Abstract
The effect of Fe3O4 nanoparticles (NPs) on chemical properties of the soil rhizosphere and on the accumulation of nutrients in common bean plants was studied for two different concentrations of Fe3O4 NPs. The root-to-leaves translocation index for micro- and macronutrients was calculated. The results showed that Fe3O4 NP treatments had a significant effect (\(P < 0.05\)) on the chemical properties of soil rhizosphere in terms of an increase in the contents of total P, extractable P, total K, extractable K, Ca, total Mn, total Fe and cation exchange capacity and of a decrease in Cl content in soil. The treatments led to a marked increase in the accumulation of nutrients in plants by revealing a higher content of total P, K, Ca, Mn and Fe in roots, stems and leaves. In addition, the plants treated with Fe3O4 NPs showed lower translocation of total Mn and Fe to stems and leaves compared with the control plants. The results indicate that Fe3O4 NPs may contribute to the conversion of the insoluble forms of total P, extractable P, total K, extractable K, Ca, total Mn and total Fe in soil into soluble forms that can dissolve in the soil solution and be taken up by plants. A greater capability in the roots, stems and leaves of Fe3O4-NP-treated plants to take up the nutrients suggests a beneficial effect for plant development and health. Likewise, the roots of plants treated with Fe3O4 NPs absorbed and accumulated the greatest quantities of Mn and Fe compared with the control plants.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nanomaterials are defined as a set of substances where at least one dimension is less than approximately 100 nm. They are of interest because at this scale unique optical, magnetic, electrical properties are detected. These properties have great impact potential in electronics, medicine, agriculture, construction, industry and other fields. In particular, the effects of engineering nanoparticles (ENPs) on plants are of considerable concern because of their crucial interface with the environment.
In an extensive review article on uptake and translocation in plants [1, 2], it is advocated that future research on plants and ENPs requires studies under natural conditions, i.e., plant–soil interaction. This study not only yields information on the effects of ENPs, when ions are released into the environment, but also quantifies the uptake and translocation of nutrients. However, few experiments on plant growth in soil have been performed hitherto [3,4,5,6]. Thus, a systematic study on uptake and accumulation of nanoparticles (NPs) in plant roots grown in soil, and under the influence of the macro- and microelement accumulation in soil, roots, stems and leaves, is still a matter of interest.
Ideal amounts of nutrients are essential for plant growth and development; however, several factors contribute to the reduction in nutrients availability. In order to increase crop yields, it is necessary to reduce nutrient losses—this can be achieved by using ENPs. A beneficial effect of NPs on plants growth and development can be found in [7]. Another study accomplished on Lactuca sativa exposed to nano-Fe/Fe3O4 and nano-Cu/CuO treatments showed changes in the uptake of different nutrients. A decrease in Mg, P and Ca was detected in leaves of plants exposed to Cu treatments, while an increase in S and Ca was found in the roots [8]. Moreover, different effects are found on the increase/decrease in a particular nutrient in plants, depending on the type of NPs [9,10,11,12], indicating that further studies are required.
The most important grain legume for direct human consumption in the world is the common bean (Phaseolusspp. L.) [13]; therefore, it was chosen as a model to study accumulation and translocation of nutrients in plants in a soil containing magnetite NPs. Thus, the aim of this work is to evaluate the influence of magnetite NPs on some soil’s chemical properties and macro- and microelement accumulation in the soil rhizosphere, roots, stems and leaves and on morphological parameters of bean plants. This study also presents a complementary analysis of the previous work reported in [14].
2 Materials and methods
Certified bean seeds (Phaseolus vulgaris L. cv. Red Guama) were genetically uniform (stated on the package by the supplier) and were provided by the Seed Laboratory of the Ministry of Agriculture in Granma Province, Cuba. Seeds without visible defects, insect damage or malformation were selected and stored in desiccators over 70% (v/v) glycerin. Seed moisture content was 10–12% on a fresh weight basis before the treatments, and final germination percentage was 90%.
