Abstract
The liver is the most common metastatic site of colorectal carcinoma. Adrenal metastasis is the second most common reason for “adrenal incidentaloma.” For malignant adrenal tumors, open adrenalectomy is the choice of operation. We present a 60-year-old man whose radiological evaluations revealed a metastatic lesion in the right adrenal gland, with a history of colorectal carcinoma. We conducted a literature analysis regarding the accuracy of imaging methods and laboratory tests. It is seen that radiological examinations and laboratory test results do not always comply with the postoperative results. Differential diagnosis and different diagnostic procedures should be considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The important part of this article is that we highlight the incompatibility between the results of the indicated diagnostic process and postoperative histological result. We question the suitability of the present guidelines made for adrenal incidentalomas.
The third most frequent cancer worldwide is colorectal cancer [1]. A metastasis had already occurred in about 20% of the patients at the time of diagnosis [1]. The liver is the organ where colorectal cancer metastasizes the most [2]. CRC metastases develop via the hematogenous route, mostly the portal venous system [2]. Localized disease had a 5-year survival rate of 90%, yet the survival rate of regional disease has been scaled down to 71% and that of distant disease has decreased to 14% [3]. Stage 4 colorectal carcinoma has a highly restricted survival rate, but unlike other carcinomas, the removal of isolated, resectable metastases might be beneficial to certain patients [2].
When an adrenal mass is found coincidentally in a radiological evaluation without any clinical symptoms of adrenal disease, it is called “adrenal incidentaloma” [4]. Radiologically, the frequency of adrenal incidentalomas ranges from 0.2 to 7%, and the discovery ratio increases with age [4]. The two most common causes of “adrenal incidentaloma” are cortical-adrenal adenomas and adrenal metastasis [5].
The presence of solitary adrenal metastasis is rare [6]. However, autopsy findings have shown that an adrenal metastasis from colorectal carcinoma is not uncommon, and its occurrence ranges from 1.9 to 17.4% in the autopsies [7]. Preoperative diagnosis of adrenal metastasis is not easy due to the asymptomatic nature of these tumors; furthermore, their morphological features are similar to those of adrenal adenomas [8]. This is the reason why adrenal metastases are not usually detected while the patient is still alive [8]. Resection is a possible method for treating adrenal metastasis [7]. In recent decades, open adrenalectomy has lost its place to laparoscopic adrenalectomy for benign adrenal tumors, yet this approach contradicts massive, possibly malignant, adrenal tumors [9].
Case Presentation
We present a 60-year-old man who applied to the emergency for gas and abdominal pain in November 2022. There were no signs of defense or rebound in the physical examination. A plain abdominal radiography was performed. The radiography revealed dilatation and air-fluid levels. A contrast-enhanced computed tomography (CT) was performed. CT revealed irregular mural tumoral thickness in the sigmoid colon. A colonoscopy was also done before the operation. It revealed colonic polyps in the descending colon. The first serum carcinoembryonic antigen (CEA) concentration was measured in December. The concentration was 190 µg/L (the reference range is 0–4.7 µg/L.)
The patient underwent an open anterior resection in February 2023. After pathological examination, the patient was diagnosed with colon adenocarcinoma involving 10% mucinous component. The microscopic examination revealed moderately differentiated adenocarcinoma infiltrating the serosa of the jejunum with lymph node metastases. The stage of the tumor was pT4b N1a Mx.
Two months after the operation, the serum CEA concentration was checked again; the result was 54.5 µg/L. From that day on, the concentration kept increasing. In October, the concentration was 578 µg/L.
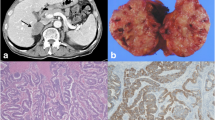
An abdominal ultrasonography (USG) was performed 5 months after the operation. The USG revealed a hypoechogenic nodular lesion 3 cm in diameter in the right adrenal gland. After this, a positron emission tomography (PET)/computed tomography was performed for re-staging. The PET/CT scan was performed 1 month after the USG. An increased fludeoxyglucose F18 (FDG) uptake was observed in the periphery of a 29 × 30 mm2 extension in the right adrenal gland (Fig. 1). A contrast-enhanced abdominal CT was performed 3 months after PET/CT. The evaluation of CT revealed a metastatic lesion with dimensional growth in the right adrenal gland (51 × 44 mm2) (Fig. 2). There were no signs of recurrence in the abdominal cavity.
A neoadjuvant radiotherapy (RT) was applied to the right adrenal gland in November 2023. One month after the RT, a contrast-enhanced CT scan was performed again. The CT scan revealed a decrease in size with a new 44 × 35 mm2 lesion in the right adrenal gland (Fig. 3).
A magnetic resonance imaging (MRI) was performed on February 12, 2024. The report mentioned a metastatic cystic nodular lesion measuring 44 × 30 mm2 in extension. An open adrenalectomy was performed (Fig. 4). Open adrenalectomy was chosen to explore the abdominal cavity for any recurrence that might have gone unnoticed during radiological evaluations. There were not any signs of recurrence in the abdominal cavity. The microscopic examination revealed mixed inflammatory cells, histiocytes, hemorrhage, and necrosis with a mucinous component (Fig. 5). There were no signs of neoplasms in the adrenal gland. On the 7th postoperative day, the patient was discharged without any complications.
