Abstract
Biosorption of copper(II) and cadmium(II) was studied using Bacillus cereus sys1 cells. The biosorption capacity of the cells has been studied as a function of pH, initial metal concentration, cell concentration and contact time. Batch studies showed that the B. cereus sys1 cells could adsorb up to 90% of both metals. For copper, total contact time of 90 min was required with 100 ppm initial metal concentration at pH 5 using 10 mg/ml cells, whereas 180 min was required for 30 ppm cadmium at pH 7. The study demonstrated correlation with Langmuir model with different concentrations of both Cu(II) and Cd(II). The adsorption capacity was found to be 225 mg/g for copper and 34.67 mg/g for cadmium. As per the Freundlich isotherm, value of 1/n, which shows the strength of adsorption, was found to be 0.5114 for copper and 0.2492 for cadmium indicating a normal and favourable adsorption intensity. The surface adsorption of metals was confirmed using SEM with EDS. It revealed that the cells treated with oil could adsorb 7.55% copper as compared to untreated cells which showed 7.29% adsorption. Similarly, for cadmium oil-treated cells showed 9.44% adsorption compared to 2.63% of untreated cells. This indicated that the cells grown in the presence of oil have a better capacity for metal ion adsorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metal pollution is one of the major concerns of the current era [1, 2]. In recent years, mushrooming of several industries has intensified the problem of metal pollution [3]. There are several heavy metals that enter into the environment through different sources. Copper(II) and cadmium(II) are two of such metal contaminants [4]. Copper enters into the environment through natural and anthropogenic sources. The contamination of air and water by copper occurs due to mining, milling, concentrating, refining of copper ores, electroplating industries, petroleum and reefing melting plants and miscellaneous units from industries, whereas cadmium can be released to the atmosphere through metal production activities, fossil fuel combustion and waste incineration [5]. The presence of copper(II) ions causes serious toxicological concerns. It is usually known to deposit in brain, skin, liver, pancreas and myocardium [1, 6]. In high doses, it can cause anaemia, liver and kidney damage and sometimes stomach and intestinal irritation [7]. Cadmium is an extremely toxic metal commonly found in industrial workplaces. Cadmium is extensively used in electroplating, although the nature of the operation does not generally lead to over exposures. As per the different studies done over the years, only a small proportion (5–10%) of ingested cadmium is absorbed by humans [8, 9]. Cadmium is capable of causing severe renal dysfunction and damage to the bone structure [1, 10]. Currently, common methods in use for metal removal are physical, chemical or mechanical in nature [11]. Due to incomplete decomposition of pollutants and high cost of operation, there is a need for the better methods of pollution control. In view of this, biosorption is becoming an increasingly popular technique for cleaning up the metal-contaminated sites. Biosorption, which is the ability of certain microbial biomaterials to bind and concentrate heavy metals from even the most dilute aqueous solutions, offers a technically feasible alternative [12]. Algae, bacteria, fungi and yeasts have proved to be potential metal biosorbents [13]. It is considered an ideal alternative method for removing contaminants from effluents as well.
The Bacillus cereus sys1 cells used in this study were isolated from oil-contaminated sites. They were found to be exopolysaccharide producers and exhibited oil degrading potential [14]. The present work delineates the use of B. cereus sys1 cells as a potential biosorbent for copper(II) and cadmium(II) metal ion removal.
2 Materials and methods
2.1 Growth of Bacillus cereus sys1
The B. cereus sys1 cells were isolated from oil-contaminated soil in and around Thane District, Maharashtra, India. These cells were grown in sterile nutrient broth as described by Gupte and Sonawdekar [14]. All the studies during the present work were carried out using freshly harvested cells. Unless otherwise stated, the amount of cells used in these studies has been indicated in terms of wet weight of the packed cells obtained after centrifugation.
