Abstract
Detection of the lactate content is of great importance in clinical diagnostics, fermentation industry, and control of the quality of food products. The work was aimed at the development of a sensitive element of the amperometric biosensor, based on enzyme lactate oxidase and carbon electrodes modified with platinum and palladium nanoparticles (Pt&Pd), for the lactate analysis in the presence of interfering substances. The voltamperometric characteristics of the modified sensor were studied, the enzyme stabilization was carried out. An influence of the medium parameters on the biosensor operation was comprehensively investigated. The working characteristics of the biosensor were thoroughly analyzed, its stability and selectivity were investigated. An increase in the bioselective membrane activity as a result of using Pt&Pd nanoparticles was shown. The developed biosensor for the measurement of lactate concentration is characterized by the linear range of 0.05–0.8 mM, the lower detection limit 0.1 µM and sensitivity of 3.03 nA mM−1 cm−2. The main interferents were shown to have no effect on the work of created lactate biosensor. The lactate content in several types of wine and must was determined with created biosensor (lactate concentration in wine ranges from 0.5 to 5 g/l), and the results were compared with those obtained by the traditional spectrophotometric method; good correlation was shown (the correlation coefficient R2 = 0.98). The developed sensor can be used in winemaking for selective detection of lactate in raw material during fermentation and control of the final quality of wine.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Grape wines are multicomponent systems, which include organic acids, carbohydrates, alcohols, etc. The concentration of ingredients in wine considerably differs depending on the grapes variety, climatic, geological, agrotechnical and other conditions. The data of qualitative and quantitative analysis of the components can be a ground to judge about naturalness of beverages and correctness of their production [1, 2].
The control of organic acids is relevant at all stages of winemaking, because the acidity is one of the main marks of the wine taste. The presence or absence of organic acids in the sample, their amount and ratio can define the quality of beverages and avoid their falsification. Thus, the control of fermentation processes allows a correlation with the taste of the final product [3, 4].
Information on the concentration of lactate is significant in wine industry because this parameter determines not only the taste and specific aroma of wine but also the bacterial activity during fermentation [5]. Reliable information on the lactate content in the must at different stages of wine production allows the control and regulation of the fermentation process [6]. Additionally, stability of wines at storage also depends on the lactate concentration [7]. Therefore, permanent, real-time, and casual monitoring of lactate concentration is actually necessary.
So-called malolactic fermentation during the wine maturation is of great importance for the organoleptic qualities of wine. It results in the transformation of malate (malic acid) into lactate, which has a milder taste and makes the wine more harmonious. This transformation is especially vital for creating high-quality red wines; however, it is of importance in production of dry and semi-dry white wines too [8].
In electrochemical biosensors, where the enzymes are integrated with electrodes, the direct electron transfer is basically difficult since the enzyme active sites are deeply buried in the protein matrix [9]. From this point, the nanomaterials have attracted much attention due to their electronic properties. Nanomaterials are suitable for acting as “electronic wires’’ to shorten the electron transfer distance, enhance the electron transfer between the redox centers of enzyme and electrode surface along with retaining the biological activity of the redox enzymes. Different nanoscaled materials including metal and carbon nanoparticles (e.g. nanotubes, graphene, nanowires) have been used for construction of sensors and biosensors [10]. ZnO nanorod arrays [11], MnO2-modified vertically aligned multiwalled carbon nanotubes [12], nitrogen-doped carbon nanotubes [13] showed great advantages over conventional materials for H2O2 detection. On the other hand, a new trend in biosensor design is the combination of two or more nanomaterials [14].
Platinum and palladium are well-known catalyst materials for electrochemical reduction of H2O2 [15,16,17,18]. Electrocatalitic activity of metals of platinum group is well studied and documented. Their binary alloys with other transition metals have been found to improve considerably the catalyst selectivity and stability. However, the catalytic activity of co-deposits of platinum and palladium was mainly investigated only with respect to fuel oxidation at the anode of fuel cell.
In the work, we developed the lactate biosensor, in which platinum and palladium nanoparticles are utilized along with an additional Nafion protective membrane. This biosensor could be used to control lactate concentration in winemaking at the stage of fermentation and in the final product, in a bottle.