Discussion
During the staging process of colorectal cancer patients, the use of CT reveals adrenal incidentalomas routinely [4]. The origin of adrenal metastasis is mostly breast cancer [10]. Most of the adrenal metastases are bilateral; however, adrenal insufficiency does not occur unless 90% of the tissue is destroyed [10].
Katayama et al. described a potential route for hematogenous spread from the original location through which the lungs reach the adrenal glands [11]. It is indeed uncommon for colorectal cancer to spread to the adrenal glands, skipping the lungs and liver.
One of the first things to do for staging CRC and revealing possible metastatic lesions is an abdominal CT [10]. It is also essential to measure serum CEA concentration and perform USG and MRI to see the situation in the adrenal glands [10].
Murakami et al. reported that resection of a solitary adrenal metastasis is beneficial for the patient [12]. The 5-year survival rate of 37 patients who had undergone adrenalectomy for metastatic disease was analyzed, and Kim et al. reported that the survival rate was 24% (median 21 months) [6]. For our patient, an adrenalectomy operation was planned after PET/CT, CT, and MRI indicated right adrenal gland metastasis.
According to Liu et al., it is necessary to test the serum CEA blood levels for prenotification of adrenal metastasis during the postoperative life of the patient [12]. However, there are situations where the serum CEA does not indicate the existence of widespread disease, particularly when the blood count is normal at the beginning [8]. Our patient’s serum CEA concentration was examined regularly after the operation. The serum CEA concentration started to increase approximately 4–5 months after anterior resection, which was consistent with the imaging findings.
Tsujimoto et al. reported that malignant and benign adrenal metastases can be separated from each other via PET/CT with a sensitivity of 93–100%, a specificity of 80–100℅, and an accuracy of 92–100% [6]. Our patient’s PET/CT showed FDG uptake in the right adrenal gland, which was compatible with the suspicion of a right adrenal gland metastatic lesion. However, microscopic examination revealed a mix of inflammatory cells and histiocytes with hemorrhage and necrosis. Castaldi et al. mentioned that the use of PET/CT is inconsistent, and the literature does not contain a comprehensive analysis of PET/CT [14].
According to Castaldi et al., a combination of delay-enhanced and unenhanced CT can be used to properly classify adrenal masses with a sensitivity of 96–98%, an accuracy of 81–96%, and a specificity of 61–92% [14]. However, Liu et al. reported that CT cannot successfully distinguish between primer lesions, secondary lesions, and incidentalomas [12]. In our patient, two enhanced CT scans were performed (one after RT), and two of them revealed metastatic lesions with different dimensions.
Franzese et al. reported that adrenal gland metastasis responds well to the use of stereotactic radiotherapy as an ablative treatment [13]. Our patient received a right adrenal gland lesion radiotherapy after the PET/CT and CT examinations, which lasted for 11 days. The lesion showed a decrease in size on CT, which was performed 1 month after RT.
According to the guidelines made for adrenal incidentalomas, a biopsy should not be performed if the imaging findings are compatible with the diagnosis. Leyendecker et al. reported that if an adrenal mass is still suspicious after imaging evaluations in a patient with a history of cancer, an adrenal biopsy is indicated if the presence of metastasis changes the treatment [15]. Adrenal biopsy has an accuracy rate of approximately 90% and a complication rate of 2.8–4.3% [16]. However, the type of biopsied mass, knowledge of radiologists, and biopsy technique can alter the diagnostic value of fine-needle aspiration (FNA) biopsy, which can be nondiagnostic in 0–28% of cases according to the abovementioned criteria [16].
It is also possible that the lack of metastatic tissue in the microscopic examination was due to the effects of radiotherapy on the cancer cells. Crook et al. mentioned that histologic resolution may take 2–3 years because of long tumor doubling times and the postmitotic cell death effect of radiotherapy [17]. This article is about prostate cancer, but we cannot disregard this theory when, in our case, all of the preoperative examinations revealed adrenal gland metastasis.
These studies and results raise the questions, “Should biopsy be indicated for situations that are uncommon, such as solitary adrenal metastases from colorectal carcinoma?” and “How should we manage the situation if the biopsy result is negative (especially after RT)?”.
Conclusion
Diagnosis made with radiological evaluations and laboratory tests might not be 100% compatible with postoperative examination results. Differential diagnosis and different diagnostic procedures should be considered, especially in uncommon situations such as solitary adrenal metastasis from colorectal carcinoma.
Data Availability
The dataset supporting the conclusion is available.
Code Availability
Not applicable.
References
Riihimäki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;15(6):29765. https://doi.org/10.1038/srep29765.
Brunicardi FC. Schwartz’s principles of surgery. New York, NY: McGraw-Hill Education; 2015.