2.2 Preparation of metal solution
Stock solutions of Cu(II) (2000 ppm) and Cd(II) (100 ppm) were prepared using copper sulphate (CuSO4·5H2O) and cadmium nitrate [Cd(NO3)2·4H2O] in deionized water, respectively. The required concentrations of both the metal ions were prepared by appropriate dilution of corresponding metal stock solutions.
2.3 Uptake studies
Biosorption studies were carried out in a batch process. For this study, B. cereus sys1 cells (10 mg/ml) were inoculated in 50 ml of an aqueous solution of copper (100 ppm) and cadmium (30 ppm) separately. The pH was adjusted to 5 for Cu(II) and to 7 for Cd(II) metal solution. The adsorption studies were carried out at constant temperature (room temperature (30 ± 2 °C) [15]. Aliquots taken at a known interval of time were centrifuged at 8000 rpm for 5 min to remove cells, and the supernatant was used for the determination of residual metal concentration at 327.4 nm for copper and 228.8 nm for cadmium using atomic absorption spectroscopy (AAS) [15,16,17,18,19,20].
2.4 Effect of different pre-treatments on metal uptake
Different pre-treatments to remove exopolysaccharide from the cells were carried out as: (a) oven drying for 20 h at 60 °C, (b) autoclaving at 15 lbs, (c) 121 °C for 20 min, (d) incubating in a boiling water bath for 60 min and (e) acetone drying [18].
2.5 Characterization of biomass
The surface characteristics of B. cereus sys1 cells were analysed before and after metal biosorption using scanning electron microscope (SEM) with energy-dispersive X-ray spectroscopy (EDAX).
3 Results and discussion
3.1 Effect of cell concentration
The cell concentration influences metal uptake capacities. To study the effect of cell concentration on biosorption of copper and cadmium, the biomass of varying concentrations (1–30 mg/ml) was incubated with aqueous solution of respective metals for 24 h and sorption studies were carried out as described earlier. Figure 1 shows the effect of cell concentration on biosorption of Cu(II) and Cd(II). Copper biosorption was found to be maximum (73%) with cell concentration of 10 mg/ml. In the case of cadmium, 10 mg/ml and 30 mg/ml cells showed 86% and 88% sorption, respectively. This can be due to the increase in the number of binding sites at higher cell/biosorbent concentrations. At very low cell concentration, the cell surface becomes saturated with the metal ions and the large amounts of metal ions remain in the solution [19]. During the study, it was observed that Cu(II) uptake was found to be reduced with 30 mg/ml cells. At very high cell concentration, the competition between the binding sites increases which might have led to desorption of copper ions. This can also be attributed to availability of solute, electrostatic interactions, interference between binding sites and reduced mixing at high biomass densities [20].
3.2 Effect of pH
pH plays an important role in metal uptake. To study the effect of pH on biosorption of copper and cadmium, the biomass of varying concentrations (1–30 mg/ml) was incubated with aqueous solution of respective metals for 24 h and sorption studies were carried out as described earlier. Due to precipitation of copper beyond pH 5, the study of effect of pH on copper sorption was conducted only till pH 5. Figure 2 shows the effect of pH on biosorption. Copper biosorption was found to be 73% at pH 5, whereas for cadmium it was found to be 88% at pH 7 with cell concentration of 10 mg/ml. It was seen that the cadmium sorption was not dependent on pH as no change in metal sorption was observed at different pH, whereas copper sorption was found to increase with increasing pH. At lower pH (< 6), concentration of proton is high, so metal binding sites become positively charged. Metal cations and protons compete for binding sites which results in lower metal uptake [21]. The studies conducted by Gupte and Nair [17] demonstrated that the removal efficiency of Cu(II) increases from the pH 3 to 5 with slight increase after pH 5. These results also suggest that ionic interaction is the main mechanism contributing to biosorption of metals. At low pH, hydrogen ions compete with metal ions, resulting in protonation of active sites. But as the pH increases, the negative charge density on the surface of adsorbent increases and the ionic point of ligands such as carboxyl, hydroxyl and amino groups is free so as to promote interaction with the metal cations [17]. Increased sorption with increasing pH can also be attributed to lower degree of hydration of hydrolysed species as it requires less energy for removal or reorientation of the hydrated water molecules upon binding.