2 Experimental
2.1 Reagents and solutions
Lactate oxidase (LOx) from Pediococcus species with activity of 20 U/mg solid was purchased from Sigma-Aldrich Co. (Steinheim, Germany); dextran, lactitol monohydrate from Sigma-Aldrich Co. (Steinheim, Germany); 98% sodium salt of L-lactic acid, bovine serum albumin (BSA) and 25% aqueous solution of glutaraldehyde (GA)—from Sigma-Aldrich Co. (St. Louis, USA); solution of Pd nanoparticles in 5% HCl, solution of Pt nanoparticles in 5% HCl—from Sigma-Aldrich Co. (Steinheim, Germany); 5% “Nafion”—from “Fluka” (Germany); Na2HPO4·7H2O and КH2PO4·H2O were from Helicon (Moscow, Russia); 3% solution of hydrogen peroxide was from Fargomed Ltd. (Teteriv, Ukraine). All chemicals were of analytical grade.
2.2 Instruments and scheme of measuring setup
All electrochemical experiments were performed using a conventional three-electrode sensor BVT AC1.W4.R1 produced by thick film technology in « BVT Technologies» (Brno, Czech Republic), which contains 1 mm carbon working electrode, platinum auxiliary electrode and Ag/AgCl reference electrode.
Amperometric measurements were carried out in a 3 ml electrochemical cell at a constant potential using a potentiostat/galvanostat PalmSens by the PalmSens PC programme (Palm Instruments BV, the Netherlands).
2.3 Determination of electrochemical characteristics of transducers
Amperometric transducers were studied with regard to their reproducibility and reliability by cyclic voltamperometry in the range of potential from 0 to + 1.0 V (speed of potential involute was 0.05 V/s). The experiments were carried out in 0.1 M phosphate buffer, pH 7.2.
2.4 Procedure of surface functionalization of the amperometric transducer by platinum and palladium nanoparticles
Surface functionalization of the working electrode was performed by electrochemical deposition of the Pt&Pd mixture in the ratio of 7:3. The electrochemical deposition by application of the potential at 0.05 V was applied for 60 s. After applying the Pt&Pd nanoparticles mixture electrodes were thoroughly washed with distilled water.
2.5 Immobilization of lactate oxidase
To prepare bioselective membranes, 10% BSA solution was mixed with 10% enzyme solution, 2% lactitol solution, and 0.2% dextran solution in 0.01 M phosphate buffer, pH 7.2. To prevent early drying of the deposited membrane, glycerol was added to the final concentration of 10%. The prepared solution was deposited on the transducer surface. For membrane polymerization, the biosensor was placed in a crystallizer in saturated GA vapour at room temperature and then air dried for 10 min.
After measurements the biosensor was carefully rinsed with distilled water, air dried and stored in refrigerator at 4 °C.
2.6 Determination of lactate in model solution
The measurements were performed in 0.1 M phosphate buffer, pH 7.2, at room temperature in an open vessel with intensive stirring. Before measurements, the transducers were kept in the buffer solution until a stable signal (baseline) was obtained. The substrate concentration was varied by the addition of aliquots of lactate stock solutions.
2.7 Determination of lactate in wine
Measurements were carried out in 0.1 M buffer solution, pH 7.0, at room temperature. To obtain the calibration curve, the aliquots of the lactate stock solution were added to the electrochemical cell. Afterwards, an aliquot (100 μl) of the wine to be tested was inserted into the cell 3 ml in volume (a 30-fold dilution). Once the responses were obtained (the response time about 1 min), the calibration curve was plotted, and lactate concentrations were determined. After each response, the sensor was washed with the buffer solution until the base signal is stabilized. The results were compared with the data of spectrophotometric method [15, 16].
The experiments were repeated at least three times. The Microsoft Origin 10 package was used for statistical analysis of the results; the average value and standard deviation were calculated; the results were considered as reliable at p < 0.05.
3 Results and discussion
3.1 Investigation of electrochemical characteristics of amperometric transducers
To obtain reproducible results, preliminary study of the electrochemical characteristics of transducers is necessary; it can be carried out by the method of cyclic voltamperometry. Therefore, the next task was to investigate the electrochemical properties and sensitivity to hydrogen peroxide of amperometric transducers, both unmodified and modified with Pt&Pd nanoparticles.
In Fig. 1 the voltamperometric characteristics of carbon electrodes before and after deposition of Pd&Pt nanoparticles are presented.
The operation of lactate-sensitive biosensor is based on the reaction:
The lactate hydrolysis is accompanied by accumulation of the electrically active substance, hydrogen peroxide, the decomposition of which results in the generation of electrons registered by the amperometric transducer.