The seeds were labeled and sown in a soil media placed in different polyethylene bags, with two replications each, identified as N0, N0 and N2. The soil in bags was a brown carbonate [15], which has a loamy texture and a pH value of 7.3 for the rhizosphere soil. It has an organic matter content of 3.4%, assimilable phosphorus 0.102 g Kg\(^{-1}\) and potassium 0.815 g Kg\(^{-1}\). Additionally, cation exchange capacity (CEC) was 42.2 meq/100g; base exchange capacity 31.8 meq/100g; total N 0.025 g Kg\(^{-1}\); Ca 1.71 g Kg\(^{-1}\); K 1.75 g Kg\(^{-1}\) ; and Mg 0.530 g Kg\(^{-1}\). According to the soil analysis, plant nutrient levels were adequate for the growth of common bean plants. Plants remained in a glasshouse environment under controlled conditions of temperature (\(\sim\) 27 \(^{\circ }\)C), relative humidity of 70% and photoperiod of 12 h.
The Fe3O4 NP treatments were applied through daily irrigation during the morning. The bags N1 and N2 were irrigated with 20 mL of water and 20 mL of suspended Fe3O4 NPs with concentrations of 1000 mg/L (T1) and 2000 mg/L (T2), respectively. The seeds in the bag N0 were grown in a soil free of Fe3O4 NPs (control) and irrigated every day with 40 mL of water. The Fe3O4 NPs, with a log-normal distribution of median diameter of 10 nm and distribution width of 0.36, were prepared by the co-precipitation method described elsewhere [16].
At 30 days after sowing, the soil rhizosphere in each treatment was sampled, dried, sieved (< 2 mm) and then homogenized in the laboratory. The pH was determined potentially in a ratio of soil to distilled water (1:2.5 w/v) suspension with a glass electrode. The soil rhizosphere cation exchangeable capacity (CEC) was determined by the ammonium acetate method [in BaCl2 0.1 M followed by a re-exchange with aqueous MgSO4 solution (0.1 M)].
Plants were allowed to grow until the vegetative stage V4 (\(\sim\) 30 days after sowing). Then, plants were removed from their polyethylene bags and rinsed well with deionized water. Finally, root length (from the root neck to the tip), plant height, root and shoot plant dry weight (after drying in a ventilated oven at 80 \(^{\circ }\)C for 72 h) were determined.
At this time, each polyethylene bag was sampled into four parts: soil rhizosphere, root, stem and leaves. First, a multi-elemental analysis for 13 elements (B, Co, Mn, Mo, Ni, Zn, Ca, Fe, K, Mg, Na, Cl and P) of each soil and dried sample was performed in an inductively coupled plasma optical emission spectrometer (ICP-OES) Spectro Arcos (Spectro). Reference solutions with a high degree of analytical purity were used to obtain the calibration curves. Deionized water (Milli-Q) was used to prepare all solutions. All samples were subjected to microwave-assisted digestion in a microwave oven (Speed Wave Four, Berghof Analytik) in a mixture of HNO3 (2 mL), HF (2 mL) and H2O2 (1 mL). The samples were digested at 200 \(^{\circ }\)C for 15 min, 170 \(^{\circ }\)C for 10 min and then 160 \(^{\circ }\)C for 5 min. The volume of the samples was then adjusted to 25 mL using deionized water before analysis.
The magnetization measurements as a function of temperature and applied magnetic field M(T, H) were taken in a commercial Quantum Design SQUID magnetometer in the roots, stems and leaves. M(T) data were obtained for both zero-field-cooled (ZFC) and field-cooled (FC) conditions, under an applied magnetic field of 500 Oe, in the temperature range 10–300 K. M(H) data were taken at 10 and 300 K, under an applied magnetic field range \(-\,70 \le H \le 70\) kOe.
3 Results and discussion
3.1 Soil
Most of the chemical interactions of both macro- and micronutrients with the soil are related to values of pH. In our study, the soil pH started weakly alkaline for Fe3O4-NP-treated soils and untreated soil, e.g., 7.2 and 7.3, respectively. These values remained stable during the experimental period of 30 days. Table 1 indicates that Na, Zn, Cu and Mo contents in soil rhizosphere were not significantly influenced by Fe3O4 NP treatments compared with the control (\(P<0.05\)). The elements Cu, Ni, B and Mo are not shown because their values are negligible. In contrast, the total P, extractable P, total K, extractable K, Ca, total Mn, total Fe and Cl contents in soil rhizosphere were significantly influenced in different ways by the Fe3O4 NP treatments compared with the control (\(P<0.05\)), as discussed below.
