Abstract
Polyvinyl acetate emulsion (PVAc) is an essential class of adhesives for woodworking applications. However, it suffers from low water resistance. In the present research, wattle tannin was incorporated in synthesised PVAc emulsion at ratios ranging from 0 to 6 wt.% in PVAc emulsion. The study was carried out in two parts. The first part focuses on understanding the effect of the presence of tannin in PVAc emulsion on its adhesive performance. The second part deals with the study of the variation in adhesive properties of tannin PVAc emulsion adhesive by introducing isocyanate crosslinker in the system. DMA was employed to understand the glass transition and rheology behaviour. FTIR was used to assess the reaction of tannin with isocyanate crosslinker. Contact angle measurement was measured using goniometry to understand the surface wettability achieved by incorporating tannin in the PVAc emulsion system. Detail study on adhesive strength was carried out by bonding wood substrates followed by measuring bonding strength in wet conditions as per BS EN 204 and heat resistance as per DIN EN 14257 (WATT 91). The presence of tannin in the system enhances water and heat resistance property which gets further improved in the presence of isocyanate crosslinkers. The observed effect is due to better wetting of substrate by adhesive, hydrogen bonding between tannin and components in adhesive, and dense crosslinking formed by the reaction of isocyanate crosslinker.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Tannin is a natural water-soluble polyphenolic compound ranging from the molecular mass of 300–3000 Da [1] found abundant in plants [2, 3]. Tannin is present in superior plants such as mimosa, chestnut and oak to protect lingo-cellulosic material against biological and radiative degradation due to their chemistry and antioxidant property [4,5,6]. Tannin is commercially essential due to its usefulness in pharmaceutical, food and nutrients-based applications. [7]. Mimosa, quebracho and pine tannins find use in commercial purposes due to their availability. The industrial grade of tannin available contains a significant portion as tannin, while sugar and hydrocolloid gum form the non-tannin part [2, 8]. The hydroxyl group of tannin reacts with the isocyanate group [9] and has different reactivity. The viscosity change of the two-component adhesive system with time shows the reaction rate and molecular growth of the polymeric chain. Wattle tannin (resorcinol), when reacted with paraformaldehyde, has a gel time of 950 (s) as compared to pine tannin extract (phloroglucinol) which has a gel time of 65 (s) [10] which makes wattle tannin a preferred choice for adhesive due to long open time, availability and its similarity with resorcinol. [7, 11]
The techniques of bonding materials using adhesives are popular since ages. However, the use of adhesive in furniture and wood bonding applications became popular from the last century. At present, adhesive systems for bonding wood for external use include urea–formaldehyde (UF), phenol–formaldehyde (PF) and one component polyurethane resin (1 K PUR). The main drive for today’s increasing interest in bio or renewable adhesives is due to environmental protection and health-related concerns about formaldehyde emissions [2, 3, 7, 8, 11, 12] and fluctuation in petroleum prices. [2, 3, 11, 12]. Tannin-based adhesives have already been used for particleboard preparations commercially [8, 13]. Though phenol–formaldehyde and melamine formaldehyde-based adhesives exhibit superior heat and water resistance properties, they have the inherent tendency of formaldehyde emission with time [2, 7, 12]. Polyurethane adhesives are equally good or even superior to PF and UF adhesives in terms of heat and water resistance properties; however, they are expensive and difficult to handle due to its hazard to human health.
Researchers have been working on the improvement of tannin-based adhesives using various routes. Kim [12] have reported a decrease in formaldehyde emission in flooring adhesive by blending PVAc adhesive with tannin paraformaldehyde adhesive. Rhazi et al. [2] have reported the commercial equivalent of Phenol formaldehyde for plywood bonding prepared by tannin lignosulfonate adhesives along with hexamine. Tondi [6] studied the effect of various crosslinkers viz. formaldehyde, hexamine, glyoxal, maleic anhydride, furfural and furfuryl alcohol when crosslinked with tannin at different pH and temperature conditions. Garhi et al. [7] prepared complete green adhesive of soy and tannin for plywood applications. Sowunmi et al. [3] modified mangrove tannin with anhydride and PF blend to obtain commercial PF equivalent adhesive for plywood applications. Elbadawi et al. [13] added tannin to UF adhesives to decrease cost and improve properties of particle boards. Kim et al. [14] studied different combinations of tannin paraformaldehyde hexamine and pMDI for preparation of particle board to keep the performance equivalent to commercial UF resins.