3.3 Effect of metal ion concentration
The metal ion concentration can influence metal uptake capacity of the cells. To study the effect of copper and cadmium concentration on biosorption, 50–250 ppm and 10–50 ppm were used, respectively, and sorption studies were carried out as described earlier. Copper sorption was found to be maximum (73%) for 100 ppm as shown in Fig. 3a. The sorption efficiency for copper was found to decrease with increase in the metal concentration. Figure 3b represents the effect of cadmium concentration. The maximum uptake (90%) was found to be at 20 ppm cadmium concentration. The efficiency of the cells to uptake cadmium was found to decrease at higher concentrations. It suggests that the biosorption is highly dependent on initial concentration of metal ion and at very high concentration of metal ions it reaches a saturation value [19].
This indicates that at higher metal concentrations, the available sites for adsorption become fewer and biosorbent sites take up the available metal more quickly at lower metal concentrations. The similar findings were reported by Khadivinia et al. [22] while studying cadmium sorption in Ochrobactrum spp.
3.4 Effect of different pre-treatments
The B. cereus sys1 cells used in the study were isolated from oil-contaminated sites and had exopolysaccharide (EPS) layer on the surface and were found to be capsular exopolysaccharides containing carboxyl group [14]. Different pre-treatments were used for removal of EPS, and the effect was studied on metal sorption. The untreated cells showed maximum metal adsorption for copper as well as cadmium (Fig. 4).
The similar study conducted by González et al. [18] using Pseudomonas aureofaciens showed that EPS-rich cells have better metal adsorption capacity than the EPS-poor cells. It was also observed that the pre-treatment of cells affected the copper sorption but did not have much effect on cadmium sorption. Similar pre-treatments were used in the metal uptake studies for Aspergillus niger [23].
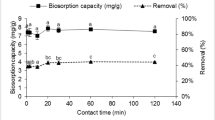
3.5 Time kinetics
The kinetic studies were carried out to determine the rate of metal sorption by B. cereus sys1 cells. Rapid removal of both the metal ions was seen within first 5 min as shown in Fig. 5. After the initial rapid uptake, it continued with slow rate until the equilibrium was attained. Equilibrium for Cu uptake was achieved at 90 min, whereas for Cd it was achieved at 180 min.
Initial higher rate of metal uptake may be attributed to the initial high metal concentration and availability of a large number of active sites on adsorbent [24]. Similar trend was observed in study conducted by Rajendran et al. [25] and showed that copper-resistant B. cereus spp. isolated from industrially polluted areas could adsorb copper in the range of 84% within 24 h. Several other studies carried out for copper adsorption [20, 26, 27] also reported maximum uptake within 4–24 h. Kulkarni et al. [28] studied the cadmium sorption capacity of Bacillus laterosporus, and the similar phenomenon of rapid adsorption in initial phase followed by slow chemical sorption phase was observed.
During the present study, it was also observed that the B. cereus sys1 cells could adsorb cadmium faster than copper. The adsorption order of the biosorbent for metal adsorbate may be related to the properties of the metal adsorbate like ionic radius and electronegativity. The ionic radius of Cd(II) is 0.98 Å, while that of Cu(II) is 0.69 Å. The smaller the ionic radius, the greater is tendency to be hydrolysed, leading to reduced biosorption of Cu(II), and the electronegativity of Cd(II) (1.69 Pauling) is lower value than that of Cu(II) (1.9 Pauling). The sequence of selectivity followed the order of decreasing electronegativity and increasing ionic radius. A similar observation was reported on biosorption of Cd(II) and Ni(II) by B. laterosporus by Kulkarni et al. [28].