Soil total P content was markedly altered by Fe3O4 NP treatments in comparison with the control, where these changes were similar in both treatments. The concentration of this element was increased by 20% in T1 and by 11% in T2 (Table 1). Likewise, higher extractable P content in soil was significantly found in Fe3O4-NP-treated soils, which had an increase of 22% in T1 and of 18% in T2 with respect to the untreated soil, without significant differences between Fe3O4 NP treatments (Table 1).
Although the soils treated with T1 (1000 mgL\(^{-1}\) Fe3O4 NPs) and T2 (2000 mgL\(^{-1}\) Fe3O4 NPs) exhibited higher amount of total P compared with the control soil, the findings do not imply a higher availability of P in the soil. The soil total P test does not measure the phosphorus in the soil solution, but rather the capacity of the soil to supply phosphorus to the soil solution [17, 18]. However, extractable P content is the concentration of soluble P in the soil solution that represents appropriately the amount of phosphorus that plants can potentially absorb during the growing season. Our results revealed that the Fe3O4-NP-treated soils contained greater extractable P amount with respect to the control soil. This is an important result, since the concentration of soluble P in the soil solution is usually very low; phosphorus is relatively immobile in the soil; and crops take up phosphorus only from the soil solution [17, 19]. It seems that Fe3O4 NPs contributed to the conversion of the insoluble forms of various P compounds into soluble forms that can dissolve in the soil solution and be taken up by plants. P is vital for bean plant growth due to the fact that it is a constituent element of bean plant tissues—P is found in every living plant cell and it is essential for biological activity. P is also involved in several key plant functions, including energy transfer, photosynthesis, transformation of sugars and starches and nutrient movement within the plant [20].
Total K and extractable K concentrations in the soil were notably enlarged by Fe3O4 NP treatments, with increases of 29 and 25% in T1 and T2 for total K and of 29 and 43% in T1 and T2 for extractable K, respectively, compared with the control (Table 1). Therefore, similar results were observed for both treatments. Regardless of the considerable increase in the K content, it is of little value in determining how well a given soil can supply potassium to growing plants. It is interesting to note that Fe3O4 NP treatments increased the extractable K concentration compared with the control, since only one to two percent of the total soil K is in an exchangeable (available) form and it is of immediate concern for plant use [21]. A higher availability of soil K is favorable for plants, considering many regulatory roles played in plant development [20]. K balances the charges of anions, increases root growth, strengthens the resistance to disease and drought and may activate enzymes. K also affects cell extension, growth through the regulation of turgor and leaf gas exchange through the control of stomatal opening and closing [20, 22]. Therefore, there is a great demand of K in bean plants growth and metabolism.
With regard to the Ca content, the Fe3O4-NP-exposed soils exhibited appreciably greater Ca concentration than the control soil (35 and 43% higher, respectively) (Table 1). This indicates a positive effect since it is the nutrient form available for plant growth; furthermore, it may improve absorption of other nutrients by plants. It suggests that Fe3O4 NPs may contribute to the solubilization of insoluble compounds of Ca with other elements in the soil, such as phosphorous, to help in stabilizing the soil structure, to replace the adsorbed sodium and to prevent damages to soil structure. Ca is vital in the growth and development of plants, and it plays an important role in various processes, such as formation of cell wall [23] and plasma membrane [24], cell growth and secretion [20].
As for the Cl content in soil, it was substantially reduced by the Fe3O4 NP treatments, with decreases of 41.4 and 30% in T1 and T2, respectively, compared with the control, and these decreases were distinctly noted in both treatments. T1 showed the lowest Cl concentration (Table 1). On the contrary, a lower soil Cl content found in the Fe3O4-NP-treated soils and the lowest Cl amount measured in T1 indicate a favorable effect for plant growth because the lower the Cl amount in the soil, the lower the likelihood of compounds with salts being formed; consequently, it counters to the increase in salinity, even though the levels of Cl content in the soil are within the sufficient limit values (10–20 ppm) for both treatments and the control.