Polyvinyl acetate emulsion (PVAc) adhesive is a popular class of non-hazardous adhesive for wood bonding application. PVAc adhesives are synthesised via emulsion polymerisation technique stabilised by colloids (polyvinyl alcohol) and surfactants [15]. The popularity of PVAc adhesive is due to its non-hazardous nature, easy application and low cost. However, PVAc chemistry shows poor water resistance, thermal stability and dimensional stability. Emulsion polymer isocyanates (EPI) are a popular class of adhesives where emulsions particularly PVAc emulsions are blended with isocyanates-based crosslinkers to improve mechanical properties and water resistance. [16, 17]
Recent studies show the improvement of water resistance and heat resistance properties of PVAc adhesive in several ways. Adamopoulos et al. [18] reported that the water resistance, as well as mechanical properties of PVAc adhesive, can be improved by treating beech wood samples with phenol–formaldehyde before applying adhesives. Gua et al. [16] have studied the effect of blending isocyanate-based polymeric MDI crosslinker in an emulsion. They found that mechanical properties of emulsion film improve on crosslinking it with isocyanate-based crosslinker. Khan et al. [19] studies show improvement of adhesive strength and toughness of PVAc adhesive by adding graphene into the system. Fang et al. [15] report improving mechanical properties of PVAC-co-NMA adhesive by incorporating exfoliated MMT. Brown and Frazier [20] show property variation in PVAC-co-NMA self-crosslink system by altering the sequence of NMA addition. Few commercial adhesives include copolymerisation products of vinyl acetate and crosslinking agents such as N methylol acrylamide. NMA crosslinks with the other NMA groups and the hydroxyl group of PVOH at lower pH conditions which produce formaldehyde as a by-product. The crosslinking improves the crosslinking density, which improves water resistance. [20, 21]. Hass et al. [22] have studied the effect of various physical parameters on the bonding of PVAc adhesive in wood bonding application. Lu et al. [23] have attempted to improve water resistance by incorporating VEVOA 10 and AAEM [acetoxy ethyl methacrylate] in PVAc system followed by crosslinking the copolymer with a diamine. Much research has been carried out for two-component PVAc adhesive crosslinked with isocyanates [17]
BS EN 204 standards [24, 25] governs and specifies wood bonding strength values. Table 1 mentions the criterion for conditioning the substrates and minimum values of adhesive bonding strength required for qualifying durability classes.
In our current research, we focus on studying the effect of incorporating wattle tannin in PVAc emulsion adhesive on its water resistance and heat resistance properties. Further, we aim to crosslink tannin PVAc emulsion adhesive with isocyanate-based crosslinker to prepare the EPI system. Due to polyphenolic and multi-hydroxyl nature of tannin, we believe tannin will act as collide, and due to the interaction of tannin with components of adhesive water resistance and heat resistance property will improve. Hydroxy group of tannin will further help in creating dense crosslinking when cured with the crosslinker.
2 Experimental
2.1 Materials
2.1.1 Emulsion preparation
Vinyl acetate monomer (VAM) was kindly donated by Pidilite Industries Limited, India. Partially saponified polyvinyl alcohol (PVOH) with a saponification value of 88% was obtained from Kuraray, Japan. Laboratory grade of potassium persulfate and sodium bicarbonate were purchased from Merck. All the chemicals mentioned were used as received without purification.
2.1.2 Formulation
Wattle tannin powder was supplied by Sriguru chemicals [% VOC 6–7%, Tannin content = 75%], India. Isocyanate-based crosslinker used was Bayhydur 304 [% NCO = 18.3%, viscosity at 25 ± 2 °C = 5000 cps] procured from Covestro, Germany. Bayhydur 304 is hydrophobically modified aliphatic isocyanate based on HDI. Henceforth in the current paper, we shall mention tannin for wattle tannin powder and crosslinker for Bayhydur 304.
