study area.
-
4.
While the iron contents were lower than some found previously in the region, iron enrichments were found directly under the coal.
-
5.
Rare earth concentrations were the highest near the coal.
-
6.
Additional work will be required to establish the mineral hosts of lithium and REEs. This in turn will guide mineral processing and extractive metallurgy research.
References
U.S. Geological Survey (2016) Mineral commodity summaries 2016: U.S. Geological Survey, pp 202. https://doi.org/10.3133/70140094
U.S. Geological Survey, 18 May 2018, “Interior releases 2018’s final list of 35 minerals deemed critical to U.S. national security and the economy”, Retrieved from: https://www.usgs.gov/news/interior-releases-2018-s-final-list-35-minerals-deemed-critical-us-national-security-and. Accessed 11 Nov 2020
Tourtelot HA, Brenner-Tourtelot EF (1977) “Lithium in flint clay, bauxite, related high-alumina materials and associated sedimentary rocks in the United States- a preliminary survey”, USGS Open File Report 77–786, pp 46
Conley JE, Brown RA, Cservenyak FJ, Anderberg RC, Kandiner HJ, Green SJ (1947) “Production of metallurgical alumina from Pennsylvania nodular diaspore clays”, U.S. Bureau of Mines Bulletin 465, pp 206
Patterson SH (1967), “Bauxite reserves and potential aluminum resources of the world”, USGS Bulletin 1228, pp 178
Patterson SH, Murray HM (1984) “Kaolin, refractory clay, ball clay, and halloysite in North America, Hawaii, and the Caribbean Region”, USGS Professional Paper 1306, pp 55
Tourtelot HA, Meier AL (1976) Lithium clayey rocks of the Pennsylvanian age, Western Pennsylvania. USGS Professional Paper 1005:128–137
Tourtelot HA, Brenner-Tourtelot EF (1978) Lithium, a preliminary survey of its mineral occurrence in flint clay and related rock types in the United States. Energy 3:263–272
Rodgers J (1970) The tectonics of the Appalachians. Wiley-Interscience, New York, p 271
Kay G (1942) Development of the northern Allegheny synclinorium and adjacent regions. Bulletin of the Geol Soc Am 53(11):1601–1658
Richardson G (1928) Structure-contour maps of the Pittsburgh-Huntingdon basin, Bulletin of the Geol. Soc Am 39:543–554
Edmunds W, Skema V, Flint N (1999) Chapter 10: Pennsylvanian, in (ed. Shultz, C.) The geology of Pennsylvania, Pennsylvania Geological Survey and Pittsburgh Geological Society, 149–170
Dodge C (2004), Bedrock Geologic Map, Coal-resource maps, and digital datasets of the Brandy Camp Quadrangle, Elk, Jefferson, and Clearfield Counties, Pennsylvania, Pennsylvania Geological Survey, 4th ser., Mineral Resource Report 99, 8-9
Berg T, Glover A (1976) Geology and mineral resources of the Sabula and Penfield Quadrangles, Clearfield, Elk, and Jefferson Counties, Pennsylvania, Pennsylvania Geological Survey, 4 ser., A074ab, A32-A35
Williams EG, Bragonier W (1985) “Origin of the Mercer high-alumina clay”, in “Central Pennsylvania geology revisited”, in Gold, D.P. (ed.), 50th Annual Field Conference of Pennsylvania Geologists, 204–290
Foose RM (1944) High-alumina clays of Pennsylvania. Econ Geol 39:557–577
Berg TM (1987) Mississippian-Pennsylvanian boundary and variability of coal-bearing facies at Curwensville Reservoir, Clearfield County, Pennsylvania. Northeastern section of the Geological Society of America, pp 43–46
Bolger R, Weitz J (1952) Mineralogy and origin of the Mercer fireclay of north-central Pennsylvania, in: American Institute of Mining and Metallurgical Engineers, Problems of clay and laterite genesis – Symposium, St. Louis, MO, 1951: New York, 81–93
Erickson ES (1963) “Mineralogical, petrographic, and geochemical relationships in some high-alumina and associated claystones from the Clearfield Basin, Pennsylvania”, Ph.D. Thesis, Penn State University, pp 190
Bragonier WA (1970) “Genesis and geologic relations of the high-alumina Mercer fireclay, Western Pennsylvania”, M.S. Thesis, Penn State University, pp 212
Weitz JH, Bolger RC (1951) Mineralogy and Nomenclature of the Mercer Fire-Clay in North-Central Pennsylvania”. Proceedings of the Pennsylvania Academy of Sciences 25:124–130
Weitz JH (1954) “The Mercer fire clays in Clinton and Centre Counties, Pennsylvania”, Ph.D. Thesis, Penn State University, pp 107
Tetra Tech, Inc (2018) “Identification and characterization of coal and coal by-products containing high rare earth element concentrations, Northern and Central Appalachia Coal Basins”, Final Report to the U.S. Department of Energy, DE-FE- 0026648, Retrieved from: https://edx.netl.doe.gov/dataset/rfp-9067-fe0026648-final-report-tetratech/resource_download/21adb635-8d71-4b86-bfe8-65863d28727d. Accessed 11 Nov 2020
Horstman EL (1957) “The distribution of lithium rubidium, and cesium in igneous and sedimentary rocks.” Geochimica et Cosmochimica Acta 12(1–2):1–28
Hofstra AH, Todorov TI, Mercer CN, Adams DT, Marsh EE (2013) Silicate melt inclusion evidence for extreme pre-eruptive enrichment and post-eruptive depletion of lithium in silicic volcanic rocks of the western United States: implications for the origin of lithium-rich brines. Econ Geol 108(7):1691–1701