Han S, Wang D, Huang Y, Zeng Z, Xu P, Xiong H, Ke Z, Zhang Y, Hu Y, Wang F, Wang J, Zhao Y, Zhuo W, Zhao G. A reciprocal feedback between colon cancer cells and Schwann cells promotes the proliferation and metastasis of colon cancer. J Exp Clin Cancer Res. 2022;41(1):348. https://doi.org/10.1186/s13046-022-02556-2.
van den Broek J, Geenen R, Heijnen L, Kobus C, Schreurs H. Adrenal incidentalomas during diagnostic work-up of colorectal cancer patients: what is the risk of metastases? Ann Surg Oncol. 2018;25(7):1986–91. https://doi.org/10.1245/s10434-018-6501-y.
Capaldi M, Ricci G, Bertolini R, Alessandroni L, Di Castro A, Saraco E, Guiducci A, Tersigni R. Colon cancer adrenal metastasis: case report and review of the literature. G Chir. 2011;32(8–9):361–3.
Tsujimoto A, Ueda T, Kuge H, Inoue T, Obara S, Nakamoto T, Sasaki Y, Nakamura Y, Koyama F, Sho M. Long-term survival after adrenal metastasectomy from colorectal cancer: a report of two cases. Surg Case Rep. 2019;5(1):61. https://doi.org/10.1186/s40792-019-0611-z.
Alvandipour M, Karami MY, Khalvati M, Khodabakhsh H. Incidentally solitary, synchronous, metastatic left adrenal mass from colon cancer. Ann Coloproctol. 2016;32(2):79–82. https://doi.org/10.3393/ac.2016.32.2.79.
Raices M, Boccalatte L, Rossi G, Wright F. Synchronous contralateral adrenal metastasis of colorectal cancer: case report. J Surg Case Rep. 2017;2017(6):rjx098. https://doi.org/10.1093/jscr/rjx098.
Kosmidis C, Efthimiadis C, Anthimidis G, Levva S, Ioannidou G, Zaramboukas T, Emmanouilides C, Baka S, Kosmidou M, Basdanis G, Fachantidis E. Adrenalectomy for solitary adrenal metastasis from colorectal cancer: a case report. Cases J. 2008;1(1):49. https://doi.org/10.1186/1757-1626-1-49.
Alberti JF, Nardi WS, Recalde M, Quildrian SD. Bilateral adrenalectomy in the context of primary adrenal insufficiency due to colorectal cancer metastasis. Ecancermedicalscience. 2022;16:1395. https://doi.org/10.3332/ecancer.2022.1395.
Liu YY, Chen ZH, Zhai ET, Yang J, Xu JB, Cai SR, Song W. Case of metachronous bilateral isolated adrenal metastasis from colorectal adenocarcinoma and review of the literature. World J Gastroenterol. 2016;22(14):3879–84. https://doi.org/10.3748/wjg.v22.i14.3879.
Murakami S, Terakado M, Hashimoto T, Tsuji Y, Okubo K, Hirayama R. Adrenal metastasis from rectal cancer: report of a case. Surg Today. 2003;33(2):126–30. https://doi.org/10.1007/s005950300028.
Franzese C, Stefanini S, Scorsetti M. Radiation therapy in the management of adrenal metastases. Semin Radiat Oncol. 2023;33(2):193–202. https://doi.org/10.1016/j.semradonc.2022.11.001.
Castaldi P, Biondi A, Rausei S, Persiani R, Mirk P, Rufini V. An unusual case of adrenal metastasis from colorectal cancer: computed tomography and fluorine 18-fluoro-deoxy-glucose positron emission tomography-computed tomography features and literature review. Case Rep Oncol. 2010;3(3):416–22. https://doi.org/10.1159/000322508.
Leyendecker JR, Dalrymple NC. Chapter 7 - computed tomography incidentalomas, In: Dalrymple NC, Leyendecker JR, Oliphant M, editors. Problem solving in abdominal imaging, Mosby. 2009;140–146. https://doi.org/10.1016/B978-0-323-04353-3.50013-6. (https://www.sciencedirect.com/science/article/pii/B9780323043533500136) (ISBN 9780323043533).
Ierardi AM, Petrillo M, Patella F, Biondetti P, Fumarola EM, Angileri SA, Pesapane F, Pinto A, Dionigi G, Carrafiello G. Interventional radiology of the adrenal glands: current status. Gland Surg. 2018;7(2):147–65. https://doi.org/10.21037/gs.2018.01.04.
Crook J, Malone S, Perry G, Bahadur Y, Robertson S, Abdolell M. Postradiotherapy prostate biopsies: what do they really mean? Results for 498 patients. Int J Radiat Oncol Biol Phys. 2000;48(2):355–67. https://doi.org/10.1016/s0360-3016(00)00637-4.
Author information
Authors and Affiliations
Contributions
AD wrote the manuscript and searched the literature. IEC reviewed and edited the manuscript. DK and DTT collected data. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to Participate
Written informed consent was obtained from the patient for participation in this case report.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Surgery
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Daş, A., Cakcak, İ.E., Karabulut, D. et al. An Adrenal Incidentaloma After Colon/Rectal Cancer Surgery: A Primer Lesion or Metastasis—A Case Report. SN Compr. Clin. Med. 6, 79 (2024). https://doi.org/10.1007/s42399-024-01712-3
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-024-01712-3