2.2 Methods
2.2.1 Adhesive synthesis
The emulsion synthesis process was utilised to synthesise the PVAc emulsion adhesive. Recipe for the synthesis of PVAc emulsion is mentioned in Table 2. The adhesive was prepared in a 5-litre glass kettle attached with a lid having five inlets immersed in a water bath with a temperature control system. The kettle was equipped with a dropping funnel, reflux condenser and an anchor-shaped stirrer.
2.2.1.1 Pre-polymerisation preparation
The calculated amount of deionised water and PVOH were taken in the kettle and stirred at 100 rpm for 2 h and at 90 °C to form a 10% non-VOC solution. A 4% non-VOC solution of potassium persulphate was prepared using deionised water. The solution was divided into three parts which consist of part A, part B and part C. Twenty per cent of solution was part A, belonging to pre-catalyst. Part B was 60% of the catalyst solution utilised during polymerisation. Remaining 20% of catalyst solution belongs to part C, used as a post catalyst. A jar with a measured quantity of VAM was attached to reaction kettle via a peristaltic pump. Peristaltic pump facilitates controlled addition of VAM during polymerisation.
2.2.1.2 PVAc polymerisation
The collide solution prepared was cooled to 80 °C, and sodium bisulphate was added as a buffer along with deionised water. Part A of catalyst solution was added to the kettle under stirring, and part B was loaded in dropping funnel. VAM and part B of catalyst solution were added dropwise for a period of 5 h. The reaction temperature was set between 80 and 84 °C and stirring was maintained between 120 and 140 rpm. After 5 h, Part C of the catalyst solution was added in the emulsion and stirred for 1 h at 85 °C. The targeted non-VOC of PVAc emulsion was 52%.
2.2.2 Formulation
Aqueous tannin solution of 40% non-VOC (weight basis) was prepared in deionised water. The adhesive solution was prepared at room temperature under stirring until the homogenous mixture was obtained. (2) The calculated amount of tannin solution was blended slowly with PVAc emulsion. Tannin solution was added dropwise to avoid any lumps and grits formation. Mixing time of 20 min was set for each sample, assuring uniform mixing by visual observation. (3) Tannin PVAc emulsion adhesive was aimed at 46% non-VOC content, and it was obtained by adjusting the non-VOC content of the blend with deionised water.
For studying the crosslinking effect, prepared tannin PVAc emulsion adhesives were further blended with different proportions of crosslinker to develop an EPI. Mechanical stirring was employed for blend preparation. Table 3 explains in detail about the variation of tannin concentration in tannin PVAc emulsion adhesive, the variation of crosslinker across the blends and nomenclature for each adhesive blend. We will follow for further discussions in this research article. For example, a sample with name PA 4/2.5 indicates 4% (w/w) of dry tannin added to 100 g [non-VOC part of PVAc emulsion] and made it to 46% non-VOC tannin PVAc emulsion adhesive. The prepared blend was mixed with 2.5 parts of crosslinker per 100 parts of tannin PVAc emulsion adhesive.
2.2.3 Tannin PVOH miscibility study
Tannin and PVOH are hydrophilic in nature, and they form hydrogen bonds when mixed. For understanding the extend of miscibility of tannin with PVOH present in the emulsion, a separate experiment was performed. In the experiment, 10% non-VOC solutions of PVOH and tannin were prepared in deionised water, respectively. In a kettle, individually prepared solutions of tannin and PVOH were mixed in different ratios to form a solution of tannin/PVOH blend. Ratios of tannin/PVOH selected were 0/100, 5/95, 10/90, 15/85, 20/80, 25/75.
2.2.4 Viscosity
The reaction between crosslinker and components of PVAc emulsion adhesive leads to rises the viscosity of the system with time. Viscosity variation was studied for PA 0/5.0 and PA 4/5.0. 400 g of PA 0/0.0 and PA 4/0.0 were measured in the respective containers, and 5 parts per hundred resin (pph) of crosslinker were added to each container. The outcome of the mixing was PA 0/5.0 and PA 4/5.0. Viscosity was measured at a regular time interval. The containers were closed with an aluminium foil to avoid loss of water. The change in viscosity was measured at 30 °C using a Brookfield RVT model with spindle no 6 or 7 at 20 rpm speed for the duration of 24 h from the time of adding the crosslinker. The rise in viscosity rise was reported as a per cent change in viscosity concerning initial viscosity.