3.6 Surface characterization
The SEM with EDX analysis confirmed the metal sorption on the cell surface. The cells grown in the presence and absence of oil were compared for their sorption efficiency. The comparative analysis of the surface elements is shown in Table 1. The study carried out on different Bacillus strains for metal biosorption showed similar results [29, 30].
3.7 Adsorption isotherm
Equilibrium adsorption isotherm studies were carried out with 10 mg/ml of biomass with copper concentration in the range of 25–1000 ppm and cadmium concentration in the rage of 10–250 ppm for 4 h (Figs. 6, 7). The uptake for construction of isotherm was calculated using the equation \(\left( {C_{i} - C_{\text{eq}} } \right) \times \frac{V}{M}\), where Ci is the initial metal concentration, Ceq is the equilibrium metal concentration, M is the mass of biomass in grams, and V is the working volume of the metal solution in litre.
3.7.1 Langmuir isotherm
The Langmuir adsorption isotherm experimental data were plotted as 1/qe on Y-axis versus 1/Ceq on X-axis, and its constants qm and KL were obtained by linear regression method as shown in Table 2 [20, 31,32,33]. The exponential data fit the Langmuir isotherm model. The maximum sorption was found to be 225 mg/g for copper and 34.67 mg/g for cadmium. Comparative data for copper and cadmium biosorption capacity of different microorganisms are shown in Table 3.
The results of this study also support the assumptions of Langmuir model that the presence of a limited number of binding sites distributes over the biosorbent surface homogeneously, thus presenting the same affinity for biosorption of a single molecular layer [20]. The separation factor, RL obtained in the current study, is 0.7049 for copper and 0.4660 for cadmium, which indicates that the adsorption process is favourable using B. cereus sys1 cereus.
3.7.2 Freundlich isotherm
The Freundlich adsorption isotherm was plotted as logqe on Y-axis versus log Ceq on X-axis. The calculated Freundlich constants were found to be as given in Table 4. The constant Kf is an approximate indicator of adsorption capacity, while 1/n is a function of the strength of adsorption in the adsorption process. As the value of 1/n reduces, heterogeneity increases. This study showed the 1/n value as 0.5114 for copper and 0.2492 for cadmium which indicates a normal adsorption. A favourable sorption process is indicated by the value of ‘n’ lying between one and ten [39]. As the value of n in the present study for both the metals was found to be higher than 1 and lesser than 10, it can be said that the adsorption intensity is favourable. Chatterjee and Ray [20] compared the copper sorption capacity of immobilized B. cereus M 116 cells. Their study showed that the B. cereus cells show normal adsorption with 1/n being lesser than 1 as also observed in the present study. Liu et al. [26] reported their findings for immobilized Bacillus subtilis cells which confirmed that Bacillus spp. usually show normal and favourable sorption at higher concentration of copper.
3.8 Kinetic models
To determine the controlling mechanism of biosorption process, two kinetic models were used to interpret the experimental data using 100 ppm copper concentration and 30 ppm cadmium concentration.
3.8.1 Lagergren’s first-order kinetic model
The correlation coefficient (R2) for the first-order kinetic plot of 100 ppm concentration for copper was found to be 0.7. It suggests that the Lagergren’s first-order plot does not adequately describe the adsorption having a low correlation coefficient (Fig. 8). Generally, the first-order kinetic model is applicable for initial stage of the biosorption processes and does not fit well with the whole range of contact time [40]. In the current study, similar findings were observed with the lower correlation coefficient. Liu et al. [26] and Chatterjee and Ray [20] also have reported that the first-order kinetic model does not fit well for copper using Bacillus cells.
Unlike copper, first-order kinetic model fits well for all tested concentrations of cadmium with correlation coefficient values ranging from 0.98 to 0.99. The same is shown in Fig. 8 for 30 ppm cadmium concentration. Similar results were observed by Kulkarni et al. [28] while studying the adsorption capacity of B. laterosporus.