With the Fe3O4 NP treatments, the treated soils showed a noticeable increase in the concentrations of soil total Mn compared with the control. T2 revealed the highest total Mn concentration raise in soil; the observed increase was 53% in T1 and of 115% in T2, respectively. Higher soil total Mn content found in Fe3O4-NP-exposed plants and the highest Mn concentration in the soil registered by T2 mean that the Fe3O4 NP treatments may contribute to the solubilization of a higher amount of Mn in soil solution. The total Mn levels in the soil are within the sufficient limit values (10–50 mg Kg\(^{-1}\)) for both treatments and the control.
The responses of the total Fe concentration in the soil resembled those of total Mn, with the highest total Fe concentration in the soil for T2. The concentration increased by 207% in T1 and by 493% in T2 with respect to the control (Table 1). Although the difficulty in correlating the soil solution concentration of iron with the soil total iron measurement, the increased soil Fe concentration and the highest concentration of this microelement in T2 may indicate that Fe3O4 NPs contribute to the solubilization of a higher amount of Fe in soil regardless of the pH level of 7.3. Normally, the availability of iron compounds in the soil may be limited in the soils with high pH, especially in calcareous soils [25, 26]. Also, Fe3O4 NPs may supply more Fe in soil through the release of free ions from such NPs. The levels of Fe in the soil for both treatments exceed the sufficiency of levels (20–100 mg Kg\(^{-1}\)). It is also necessary to highlight that a higher bioavailability of iron in the soil may favor the soil microorganisms’ growth. Molecular evidence suggests that iron oxide magnetic NPs may facilitate C and N cycling in the soil by influencing the soil bacterial community [27].
The Fe3O4 NP treatment of soils further presented a favorable result for the cation exchange capacity (CEC). The performance was higher for T2 (49.2 meq/Kg\(^{-1}\)) with an increase of 17% when compared with the control soil (42.2 meq/100 g) versus 9% in T1 (46.2 meq/100 g). CEC characterizes the number of negatively charged sites in the soil that are able to adsorb exchangeable cations. This is an important parameter because it provides a good indication of the ability of the soil to adsorb nutrients and therefore an indication of the quality and productivity of the soil.
3.2 Plant organs: root, stem and leaves
The nutrients accumulation levels in bean plant organs (roots, stems and leaves) growing naturally at the sampling site are presented in Table 2. These results showed that Fe3O4 NP treatments modulated the nutrient uptake in the bean plant tissues of exposed plants, indicating their different capacities for nutrients uptake. Among the analyzed elements, a significant difference for total P, total K, Ca, total Mn, and total Fe contents was found in plant organs exposed to Fe3O4 NPs compared with the control plants, as described below for each plant organ separately. On the contrary, Mg, Na, Zn and Cl contents in plant tissues were not significantly different between Fe3O4-NP-exposed plants and the control plants. Due to their negligible values, Cu, Ni and Mo contents are not provided. Furthermore, no toxicity on plants growth has been detected visually as well as no evidences of stubby roots and Fe deficiency in the leaves.
As can be seen from the data in Table 2, improved concentration of several elements was found in the roots due to Fe3O4 NP treatments, with an increase in total P by 14% and 11% (no differential effects), in total K by 32% and 21%, in Ca by 22% and 17% and in Mn by 10% and 17% (no differential effects), for T1 and T2, respectively. Also, the roots registered a considerable increase of 192% in T1 and 277% in T2 in the concentrations of total Fe content with respect to the control plants. The highest concentration of total Fe was found in T2 (462.0 ± 0.1 mg Kg\(^{-1}\)), which differs significantly from T1 (356.9 ± 0.1 mg Kg\(^{-1}\)). Our results are in conformity with those reported by Antisari et al. [28] who observed an increased concentration of Ca in roots of tomato plants treated with 20 \(\upmu\)g mL\(^{-1}\) of Fe3O4 NPs. Iannone et al. [29] reported that the Fe3O4 NPs had the ability to increase the Fe content in roots of wheat plants treated with 20 mg L\(^{-1}\) of Fe3O4 nanoparticles compared with the control plants.