2.2.5 Fourier transform infrared spectroscopy (FTIR)
FTIR is suitable for studying the interaction between the chemical groups. FTIR spectroscopy predicts the reaction of the crosslinker with the blend. Fifteen gram of aqueous tannin solution (40% weight basis) was mixed with 1 g crosslinker, and the mixture was allowed to react for 7 days at room temperature. The reaction provides a hard material on curing, which was washed with deionised water twice and dried in an oven at 105 °C for 4 h. FTIR spectrum was recorded on PerkinElmer FTIR spectrophotometer of spectrum 100 assisted by diamond ATR.
2.2.6 Dynamic mechanical analysis (DMA)
DMA is an accurate way of determining glass transition temperature (Tg). Following are three ways to determine Tg from DMA analysis: (a) The peak of the tan delta curve denotes Tg; (b) The peak of loss modulus curve indicates the onset of segmental motion and Tg; (c) From slope intersection of storage modulus curve [26]. We aim to compare the change in Tg for tannin PVAc blends, and hence, we refer peak of tan delta curve for Tg calculation. Since tan delta value is the ratio of loss modulus to storage modulus, dimensional stability factor in the calculation of Tg is eliminated. PA 0/0.0, PA 2/0.0, PA 4/0.0 and their respective EPI prepared by adding 2.5 and 5 pph of crosslinker were tested for DMA analysis. Films of the samples were cast on a silicon paper and dried for 7 days before testing at 25 ± 2 °C and 65 ± 5% of relative humidity (RH). The casting was done with a film applicator of 200 µm coating thickness to obtain dry film thickness was between 80 and 100 µm. DMA analysis was carried using a TA Instruments Q800 dynamic mechanical analyser (USA) in a film-tension geometry mode at a frequency of 1 Hz. The temperature ranged from − 30 to 150 °C at a heating ramp rate of 5 °C/min.
2.2.7 Goniometry
The water contact angle was measured to understand the effect of tannin on surface wetting properties of PVAc tannin blend. Tannin PVAc emulsion adhesives were coated on a glass plate using 200-micron applicator. Films were dried for 7 days at 25 ± 2 °C and 65 ± 5% RH. Five measurements for each sample were taken. The droplet size was kept constant at 10 µL. The test was carried on the Rame-Hart Goniometer instrument.
2.2.8 Bonding strength
Tannin PVAc adhesive was applied on a step-cut unsteam beech wood substrates with a bonding area of (2 × 2) sq. cm followed by applying static pressure of 1 MPa. The adhesive was applied on the substrates at 25 ± 2 °C and 65 ± 5% RH, followed by the conditioning of the substrates for 7 days at the same temperature and humidity condition. After 7 days, samples were released from the clamps and were conditioned again at 25 ± 2 °C and 65 ± 5% RH for 7 days. The substrates were conditioned as per BS EN 204, D1, D2, D3 [24] and DIN 14257/WATT 91 [27], respectively. Henceforth, in this research article, bonding strength for the substrates conditioned as per BS EN 204/D1 termed as D1 bonding strength and similarly for substrate conditioned as per BS EN 204/D2, D3 and DIN 14257/WATT91 will be termed as D2 bonding strength, D3 bonding strength and heat resistance bonding strength, respectively. For each test, at least five samples were prepared, and standard deviations were calculated. Bond strength evaluation was carried on a universal testing machine (UTM) of making Amil technologies. Load cell capacity of UTM was 25KN, and crosshead speed was 5 mm/min. Figure 1 shows the side view and top view of the bonded substrate.
3 Results and discussion
The synthesised polyvinyl acetate emulsion has a milky white appearance, viscosity of 598 poise, non-volatile content of 51.60% and pH of 4.52. The prepared emulsion was used for further formulations with tannin and crosslinker.