Firstly, we measured the biosensor responses before using the Pt&Pd nanoparticles as the modificator of the electrode surface and then compared the obtained results with the responses after modification (Fig. 2). It was shown that the transducer’s sensitivity to hydrogen peroxide has significantly increased.
3.2 Determination of lactate in model solutions and comparison of sensitivity of biosensors with unmodified and modified electrodes
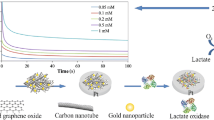
Measurements were performed in 0.1 M phosphate buffer solution, pH 7.2 at room temperature in a cell with intense stirring. The substrate concentrations were varied by the addition of certain aliquots of lactate stock solutions. The graphs (Fig. 3) clearly show that the biosensor with electrodes modified by Pt&Pd nanoparticles had a higher sensitivity to lactate compared to the biosensor with unmodified electrodes. This phenomenon can be explained by the changes in the catalytic activity and surface morphology of electrodes after their coating with the Pt&Pd nanoparticles [12].
As a result of the next experiment, we received the confirmation of our hypothesis that responses to lactate will be enhanced noticeably due to an increase in the transducer’s sensitivity to H2O2 (Fig. 2). The Fig. 3 shows that electrochemical modification of the electrode surface led to the improvement of analytical characteristics of the created biosensor.
3.3 Investigation of dependence of biosensor response on concentrations of background electrolyte and buffer solution
The effect of buffer capacity and ionic strength on the basic working characteristics of biosensors is an issue of investigation, considering that physiological and culture fluids have high ionic strength and buffer capacity. In Fig. 4 the dependence of biosensor response on the ionic strength and buffer concentration is shown.
Noteworthy, salt Na2SO4 was used in the experiments because it contains the inorganic cations and anions common for wines.
As seen, the response of the developed biosensor with modified electrodes does not depend on the buffer capacity and concentration of background electrolyte [19,20,21]. This enables further using of developed biosensor for lactate determination in biological fluids and in the food industry.
3.4 pH effect on operation of lactate biosensor
It is known that the rate of enzymatic reactions in a homogeneous solution strongly depends on the value of its pH. This is due to the fact that the functional protein groups involved in the catalysis can be either protonated or deprotonated depending on the solution pH but commonly only one of these forms is reactive. Noteworthy, according to the manufacturer the optimum pH for native LOx is 6.5. As a result of the work, it was shown that the optimal pH value for the LOx-based amperometric biosensor is 7.0 (Fig. 5).
3.5 Biosensor stability
To increase the biosensor stability, the enzyme stabilizers, lactitol and dextran, were added to the enzyme membrane. It resulted in a higher stability, though the biosensor activity dropped by 30% (Fig. 6). Nevertheless, the residual activity was sufficient for the real samples analysis.
The biosensor stability during storage was studied; it was shown that after 28 days of operation the biosensor still retained 30% of its activity towards LOx, which enabled reliable measurements (Fig. 7).
3.6 Selectivity of the developed biosensor
The biosensor with high working potential, used in the work, creates the prerequisites for the oxidation of a number of electrically active compounds other than tested, which are present in biological samples.
Application of additional membranes for improvement of selectivity could make solution of problems of the diffusion control, mechanical protection and reduce the influence of interfering substances. There are various commercial polymers (PVC, polyethylene, polymethacrylate, polyurethane and Nafion).
Based on the literature data and previous researches [22], the effect of deposition of Nafion membrane over the enzyme membrane on the biosensor characteristics was studied (Fig. 8). As seen, there was no influence on the linear part of the calibration curve; however, at the lactate concentrations higher than 2 mM the substrate saturation was observed.
Figure 8 displays the calibration curve for lactate in the concentration range of 0.05 mM–0.8 mM with a linearity of R2 = 0.99 and a sensitivity of 3.03 nA mM−1 cm−2. The biosensor demonstrated the fast response (5 s) with detection limit of 0.1 µM (s/n = 3).
As seen (Fig. 8), the responses of the biosensors with Nafion-modified membranes were lower compared to those of the biosensors without using Nafion whereas the biosensor sensitivity on the linear part of calibration curve remained the same, which enables further using of this design for analysis of real samples.
An influence of interfering substances on the biosensor signal is a challenge when analyzing real samples like biological liquids and food products. Therefore, the effect of ascorbic acid, cysteine, urea, glutamic acid on the biosensor work was investigated (Fig. 9).