3.8.2 Ho’s second-order kinetic model
Second-order plots for all the concentrations of copper as well as cadmium were found to be linear with correlation coefficients higher than 0.9, which indicates that the second-order kinetic model fits well with the experimental data. Figure 9 shows the second-order plot for 100 ppm copper and 30 ppm cadmium. Table 5 represents the rate constants (K1, K2) and adsorption capacity (qe) correlation coefficient for the same.
The second-order kinetic model involves valency forces through sharing or exchange of electrons between adsorbent and adsorbate [41]. In chemisorption, the metal ions form a chemical bond and stick to the adsorbent surface. They usually tend to form a covalent bond and find sites that help them enhance the affinity towards absorbent surface by increasing their coordination number with the surface [42]. The pseudo-second-order kinetic analysis reveals that value of initial adsorption rates increases with increase in the initial metal ion concentration.
In the present study, it was observed that the metal adsorption rate increases from 100 to 1000 ppm for copper and 10 to 30 ppm for cadmium. The coefficient of correlation for pseudo-second-order kinetic model for different copper and cadmium concentrations was found to be 0.98 to 1.0. Both factors suggest that the biosorption of copper and cadmium ions followed the pseudo-second-order kinetic model, indicating that the rate-limiting step was a chemical biosorption process between metal ions and biosorbent through the exchange of electrons between the particles involved [43,44,45,46].
4 Conclusion
The present study evaluates possibility of using B. cereus sys1 cells for biosorption of copper and cadmium. Effect of various parameters like pH, cell concentration and metal concentration on biosorption was studied for process optimization. The comparison between different pre-treatments revealed that the adsorption capacity is dependent on the functional groups present on the surface and they might be affected due to the pre-treatments. Freundlich and Langmuir models as well as pseudo-second-order plot exhibited good fit to Cu(II) and Cd(II) sorption which indicated a favourable and normal adsorption. The results from the study would contribute to understanding the potential of oil degrading strain of B. cereus sys1 as a biosorbent.
References
Rajeswari TR, Namburu S (2014) Impact of heavy metals on environmental pollution. National seminar on impact of toxic metals, minerals and solvents leading to environmental pollution-2014 JCHPS Special Issue 3, pp 175–181
Perpetuo EA, Souza CB, Nascimento CAO (2011) Engineering bacteria for bioremediation. In: Carpi A (ed) Progress in molecular and environmental bioengineering-from analysis and modelling to technology applications. IntechOpen, Rijeka, pp 605–632
Patnaik R (2018) Impact of industrialization on environment and sustainable solutions—reflections from a south Indian region. IOP Conf Ser Earth Environ Sci 120:1–8. https://doi.org/10.1088/1755-1315/120/1/012016
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. Exp Suppl 101:133–164. https://doi.org/10.1007/978-3-7643-8340-4_6
Brad HB (2004) Adsorption of metal ions on soils and soil constituents. J Colloid Interface Sci 277:1–18. https://doi.org/10.1016/j.jcis.2004.04.005
Davis TA, Volesky B, Vierra RHSF (2000) Sargassum seaweed as biosorbent for heavy metals. Water Res 34(17):4270–4278. https://doi.org/10.1016/S0043-1354(00)00177-9