Following the same trend, the data in Table 2 suggest that the content of several elements, such as total P and total K, increased appreciably in the stems of plants in T1 and T2 with respect to the control plants, although these increases were not significantly different in both treatments. An increase of 22% in T1 and 20% in T2 was found for total P contents and of 24% in T1 and 21% in T2 for the total K concentration in the stems. A differential effect between the two Fe3O4 NP treatments was found in the total Mn concentration in the stems: It was improved by 41% and 68% in T1 and T2 with respect to the untreated plants, respectively. In the same way, the stems registered a considerable increase of 221% in T1 and 335% in T2 in the concentrations of total Fe content with respect to the control plants. The highest concentration of total Fe was found in T2 (384.7 ± 1.4 mg Kg\(^{-1}\)), which differs significantly from T1 (283.9 ± 1.9 mg Kg\(^{-1}\)).
Significant results were also found for the leaves in the bean plants treated with Fe3O4 NPs, as indicated in Table 2; however, the effect of Fe3O4 NP suspension concentration on the analyzed elements was different. Total K concentrations in leaves were notably enlarged by Fe3O4 NP treatments, which increased by 32% and 17% in T1 and T2 compared with the control, respectively.
We have found that the increase in the total P and Ca contents was different from treatments T1 and T2. For example, the leaf total P content was markedly altered by Fe3O4 NP treatments in comparison with the control, increasing by 73% in T1 and 62% in T2; the increase in Ca was 26% and 25% for T1 and T2, respectively. The results of P are consistent with those reported by Zahra et al. [30] who found higher P content in shoots than that in roots in lettuce plants treated with Fe3O4 NPs. Jalali et al. [31] revealed a significant increase in shoot Ca content in maize plants treated with Fe3O4 NPs compared with the control plants.
It was also found that plants treated with Fe3O4 NPs showed a noticeable increase in the concentrations of leaf total Mn. T2 revealed the highest total Mn concentration (increased by 78%) and T1 the lowest (53%). It suggests that Fe3O4 NPs induced greater capability for roots, stem and leaves to take up Mn in treated plants for bean plant growth. Manganese may also serve as a cofactor, activating numerous enzymes involved in the catalysis of oxidation reduction, decarboxylation and hydrolytic reactions in plant growth processes. It is also involved in metabolic processes such as respiration, photosynthesis, synthesis of amino acids and hormone activation [20, 32, 33].
The responses of the total Fe concentration in leaves resembled those of Mn, increasing in leaves by 329% in T1 and by 448% in T2 when compared with the control. This fact indicates that magnetite NPs induced greater ability for plant roots, stems and leaves to extract total Fe from soils, which may lead to an enhancement of both iron mobilization in the rhizosphere and the uptake rate of iron. There is evidence that iron may serve as an activator for biochemical processes such as respiration, photosynthesis and symbiotic nitrogen fixation and protein metabolism involved in the plant growth [20]. These results are in agreement with the findings of El-Nasr et al. [34] who found an increase in iron leaf content of pear saplings plants due to foliar spray with magnetite nanoparticles compared with control plants.
On the other hand, more total Fe was taken up into roots than into shoots (1.25 times higher than stems and 1.85 times higher than leaves in T1 while 1.20 times higher than stems and 1.88 times higher than leaves in T2), probably because Fe is absorbed into plants via the roots. Despite some reports showing no difference in the concentration of Fe after treatment with iron nanoparticles [35, 36], in this study it is evident that utilizing magnetite NPs has dramatically increased Fe content of the root, stem and leaf. Trujillo-Reyes et al. reported that applying Fe/Fe3O4 nanoparticles to lettuce results in retaining Fe in the roots as insoluble compounds [8]. Jalali et al. revealed that the Fe3O4 NPs promoted increased shoot total iron content in maize plants treated with Fe3O4 NPs [31]. Yuan et al. [37] found an increase in total iron content in the roots and stems of Capsicum annuum plants exposed to 0.05 mM/L of Fe NPs. Likewise, in many other investigations application of nano-iron oxides has been identified as an outstanding contributing element for enhancing Fe content in plants [38, 39]. Unfortunately, there is a lack of understanding in the current literature on how nanoparticles modify nutrient uptake in plants.