Starkey HC (1982) “The role of clays in fixing lithium”, U.S. Geological Survey Bulletin 1278-F
Anders E, Grevesse N (1989) Abundances of the elements, meteoric and solar. Geochim Cosmochim Acta 53:197–214
Krishnamurthy N, Gupta CK (2016) “The extractive metallurgy of rare earths”, CRC Press, pp 839
U.S. Geological Survey, 2002, “Rare earth elements—critical resources for high technology”, USGS Fact Sheet 087–02, Retrieved from http://pubs.usgs.gov/fs/2002/fs087-02/fs087-02.pdf. Accessed 11 Nov 2020
Schatzel SJ, Stewart BW (2003) Rare element sources and modification in the lower Kittanning coal bad, Pennsylvania: implications for the origin of coal mineral matter and rare earth element exposure in underground mines. Int J Coal Geol 54:223–251
David DM (2019) “Geometallurgy”, in Dunne RC, Kawatra SK, and Young CA, (eds.), “SME mineral processing and extractive metallurgy handbook”, Society of Mining, Metallurgy & Exploration, 173–184
Kogel JE (2017) Mining and processing kaolin. Elements 10:189–193
Prasad MS, Reid KJ, Murray HH (1991) Kaolin: processing, properties and applications. Appl Clay Sci 6:87–119
Asdell BK (1967) Wet processing of Kaolin. AIME Transactions 238:467–474
Smith DT (1938) “A preliminary examination of the removal of iron from a flint clay by froth Flotation”, B.S. Thesis, Penn State University, pp 35
Green JF (1940) “A study in the beneficiation of refractory and face-brick clays by froth flotation”, B.S. Thesis, Penn State University, pp 29
Wilson IR (2004) Kaolin and halloysite deposits of China. Clay Miner 39:1–15
Bu X, Evans G, Xie G, Peng Y, Zhang Z, Nie C, Ge L (2017) Removal of fine quartz from coal-series Kaolin by flotation. Appl Clay Sci 143:437–444
Gibson B, Wonyen DG, Chelgani SC (2017) A review of pretreatment of diasporic bauxite ores by flotation separation. Miner Eng 114:64–73
Zhang N, Nguyen AV, Zhou C (2018) A review of the surface features and properties, surfactant adsorption and floatability of four key minerals of diasporic bauxite resources. Adv Coll Interface Sci 254:56–75
Jiang Y-R, Zhao B-N, Zhou X-H, Zhou L-Y (2010) Flotation of diaspore and aluminosilicate minerals applying novel carboxyl hydroxamic acids as collector. Hydrometallurgy 104:112–118
Skelly DC (1950) “A study of the separation of free silica from fireclays by electrostatic methods”, B.S. Thesis, Penn State University
Murray HH (1989) “Clay minerals for advanced ceramics”, Mining Engineering, Volume 41, Number 11, 1123–1125
Powell HE, Calhoun WA, Miller CK (1963) “Beneficiation of refractory clay”, U.S. Bureau of Mines Report of Investigations 6142, pp 46
Fetterman JW (1961) “Alumina extraction from a Pennsylvania diaspore clay by an ammonium sulfate process”, Ph.D. Thesis, The Pennsylvania State University, pp 150
Chao T (1966) “Sulfatization of alumina with gaseous sulfur trioxide and its kinetics”, Ph.D. Thesis, Penn State University, pp 156
Chao T, Sun SC (1967) Study on sulfatization of alumina with gaseous sulfur trioxide. AIME Transaction 238:420–429
Demol J, Ho E, Soldenhoff K, Senanayake G (2019) The sulfuric acid bake and leach route for processing of rare earth ores and concentrates. Hydrometallurgy 188:123–139
Ausenco Services Pty Ltd (2018) “Technical report on the feasibility study for the Sonora lithium project, Mexico”, Report to Bacanora Minerals Ltd., Retrieved from https://www.goldplat.com/pdfs/Bacanora-FS-Technical-Report-25-01-2018.pdf. Accessed 11 July 2018
Hazen N, Robertson J (2019) “Research and development”, in Dunne R.C., Kawatra SK, and Young CA (eds.), “SME mineral processing and extractive metallurgy handbook”, Society of Mining, Metallurgy & Exploration, 325–332
Taylor SR and McLennan SM (1985) The Continental Crust; Its composition and evolution; an examination of the geochemical record preserved in sedimentary rocks. Blackwell, Oxford. pp 312
O’Neill BL, Lapham DM, Jaron MG, Socolow AA, Thomson RD, Hamlin HP (1965) “Properties and uses of Pennsylvania shales and clays”, Pennsylvania Geological Survey, 4th ser., Mineral Resource Report 51, pp 448
Acknowledgements
Work reported here has been conducted by the Penn State University Center for Critical Minerals. Safiya Alpheus and Victor Garcia collected samples and conducted X-ray diffraction analyses.
Funding
This study was funded internally by the Center for Critical Minerals at the Pennsylvania State University.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material selection, collection preparation, data collection, and analysis were performed by Maureen Feineman, Timothy White, Nicholas Crescenzo, Alan Larson, and Peter Rozelle. The first draft of the manuscript was written by Peter Rozelle, and all authors commented on previous versions and contributed to the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rozelle, P.L., Feineman, M.D., White, T.S. et al. The Mercer Clay in Pennsylvania as a Polymetallic Mineral Resource: Review and Update. Mining, Metallurgy & Exploration 38, 2037–2054 (2021). https://doi.org/10.1007/s42461-021-00452-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-021-00452-5