From the tannin PVOH blend, we observed a smooth homogenous solution until the tannin/PVOH ratio was 15/85. At the ratio of 20/80, gelling was observed, and at the ratio of 25/75, coagulation was observed in the blend. The observation is attributed to the formation of dense hydrogen bonding which helps tannin and polyvinyl alcohol to bind tightly and forms coagulation in the solution [28]. Figure 2 shows the smooth homogenous blend of tannin/PVOH (10/90) and coagulated particles in tannin/PVOH (25/75).
For simplifying the observations and discussions, we have divided the section into two parts. Part A discusses about the property variations of tannin PVAc blend by varying tannin concentration. For discussion in part A, we consider properties of PA 0/0.0, PA 1/0.0, PA 2/0.0, PA 4/0.0 and PA 6/0.0. PA 0/0.0 is a control sample. Part B deals with the effect on properties by adding isocyanate hardener to form an EPI blend. We considered all the samples mentioned in Table 2 for evaluation. PA 0/0.0, PA 0/2.5, PA 0/5.0 series is considered being a control sample series.
3.1 Part A
3.1.1 Formulation
Addition of tannin in the synthesises PVAc emulsion gives a smooth blend product. After adding 6 pph (on dry basis) of tannin, small particles were observed. These particles were similar to the coagulations we found in tannin/PVOH blend. This observation is attributed to the formation of hydrogen bonding between tannin and polyvinyl alcohol present as a collide in PVAc emulsion.
3.1.2 Dynamic mechanical analysis (DMA)
Referring to Fig. 3, we observed that Tg increases with increase in tannin concentration. Tg increases from 40.70 °C for the control sample PA 0/0.0 to 42.78 °C for PA 4/0.0. The sharp rise in Tg was observed between PA 0/0.0 and PA 2/0.0. Increase in Tg is attributed to the formation of secondary hydrogen bonding of tannin with the hydroxyl of PVOH and carbonyl group. Secondary interactions also improve the elasticity (storage modulus) of the blend. Decrease in tan delta peak (Fig. 3) indicates the improvement in the elastic properties of the blend.
3.1.3 Goniometry
Measurement of contact angle provides essential information on surface wetting property of tannin PVAc emulsion adhesive. We observed a decrease in contact angle with an increase in tannin concentration in the blend. For control sample PA 0/0.0, value of surface contact angle is 44.02 θ (SD = 0.62), whereas for PA 6/0.0, contact angle value is 40.01 θ (SD = 0.52). Figure 4 shows the trend of contact angle obtained by goniometry, and Fig. 5 shows the shape of water droplet while measuring the contact angle. The improvement in surface wetting property of the adhesive provides better penetration of adhesive in the wood substrate, which improves bonding strength [29, 30].
3.1.4 Bonding strength
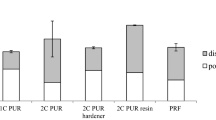
From Fig. 6 and Table 5, we observe that D1 bonding strength values slightly increase from 9.61 N/mm2 (SD = 0.52) control sample PA 0/0.0 to 10.24 N/mm2 (SD = 0.29) for PA 4/0.0. However, the increase in D2 bonding strength values is significant. D2 bond strength values for a control sample of tannin PA 0/0.0 were 5.20 N/mm2 (SD 0.41) which increases to 8.09 N/mm2 (SD = 0.40) for PA 4/0.0. The observed trend could be due to the presence of tannin, which makes adhesive more polar, which facilitates better wetting of wood surfaces and better penetration of adhesive in wood. The observation can be supported by goniometry results which indicate a decrease in water contact angle on increasing tannin content in tannin PVAc emulsion adhesive. Also, the presence of tannin provides strong hydrogen bonding which makes an adhesive compact and less susceptible towards the water. We observe PA 4/0.0, and PA 6/0.0 qualifies D2 bonding strength criterion as per EN 204. The heat resistance improves slightly with an increase in tannin content. Bond strength values for heat resistance test are 2.99 N/mm2 (SD = 0.32) for PA 6/0.0 from 2.10 N/mm2 (SD = 0.41) for PA 0/0.0. Improvement in heat resistance can be attributed to secondary bonding between the tannin hydroxyl group and the carbonyl group of PVAc [31] and between the hydroxyl group of PVOH and a tannin hydroxyl group.