In addition to the above elemental analysis for each plant organ, it was also investigated the translocation index. The root-to-stem translocation index can be estimated by the expression [40]:
where RCN, SCN and LCN are the root, stem and leaves concentration of nutrients (micro and macro), respectively. The results reported in Table 3 showed that TI was unaffected by Fe3O4 NPs, in Ca, Mg, Na, Zn and Cl (Table 3). It was found that TI for total P, total Mn and total Fe was significantly higher in plants treated with Fe3O4 NPs (28 in T1 and 25% in T2 for total P, 18% in T1 and 31% in T2 for total Mn and of 20% in T1 and 28% in T2 for total Fe) when compared with the control plants, implying that significant amounts of phosphorus, manganese and iron could translocate to the shoot parts. These results are consistent with those of Antisari et al. [28], who reported a decrease in translocation of Fe to leaves in tomato plants treated with Fe3O4 NPs.
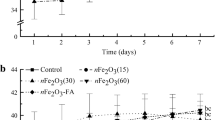
Finally, Fig. 1 shows values of the total Fe concentration (Fig. 1a) and the saturation magnetization (Fig. 1b) in bean plant organs irrigated with different concentrations of Fe3O4 NPs. Values of saturation magnetization, \(M_{\mathrm{s}}\), were extracted from Table 1 of Ref. [14]. Notice that both parameters yield direct and undirect quantitative information, respectively, on the amount of iron accumulated in different parts of the plants, respectively. The obtained results indicate that the Fe concentration decreases from root to leaves and the qualitative behavior for both treatments is quite similar. Such a result has its counterpart in the behavior of the saturation magnetization (see Fig. 1b). This finding confirms that this experimental technique can be used to study the uptake, translocation and accumulation of magnetic nanoparticles in plants as also reported recently [41].
4 Conclusions
In summary, the Fe3O4 NP (1000 mg/L and 2000 mg/L) treatments had a positive effect on the chemical properties of soil rhizosphere and on nutrient uptake by bean plants grown in soil. A remarkable increase was found in the contents of total P, extractable P, total K, extractable K, Ca, total Mn, total Fe and cation exchange capacity in soils irrigated with Fe3O4 nanoparticles, while a marked decrease was found in Cl content in soils. Likewise, the Fe3O4-NP-treated plants revealed higher contents of Ca, total K, total P, total Fe and total Mn in the root, stem and leaf tissues. A greater capability in the roots, stems and leaves of Fe3O4-NP-treated plants to take up these nutrients suggest a beneficial effect for plant development and health. Furthermore, the lower values of the root-to-leaves translocation of total Mn and total Fe indicate that the roots of plants exposed to Fe3O4 NPs uptake, translocate and accumulate the greatest quantities of Mn and Fe compared with the control plants.
References
Dietz K, Herth S (2011) Plant nanotoxicology. Trends Plant Sci 16:582–589
Sandeep KV, Ashok KD, Manoj KP, Ashish S, Vinay K, Saikat G (2018) Engineered nanomaterials for plant growth and development: a perspective analysis. Sci Total Environ 630:1413–1435
El-Temsah Y, Joner E (2010) Impact of Fe and Ag nanoparticles on seed germination and differences in bioavailability during exposure in aqueous suspension and soil. Environ Toxicol 27:42–49
Sheykhbaglou R, Sedghi M, Shishevanand M, Shari RS (2010) Effects of nano-iron oxide particles on agronomic traits of soybean. Nat Sci Biol 2:112–113
Priester JH, Ge Y, Mielke RE, Horst AM, Moritz SC, Espinosa K, Gelb J, Walker SL, Nisbet RM, An Y-J, Schimel JP, Palmer RG, Hernandez-Viezcas JA, Zhao L, Gardea-Torresdey JL, Holden PA (2012) Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc Natl Acad Sci 109:E2451–E2456