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: a review. J Chem Sci Technol 3(4):79–102
Bernhoft R (2013) Cadmium toxicity and treatment. Sci World J. https://doi.org/10.1155/2013/394652
Godt J, Scheidig F, Grosse-Siestrup C, Esche V, Brandenburg P, Reich A, Groneberg D (2006) The toxicity of cadmium and resulting hazards for human health. J Occup Med Toxicol 1(22):1–6. https://doi.org/10.1186/1745-6673-1-22
Bernard A (2008) Cadmium and its adverse effects on human health. Indian J Med Res 128:557–564
Gunatilake S (2015) Methods of removing heavy metals from industrial wastewater. J Multidiscip Eng Sci Stud 1(1):12–18
Ahalya N, Ramachandra TV, Kanamadi RD (2003) Biosorption of heavy metals. Res J Chem Environ 7:71–79
Volesky B (1986) Biosorbent materials. Biotechnol Bioeng Symp 16:121–126
Gupte A, Sonawdekar S (2016) Production and characterization of exopolysaccharide produced by oil emulsifying bacteria. Int J Curr Microbiol Appl Sci 5(2):254–262. https://doi.org/10.20546/ijcmas.2016.502.028
Arivalagan P, Kaliannan T, Singaraj D, Haridass V (2014) Removal of cadmium from aqueous solution by batch studies using Bacillus cereus. Ecol Eng 71:728–735
Rajesh N, Manasi Rajesh V, Kumar ASK (2014) Biosorption of cadmium using a novel bacterium isolated from an electronic industry effluent. Chem Eng J 235:176–185. https://doi.org/10.1016/j.cej.2013.09.016
Gupte A, Nair J (2013) Biosorption of copper by the yeast Kluyveromyces marxianus grown on whey. D Y Patil J Health Sci 1(1):35–38
González AG, Shirokova LS, Pokrovsky OS, Emnova EE, Martinez RE, Santana-Casiano JM, Gonzalez-Davila M, Pokrovski GS (2010) Adsorption of copper on Pseudomonas aureofaciens: protective role of surface exopolysaccharides. J Colloid Interface Sci 350:305–314. https://doi.org/10.1016/j.jcis.2010.06.020
Mukhopadhyay M, Noronha SB, Suraishkumar GK (2007) Kinetic modelling for the biosorption of copper by pretreated Aspergillus niger biomass. Bioresour Technol 98(9):1781–1787. https://doi.org/10.1016/j.biortech.2006.06.025
Chatterjee A, Ray L (2008) Biosorption of Cu(II) by immobilized biomass of Bacillus cereus M from aqueous solution. J Sci Ind Res 67:629–634
Aksu Z, Sag Y, Kutsal T (1992) The biosorption of copper by C. vulgaris and Z. ramigera. Environ Technol 13:579–586
Khadivinia E, Sharafi H, Hadi F (2014) Cadmium biosorption by a glyphosate-degrading bacterium, a novel biosorbent isolated from pesticide-contaminated agricultural soils. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2014.01.037
Kapoor A, Viraraghavan A (1998) Biosorption of heavy metals on Aspergillus Niger: effect of pre-treatment. Bioresour Technol 63:109. https://doi.org/10.1016/S0960-8524(97)00118-1
Merandi R (2011) Bioextraction of Cu(II) ions from acid mine drainage by Bacillus thuringiensis. Int J Biol Eng 1(1):11–17. https://doi.org/10.5923/j.ijbe.20110101.03
Rajendran R, Jamaldheen S, Krishnamurthy M (2013) Biosorption of Cu(II) ions by indigenous copper-resistant bacteria isolated from polluted coastal environment. Toxicol Environ Chem 95:590–604. https://doi.org/10.1080/02772248.2013.801979
Liu YG, Liao T, He ZB, Li TT, Wang H, Hu XJ, Guo YM, He Y (2013) Biosorption of copper(II) from aqueous solution by Bacillus subtilis cells immobilized into chitosan beads. T Nonferr Metal Soc China 23:1804–1814. https://doi.org/10.1016/S1003-6326(13)62664-3
Babarinde NA, Oyesiku OO, Dairo OF (2007) Isotherm and thermodynamic studies of the biosorption of copper(II) ions by Erythrodontium barteri. Int J Phys Sci 2(11):300–304