3.2 Part B
3.2.1 Viscosity
From Fig. 7, we observed that the control sample PA 0/5.0 shows rise of 120% from its initial viscosity in 24 h, whereas the viscosity of PA 4/5.0 shows an increase of 222% from its initial value in the same time. The rate of rising in viscosity for PA 4/5.0 is higher than PA 0/5.0. The observed phenomenon is attributed to the faster reaction of isocyanate with the phenolic hydroxyl group of tannin to form a denser crosslinked structure. The crosslinking facilitates the increment in viscosity of the adhesive blend.
3.2.2 Fourier transform infrared spectroscopy (FTIR)
From Fig. 8a, we observe, in tannin spectrum, a broad peak resembling hydroxyl groups in the region of 3000 and 3400 cm−1. On reacting tannin with a crosslinker, the intensity of hydroxyl peak is reduced, indicating that the hydroxyl group of tannin reacted with the isocyanate group of crosslinker. From Fig. 8b, complete reaction of the isocyanate group was confirmed by the disappearance of isocyanate (NCO) peak at 2255 cm1.
3.2.3 Dynamic mechanical analysis (DMA)
Table 4 shows the variation of Tg on adding crosslinker in tannin PVAc emulsion adhesive system. We observed that the Tg increases on the addition of crosslinker. For control sample series of PA 0, improvement in Tg is from 40.70 °C for PA 0/0.0 to 42.90 °C, for PA 0/5.0, i.e. the difference in Tg is 2.2 °C, whereas, for PA 4 series, with the addition of crosslinkers, Tg improves from 40.70 °C for PA 4/0.0 to 46.18 °C for PA 4/5.0, i.e. increment of 3.4 °C in Tg value. The observed improvement is attributed to better crosslinking in tannin PVAc emulsion adhesive system. This difference in Tg is small to compare. However, it indicates the increment in crosslinking in tannin PVAc EPI blend.
3.2.4 Bonding strength
The bonding strength of PVAc tannin EPI blend was assessed on the substrates conditioned as per EN 204 for water resistance and WATT 91 for heat resistance. From Fig. 9 and Table 5, we observed, for control series of PA 0, D2 bonding strength increase from 5.20 N/mm2 (SD = 0.41) for PA 0/0.0 to 8.09 N/mm2 (SD = 0.56) for PA 0/5.0. Incorporation of tannin in PVAc emulsion further improves the D2 bonding strength. For PA 4 and PA 6 series, as observed in part A, D2 bonding strength is 8.09 N/mm2 (SD = 0.40) and 8.19 N/mm2 (SD = 0.52), respectively. Addition of crosslinker into the blend further improves D2 bonding strength to 10.20 N/mm2 (SD = 0.39) and 10.82 N/mm2 (SD = 0.71) for PA 4/5.0 and PA 6/5.0, respectively. From Fig. 10 and Table 5, we observed variation in D3 bonding strength values. For the samples without crosslinker, we could not measure the bonding strength values. Samples were too weak to handle the stress developed while testing the samples. Bonding strength improved on adding crosslinker. For PA0/5.0, we observed D3 bonding strength of 1.52 N/mm2 (SD = 0.41), whereas for PA 4/5.0 and PA 6/5.0 D3 bonding strength was measured 2.12 N/mm2 (SD = 0.27) and 2.01 N/mm2 (SD = 0.19), respectively. We summarise that the prepared sample of PVA emulsion qualifies D1 criterion. Addition of minimum 4 pph tannin on a dry weight basis of emulsion qualifies the same emulsion to D2 criterion. Addition of crosslinker further improves the bonding properties, which converts the adhesive to qualify D3 criterion. From Table 5, We observed the balance between tannin content and a crosslinker. Samples PA 1/2.5 and PA 2/2.5 qualify D2 criterion. If we expect to convert the synthesised adhesive for passing D2 bonding strength, three options could be utilised. One is adding 4% tannin in the PVAc emulsion (PA 4/0.0) and second is adding either 1 or 2% of tannin followed by addition of 2.5 pph of crosslinker (PA 1/2.5, PA 2/2.5). The third option is adding 5 pph of crosslinker in the prepared PVAc emulsion(PA 0/5.0). The observation indicates that the addition of tannin decreases the amount of crosslinker required in the system.