Wang Y, Westerhoff P, Hristovski K (2012) Fate and biological effects of silver, titanium dioxide, and C60 (fullerene) nanomaterials during simulated wastewater treatment processes. J Hazard Mater 201–202:16–22
Zhu H, Han J, Xiao JQ, Jin Y (2008) Uptake, translocation, accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J Environ Monit 10:713–717
Trujillo-Reyes J, Majumdar S, Botez CE, Peralta-Videa JR, Gardea-Torresdey JL (2014) Exposure studies of core-shell Fe/Fe3O4 and Cu/CuO NPS to lettuce (Lactuca sativa) plants: are they a potential physiological and nutritional hazard? J Hazard Mater 267:255–263
Jacob DL, Borchardt JD, Navaratnam L, Otte ML, Bezbaruah AN (2013) Uptake and translocation of Ti from nanoparticles in crops and wet land plants. Int J Phytoremediat 15:142–153
Peralta-Videa JR, Hernandez-Viezcas JA, Zhao L, Diaz BC, Ge Y, Priester JH, Holden PA, Gardea-Torresdey JL (2014) Cerium dioxide and zinc oxide nanoparticles alter the nutritional value of soil cultivated soybean plants. Plant Physiol Biochem 80:128–135
Zhao L, Sun Y, Hernandez-Viezcas JA, Hong J, Majumdar S, Niu G, Duarte-Gardea M, Peralta-Videa JR, Gardea-Torresdey JL (2015) Monitoring the environmental effects of CeO2 and ZnO nanoparticles through the life cycle of corn (Zea mays) plants and in sit μ-XRF mapping of nutrients in kernels. Environ Sci Technol 49:2921–2928
Dimkpa CO, White JC, Elmer WH, Gardea-Torresdey J (2017) Nanoparticle and ionic Zn promote nutrient loading of sorghum grain under low NPK fertilization. J Agric Food Chem 65(39):8552–8559
Broughton W, Hernandez G, Blair M, Beebe S, Gepts P, Vanderleyden J (2003) Beans (Phaseolus spp.) model food legumes. Plant Soil 252:55–128
Govea-Alcaide E, Masunaga SH, De Souza A, Fajardo-Rosabal L, Effenberger FB, Rossi LM, Jardim RF (2016) Tracking iron oxide nanoparticles in plant organs using magnetic measurements. J Nanopart Res 18:305–318
IUSS Working Group W.R.B (2006) World reference base for soil resources 2006. In: World soil resources Reports No. 103. FAO, Rome
Rossi LM, Vono LL, Silva FP, Kiyohara PK, Duarte EL, Matos J (2007) A magnetically recoverable scavenger for palladium based on thiol-modified magnetite nanoparticles. Appl Catal A Gen 330:139–144
Cade-Menun BJ, O’Halloran IP (2007) Total and organic phosphorus. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton, pp 265–292
Schoenau J, O’Halloran I (2007) Sodium bicarbonate-extractable phosphorus. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton, pp 89–94
Sharpley A, Kleinman PJA, Weld J (2007) Environmental soil phosphorus indices. In: Carter MR, Gregorich EG (eds) Soil sampling and methods of analysis, 2nd edn. CRC Press, Boca Raton, pp 141–159
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic Press, London
Hendershot WH, Lalande H, Duquette M (1993) Ion Exchange and exchangeable cations. In: Carter MR (ed) Soil sampling and methods of analysis for Canadian society of soil science. Lewis, Boca Raton, pp 167–176
Mengel S (2007) Potassium. In: Barker AV, Pilbean DJ (eds) Handbook of plant nutrition. CRC, New York, pp 395–402
Kobayashi M, Nakagawa H, Asaka T, Matoh T (1999) Borate rhamnogalacturonan II bonding reinforced by Ca2+ retains pectic polysaccharides in higher-plant cell walls. Plant Physiol 119:199–204
Legge RL, Thompson JE, Bake JE, Lieberman M (1982) The effect of calcium on the fluidity and phase properties of microsomal membranes isolated from post climacteric golden delicious apples. Plant Cell Physiol 23:161–169
Lindsay WL, Schwab AP (1982) The chemistry of iron in soils and its availability to plants. J Plant Nutr 5:821–840
Kabata-Pendias A, Pendias H (2010) Trace elements in soils and plants, 2nd edn. CRC Press, Boca Raton