Dependence of responses of biosensor modified with Pt&Pd nanoparticles with and without Nafion membrane on the concentration of interfering substances in the working solution. Measurements were performed in 0.1 M phosphate buffer, pH 7.2. Working potential of 0.6 V versus Ag/AgCl reference electrode
As shown in Fig. 9 the biosensors with Nafion layer almost do not react with the interfering substances. Therefore, the transducers of such design are suitable for the lactate determination in wine.
3.7 Analysis of real samples
At the next stage, the lactate concentrations in 11 wine samples were analyzed (Fig. 10) using the developed amperometric biosensors modified with Pt&Pd nanoparticles and additional Nafion membrane (axis Y). The results were compared with the data of spectrophotometric method (axis X) [23, 24].
The results of lactate analysis in the wine samples tested are shown in Fig. 10. These were in good agreement with the data of spectrophotometric method (the correlation coefficient for lactate R2 = 0.98).
4 Conclusion
The performed research resulted in the improvement of the lactate biosensor sensitivity and selectivity due to the functionalization of amperometric transducer with Pt&Pd nanoparticles and Nafion. The analytical characteristics of amperometric lactate biosensor are investigated, including the sensitivity to ionic strength, buffer capacity and pH of a buffer solution. The biosensor demonstrated the fast response (5 s), high sensitivity and selectivity, linear working range of lactate determination 0.05–0.8 mM, sensitivity of 3.03 nA mM−1 cm−2, detection limit 0.1 µM (s/n = 3). Application of bionanocomposites with promising properties opens new possibilities for the enzyme immobilization and the development of new electrochemical biosensors.
References
Saurina J (2010) Characterization of wines using compositional profiles and chemometrics. Trends Anal Chem 29:234–245. https://doi.org/10.1016/j.trac.2009.11.008
Zeravik J, Hlavacek A, Lacina K, Skladal P (2009) State of the art in the field of electronic and bioelectronic tongues: towards the analysis of wines. Electroanalysis 21:2509–2520. https://doi.org/10.1002/elan.200900285
Valuiko G, Kostiura V (2000) Handbook on winemaking, 2nd edn. Simferopol, Taurida
Rodopulo A (1971) Biochemistry of winemaking. Food Industry, Moscow
Avramescu A, Noguer T, Magearu V, Marty J-L (2001) Chronoamperometric determination of d-lactate using screen-printed enzyme electrodes. Anal Chim Acta 433:81–88. https://doi.org/10.1016/S0003-2670(00)01386-6
Parra A, Casero E, Vázquez L, Pariente F, Lorenzo E (2006) Design and characterization of a lactate biosensor based on immobilized lactate oxidase onto gold surfaces. Anal Chim Acta 555:308–315. https://doi.org/10.1016/j.aca.2005.09.025
Esti M, Volpe G, Micheli L, Delibato E, Compagnone D, Moscone D, Palleschi G (2004) Electrochemical biosensors for monitoring malolactic fermentation in red wine using two strains of Oenococcus oeni. Anal Chim Acta 513:357–364. https://doi.org/10.1016/j.aca.2003.12.011
Katrlik J, Pizzariello A, Mastihuba V, Svorc J, Stred’ansky M, Miertus S (1999) Biosensors for l-malate and l-lactate based on solid binding matrix. Anal Chim Acta 379:193–200. https://doi.org/10.1016/S0003-2670(98)00610-2
Lu Q, Dong X, Li LJ, Hu X (2015) Direct electrochemistry-based hydrogen peroxide biosensor formed from single-layer graphene nanoplatelet-enzyme composite film. Talanta 82:1344–1348. https://doi.org/10.1016/j.talanta.2010.06.061
Zhao X, Mai Z, Kang X, Zou X (2008) Direct electrochemistry and electrocatalysis of horseradish peroxidase based on clay–chitosan-gold nanoparticle nanocomposite. Biosens Bioelectron 23:1032–1038. https://doi.org/10.1016/j.bios.2007.10.012
Wang J, Xu M, Zhao R, Chen G (2010) A highly sensitive H2O2 sensor based on zinc oxide nanorod arrays film sensing interface. Analyst 135:1992–1996. https://doi.org/10.1039/c0an00041h