From Fig. 11 and Table 5, we observed variation in bonding strength test for assessing heat resistance property of the blend. For PA 0 series, bonding strength improves from 2.10 N/mm2 (SD = 0.41) for PA0/0.0 to 2.83 N/mm2 (SD = 0.35) for PA 0/5.0, whereas for PA 4 ad PA 6 series, heat resistance bonding strength was 2.91 N/mm2 (SD = 0.21) for PA 4/0.0 and 2.99 N/mm2 (SD = 0.32) for PA 6/0.0 which increases to 3.79 N/mm2 (SD = 0.40) and 3.72 N/mm2 (SD = 0.29) for PA 4/5.0 and PA 6/5.0, respectively. We observe that PA 4/5.0 and PA 6/5.0 qualify D3 criterion.
The overall improvement in bonding strength is attributed to the following observations which include better wetting property due to the presence of tannin measured by goniometry (Fig. 4), hydrogen bonding and dense crosslinking provided by crosslinking of multi-hydroxyl tannin, PVOH and isocyanate group.
4 Conclusion
Wattle tannin extract helps in improving the water resistance and heat resistance property of polyvinyl acetate emulsion adhesive. From our research understanding, we can conclude
-
1.
Tannin enhances the wettability of PVAc emulsion adhesive, which is observed from contact angle measurement using a goniometer.
-
2.
Surface wetting property improves bonding strength, water resistance and heat resistance property.
-
3.
Incorporating tannin converts a BS EN 204 D1 compliance adhesive to BS EN 204 D2 compliance adhesive.
-
4.
By incorporating crosslinker, the viscosity of the system rises quickly due to faster reaction of the crosslinker with tannin.
-
5.
Water resistance and heat resistance further improve in the presence of tannin and crosslinker converting a BS EN 204 D1 compliance PVAc adhesive to BS EN 204 D3 compliance adhesive.
The methods mentioned in the research provides a simple and cost-effective way to improve the PVAc adhesive performance by incorporating tannin in PVAc emulsion adhesive.
References
Khanbabaee K, van Ree T (2001) Tannins: classification and definition. Nat Prod Rep 18(6):641–649
Rhazi N, Oumam M, Sesbou A, Hannache H, Charrier-El Bouhtoury F (2017) Physico-mechanical properties of plywood bonded with ecological adhesives from Acacia mollissima tannins and lignosulfonates. Eur Phys J Appl Phys 78(3):34813
Sowunmi S, Ebewele RO, Conner AH, River BH (1996) Fortified mangrove tannin-based plywood adhesive. J Appl Polym Sci 62(3):577–584
El-Din HMF, El-Messery TM, Mehanna NS (2015) Interaction between some plants tannins and milk protein. Int J Food Nutr Sci 4(1):16–20
Deshpande SS, Cheryan M, Salunkhe DK, Luh BS (1986) Tannin analysis of food products. Crit Rev Food Sci Nutr 24(4):401–449
Tondi G (2017) Tannin-based copolymer resins: synthesis and characterisation by solid state 13C NMR and FT-IR spectroscopy. Polymers 9(6):223
Ghahri S, Pizzi A, Mohebby B, Mirshokraie A, Mansouri HR (2018) Soy-based, tannin-modified plywood adhesives. J Adhes 94(3):218–237
Navarrete P, Pizzi A, Bertaud F, Rigolet S (2011) Condensed tannin reactivity inhibition by internal rearrangements: detection by CP-MAS 13C NMR. Maderas Cienc Tecnol 13(1):59–68
Celzard A, Szczurek A, Jana P, Fierro V, Basso MC, Bourbigot S, Pizzi A (2015) Latest progresses in the preparation of tannin-based cellular solids. J Cell Plast 51(1):89–102
Pizzi A (1982) Pine tannin adhesives for particleboard. Holz als Roh-und Werkst 40(8):293–301