Kulkarni RM, Shetty VK, Shrinikethan G (2013) Cadmium(II) and nickel(II) biosorption by Bacillus laterosporus (MTCC 1628). J Taiwan Inst Chem Eng. https://doi.org/10.1016/j.jtice.2013.11.006
Syed S, Chinthala P (2015) Heavy metal detoxification by different Bacillus species isolated from solar salterns. Scientifica 2015:319760. https://doi.org/10.1155/2015/319760
Zolgharnein H, Karami K, Asadi MM, Sohrab D (2010) Investigation of heavy metals biosorption on Pseudomonas aeruginosa strain MCCB 102 isolated from the Persian Gulf. Asian J Biotechnol 2(2):99–109. https://doi.org/10.3923/ajbkr.2010.99.109
Dada AO, Olalekan AP, Olatunya AM, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. J Appl Chem 3(1):38–45. https://doi.org/10.9790/5736-0313845
Hameed BH, Foo KY (2010) Insights into the modelling of adsorption isotherm systems. Chem Eng J 156:2–10. https://doi.org/10.1016/j.cej.2009.09.013
Hameed BH, Mahmoud DK (2008) Equilibrium modelling and kinetic studies on the adsorption of basic dye by a low-cost adsorbent: coconut (Cocos nucifera) bunch waste. J Hazard Mater 158(1):65–72. https://doi.org/10.1016/j.jhazmat.2008.01.034
Tunali S, Çabuk A, Akar T (2006) Removal of lead and copper ions from aqueous solutions by bacterial strain isolated from soil. Chem Eng J 115:203–211. https://doi.org/10.1016/j.cej.2005.09.023
Nakajima A, Yasuda M, Yokoyama H, Ohya-Nishiguchi H, Kamada H (2001) Copper biosorption by chemically treated Micrococcus luteus cells. World J Microbiol Biotechnol 17:343–347. https://doi.org/10.1023/A:1016638230043
Ray L, Ganguly A, Guha AK (2011) Adsorption behaviour of cadmium by Bacillus cereus M 116 : some physical and biochemical studies. Chem Speciat Bioavailab 23(3):175–182. https://doi.org/10.3184/095422911X13103800616341
Yilmaz EI, Ensari NY (2005) Cadmium biosorption by Bacillus circulans strain EB1. World J Microbiol Biotechnol 21:777–779. https://doi.org/10.1007/s11274-004-7258-y
Zouboulis AI, Loukidou MX, Matis KA (2004) Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem 39:909–916. https://doi.org/10.1016/S0032-9592(03)00200-0
Kumar U, Bandyopadhyay M (2006) Sorption of cadmium from aqueous solution using pre-treated rice husk. Bioresour Technol 97:104–109. https://doi.org/10.1016/S1003-6326(13)62664-3
Chatterjee S, Woo SH (2009) The removal of nitrate from aqueous solutions by chitosan hydrogel beads. J Hazard Mater 164:1012–1018. https://doi.org/10.1016/j.jhazmat.2008.09.001
Sağ Y, Aktay Y (2002) Kinetic studies on sorption of Cr(VI) and Cu(II) ions by chitin, chitosan and Rhizopus arrhizus. Biochem Eng J 12(2):143–153. https://doi.org/10.1016/S1369-703X(02)00068-2
Atkins PW (1995) Physical chemistry, 5th edn. Oxford University Press, Oxford
Wong KK, Lee CK, Low KS, Haron MJ (2003) Removal of Cu(II) and Pb(II) by tartaric acid modified rice husk from aqueous solutions. Chemosphere 50:23–28. https://doi.org/10.1016/S0045-6535(02)00598-2
Dundar M, Nuhoglu C, Nuhoglu Y (2007) Adsorption of Cu(II) ions onto natural trembling poplar forest. J Hazard Mater 151(1):86–95. https://doi.org/10.1016/j.jhazmat.2007.05.055
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735. https://doi.org/10.1016/S0043-1354(99)00232-8
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sonawdekar, S., Gupte, A. Biosorption of copper(II) and cadmium(II) by Bacillus cereus sys1 isolated from oil-contaminated site. SN Appl. Sci. 2, 1254 (2020). https://doi.org/10.1007/s42452-020-3062-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-3062-z