Xu B, Ye M-L, Yu Y-X, Zhang W-D (2010) A highly sensitive hydrogen peroxide amperometric sensor based on MnO2-modified vertically aligned multiwalled carbon nanotubes. Anal Chim Acta 674:20–26. https://doi.org/10.1016/j.aca.2010.06.004
Xu E, Wei J, Wang K, Li Z, Gui X, Jia Y, Zhu H, Wu D (2010) Doped carbon nanotube array with a gradient of nitrogen concentration. Carbon 48:3097–3102. https://doi.org/10.1016/j.carbon.2010.04.046
Xiao Y, Li CM (2008) Nanocomposites: from fabrications to electrochemical bioapplications. Electroanalysis 20:648–662. https://doi.org/10.1002/elan.200704125
Palmisano F, Rizzi R, Centonze D, Zambonin PG (2000) Simultaneous monitoring of glucose and lactate by an interference and cross-talk free dual electrode amperometric biosensor based on electropolymerized thin films. Biosens Bioelectron 15:531–539. https://doi.org/10.1016/S0956-5663(00)00107-X
Suman S, Singhal R, Sharma AL, Malthotra BD, Pundir CS (2005) Development of a lactate biosensor based on conducting copolymer bound lactate oxidase. Sen Actuat B Chem 107:768–772. https://doi.org/10.1016/j.snb.2004.12.016
Romero MR, Garay F, Baruzzi AM (2008) Design and optimization of a lactate amperometric biosensor based on lactate oxidase cross-linked with polymeric matrixes. Sens Actuat B Chem 131:590–595. https://doi.org/10.1016/j.snb.2007.12.044
Parra-Alfambra AM, Casero E, Petit-Domínguez MD, Barbadillo M, Pariente F, Vázquez L, Lorenzo E (2011) New nanostructured electrochemical biosensors based on three-dimensional (3-mercaptopropyl)-trimethoxysilane network. Analyst 136:340–347. https://doi.org/10.1039/C0AN00475H
Goriushkina TB, Shkotova LV, Gayda GZ, Klepach HM, Gonchar MV, Soldatkin AP, Dzyadevycha SV (2010) Amperometric biosensor based on glycerol oxidase for glycerol determination. Sens Actuat B 144:361–367. https://doi.org/10.1016/j.snb.2008.11.051
Shkotova LV, Goriushkina TB, Tran-Minh C, Chovelon J-M, Soldatkin AP, Dzyadevych SV (2008) Amperometric biosensor for lactate analysis in wine and must during fermentation. Mater Sci Eng C 28:943–948. https://doi.org/10.1016/j.msec.2007.10.038
Shkotova LV, Piechniakova NY, Kukla OL, Dzyadevych SV (2016) Thin-film amperometric multibiosensor for simultaneous determination of lactate and glucose in wine. Food Chem 197:972–978. https://doi.org/10.1016/j.foodchem.2015.11.066
Shkotova LV, Soldatkin AP, Dzyadevych SV (2004) Adaptation of amperometric enzyme biosensor for glucose analysis in wine Ukrain’skyi. Biokhimichnyi Zhurnal 76(3):114–121
Gonchar M, Osmak G. (2009) A method of qualitative determination of lactate in food and biological fluids. Patent of Ukraine 45283
Gonchar M, Smutok O, Os’mak H. (2009) Flavocytochrome b2-based enzymatic composition, method and kit for l-lactate. International PCT Patent Application (No PCT/US2008/069637). International publication WO/2009/009656. publ. http://www.wipo.int/pctdb/en/wo.jsp?WO=2009009656
Acknowledgements
The authors gratefully acknowledge the financial support of this study by the National Academy of Sciences of Ukraine in the frame of Scientific and Technical Program “Intelligent” sensory devices of a new generation based on modern materials and technologies”.
Author information
Authors and Affiliations
Contributions
LS and AB performed the functionalization of amperometric transducer with Pt&Pd nanoparticles and Nafion and studied the analytical characteristics of obtained lactate biosensor. LS, IV and SD processed the obtained results, wrote, and arranged the article. OS performed the lactate analysis in wine by the spectrophotometric method. LV proposed the idea of the modified amperometric lactate biosensor with platinum and palladium nanoparticles. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shkotova, L., Bohush, A., Voloshina, I. et al. Amperometric biosensor modified with platinum and palladium nanoparticles for detection of lactate concentrations in wine. SN Appl. Sci. 1, 306 (2019). https://doi.org/10.1007/s42452-019-0315-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-019-0315-9