Ugovsek A, Kariz M, Sernek M (2010). Bonding of beech wood with an adhesive mixture made of liquefied wood and phenolic resin. In: Proceedings of the “Hardwood Science and Technology”—the 4th conference on hardwood research and utilisation in Europe. Sopron, Hungary, pp 17–18
Kim S (2009) Environment-friendly adhesives for surface bonding of wood-based flooring using natural tannin to reduce formaldehyde and TVOC emissions. Biores Technol 100(2):744–748
Elbadawi M, Osman Z, Paridah T, Nasroun T, Kantiner W (2015) Mechanical and physical properties of particleboards made from Ailanthus wood and UF resin fortified by Acacias tannins blend. J Mater Environ Sci 6(4):1016–1021
Kim S, Lee JH, Kim J (2012). Application of tannin as green adhesive for environment-friendly furniture materials. In: 10th International conference on healthy buildings 2012, pp 931–935
Fang Q, Cui HW, Du GB (2013) Preparation and characterisation of PVAc–NMA–MMT. J Thermoplast Compos Mater 26(10):1393–1406
Guo J, Hu H, Zhang K, He Y, Guo X (2018) Revealing the mechanical properties of emulsion polymer isocyanate film in humid environments. Polymers 10(6):652
Grøstad K, Pedersen A (2010) Emulsion polymer isocyanates as wood adhesive: a review. J Adhes Sci Technol 24(8–10):1357–1381
Adamopoulos S, Bastani A, Gascón-Garrido P, Militz H, Mai C (2012) Adhesive bonding of beech wood modified with a phenol formaldehyde compound. Eur J Wood Wood Prod 70(6):897–901
Khan U, May P, Porwal H, Nawaz K, Coleman JN (2013) Improved adhesive strength and toughness of polyvinyl acetate glue on addition of small quantities of graphene. ACS Appl Mater Interfaces 5(4):1423–1428
Brown NR, Frazier CE (2007) Cross-linking poly [(vinyl acetate)-co-N-methylolacrylamide] latex adhesive performance part I: n-methylolacrylamide (NMA) distribution. Int J Adhes Adhes 27(7):547–553
Bufkin BG, McGrawe JR (1978) J Coat Technol 50(641):41–55
Hass P, Wittel FK, Mendoza M, Herrmann HJ, Niemz P (2012) Adhesive penetration in beech wood: experiments. Wood Sci Technol 46(1–3):243–256
Lu J, Easteal AJ, Edmonds NR (2011) Crosslinkable poly (vinyl acetate) emulsions for wood adhesive. Pigm Resin Technol 40(3):161–168
EN 204/2001: Classification of thermoplastic wood adhesives for non-structural applications
Iwata R, Inagaki N (2006) Durable adhesives for large laminated timber. J Adhes Sci Technol 20(7):633–646
Menard KP (1999) Dynamic mechanical analysis: a practical introduction. CRC Press, Boca Raton
DIN EN 14257 (WATT 91): Determination of tensile strength of lap joints at elevated temperature
Chen YN, Peng L, Liu T, Wang Y, Shi S, Wang H (2016) Poly (vinyl alcohol)–tannic acid hydrogels with excellent mechanical properties and shape memory behaviors. ACS Appl Mater Interfaces 8(40):27199–27206
Moredo CC Jr, Sakuno T, Kawada T (1996) The improvement of bond strength properties and surface characteristics of resinous woods. J Adhes 59(1–4):183–195
Wolkenhauer A, Avramidis G, Hauswald E, Loose S, Viöl W, Militz H (2009) Investigations on the drying behaviour of adhesives on plasma-treated wood materials. Wood Res 54:56–66
Huang MW, Kuo SW, Wu HD, Chang FC, Fang SY (2002) Miscibility and hydrogen bonding in blends of poly (vinyl acetate) with phenolic resin. Polymer 43(8):2479–2487
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prabhu, R., Jagtap, R. & Digar, M. Study on incorporating wattle tannin in polyvinyl acetate emulsion and its effect on properties for wood bonding application. SN Appl. Sci. 2, 1722 (2020). https://doi.org/10.1007/s42452-020-03516-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-03516-1