He S, Feng Y, Ren H, Zhang Y, Gu N, Lin X (2011) The impact of iron oxide magnetic nanoparticles on the soil bacterial community. J Soils Sediments 11:1408–1417
Antisari LV, Carbone S, Gatti A, Vianello G, Nannipieri P (2015) Uptake and translocation of metals and nutrients in tomato grown in soil polluted with metal oxide (CeO2, Fe3O4, SnO2, TiO2) or metallic (Ag Co, Ni) engineered nanoparticles. Environ Sci Pollut Res 22:1841–1853
Iannone MF, Groppa MD, de Sousa ME, Fernndez van Raap MB, Benavides MP (2016) Impact of magnetite iron oxide nanoparticles on wheat (Triticum aestivum L.) development: Evaluation of oxidative damage. Environ Exp Bot 131:77–88
Zahra Z, Arshad M, Rafique R, Mahmood A, Habib A, Qazi IA, Khan SA (2015) Metallic nanoparticle (TiO2 and Fe3O4) application modifies rhizosphere phosphorus availability and uptake by Lactuca sativa. J Agric Food Chem 63:6876–6882
Jalali M, Ghanati F, Modarres-Sanavi AM, Khoshgoftarmanesh AH (2017) Physiological effects of repeated foliar application of magnetite nanoparticles on maize plants. J Agro Crop Sci 203:593–602
Burnell JN (1988) The biochemistry of manganese in plants. In: Graham RD, Hannam J, Uren NC (eds) Manganese in soils and plants. Kluwer, Dordrecht, pp 125–133
Loneragan JF (1997) Plant nutrition in the 20th and perspectives for the 21st century. Plant Soil 196:163–174
Abou El-Nasr MK, El-Hennawy HM, El-Kereamy AMH, Abou El-Yazied A, Salah Eldin AT (2015) Effect of magnetite nanoparticles (Fe3O4) as nutritive supplement on pear saplings. Middle East J Appl Sci 5:777–785
Nasibova AN, Fridunbayov IY, Khalilov RI (2017) Interaction of magnetite nanoparticles with plants. Eur J Biotechnol Biosci 5:14–16
Wang H, Kou X, Pei Z, Xiao JQ, Shan X, Xing B (2011) Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 5:30–42
Yuan J, Chen Y, Li H, Lu J, Zhao H, Liu M, Nechitaylo GS, Glushchenko NN (2018) New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci Rep 8:3228
Dhoke SK, Mahajan P, Kamble R, Khanna A (2013) Effect of nanoparticles suspension on the growth of mung (Vigna radiata) seedlings by foliar spray method. Nanotechnol Dev 3:e1–e5
Monsef Afshar R, Hadi H, Pirzad A (2012) Effect of Nano-iron foliar application on qualitative and quantitative characteristics of cowpea, under end season drought stress. Int Res J Appl Basic Sci 3:1709–1717
Cannata MG, Bertoli AC, Carvalho R, Bastos ARR, Freitas MP, Augusto AS (2014) Effects of cadmium on the content, accumulation, and translocation of nutrients in bean plant cultivated in nutritive solution. Commun Soil Sci Plan 45:223–235
Tombuloglu H, Tombuloglu G, Slimani Y, Ercan I, Sozeri H, Baykal A (2018) Impact of manganese ferrite (MnFe2O4) nanoparticles on growth and magnetic character of barley (Hordeum vulgare L.). Environ Pollut 243:872–881
Funding
The authors acknowledge the financial support provided by Brazil’s agencies Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Grant Nos. 2014/12392-3, 2014/19245-6 and 2013/07296-2) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Grant Nos. 168255/2014-6, 444712/2014-3, 501446/2014-1 and 308706/2007-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This research paper does not contain any studies with human participants or animals performed by any of the authors.
Ethical standard
This study is in full compliance with all applicable ethical standards.
Informed consent
All authors read and approved the final manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Souza, A., Govea-Alcaide, E., Masunaga, S.H. et al. Impact of Fe3O4 nanoparticle on nutrient accumulation in common bean plants grown in soil. SN Appl. Sci. 1, 308 (2019). https://doi.org/10.1007/s42452-019-0321-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0321-y