Abstract
A rare case of primary osseous solitary fibrous tumor (SFT) located in the calvaria is described. SFTs, previously called hemangiopericytomas (HPC), are highly vascularized extra-axial tumors that may originate from the soft tissue, intracranial meninges, or the skeletal system. Primary osseous SFT located in the skull is an exceedingly rare occurrence. We report on a case of a 64-year-old man that presented with an important osteolytic and vascular bone tumor of the left frontal bone that proved to be a grade III SFT. We also review all the cases of primary skull SFT reported in the literature. Initial embolization and then complete surgical removal of the tumor were performed. Given the histopathological result and the high mitotic index, radiation therapy was performed. Primary osseous SFT is an extremely rare form of bone tumor. This diagnostic should be taken into consideration in cases of hypervascular and highly destructive lesions of the bone. Complete surgical resection when possible is the treatment of choice. The place of radiotherapy as a primary or adjuvant treatment is yet to be determined. Long-term follow-up is highly required due to the risk of distant metastasis and local recurrence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Solitary fibrous tumors, previously called hemangiopericytomas, are highly vascularised extra-axial tumors of mesenchymal origin. The latest World Health Organization (WHO) classifications considered the term hemangiopericytoma obsolete, and instead, the term “solitary fibrous tumor” is now preferred. Solitary fibrous tumors were the most common nonmeningothelial mesenchymal neoplasm and shared the common molecular feature of NAB2-STAT6 gene fusions. They were assigned three grades, grade III corresponding to the anaplastic hemangiopericytoma on the ancient classification [1].
Initially described by A. Stout and M. Murray in 1942, they have been shown to arise from Zimmermann pericytes, contractile spindle cells surrounding capillaries and postcapillary venules in any part of the human body [2]. Solitary fibrous tumors often arise in the musculoskeletal system particularly in the inferior extremities, retroperitoneum, pelvic region, and, in almost 25% of the case, in the head and neck [3]. They account for less than 1% of intracranial tumors. They are associated with high rates of recurrence and have metastatic potential, usually in the bones, lungs, liver, and less frequently in the vertebras [4].
Osseous involvement by SFT occurs far more often as secondary to a previous soft tissue SFT. Primary osseous SFT is an exceedingly rare occurrence. They arise from the capillary pericytes of the skeletal system [5, 6]. Primary osseous SFT of the skull are particularly scarce, most of them being described in the skull base and a couple of isolated cases in the calvaria. To date, only ten previous cases were reported as primary osseous skull SFT (Table 1).
However, the differential diagnosis of bone invasion by a meningeal SFT should be taken into consideration. As histologically osseous SFT is identical to soft tissue SFT, the bone origin is proven primarily by the radiological, surgical, and by some anatomopatholodiagnogical findings.
The present clinical case of a man with a frontal bone osteolytic tumor that turned out to be an SFT prompted us to report it and to review the international literature on primary osseous skull SFT.
Case Report
Medical History
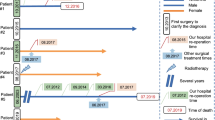
A 64-year-old man without any oncological and medical history suffered from a painful frontal tumefaction, which increased in volume over the last year before admission. The clinical examination showed a slightly sensible fixed subcutaneous mass of hard consistency surrounded by engorged scalp veins. The skin overlaying the lesion was intact and showed no signs of redness or discoloration. The initial neurologic examination did not show any deficit.
The patient underwent a complete panel of radiological investigations including cerebral and full-body CT scans, brain MRI, and four-vessel digital subtraction angiography (DSA). The head CT examination in bone window settings showed a well-defined, expansile lesion, located in the left frontal bone. The mass, measuring 45 × 37 × 39 mm, was extensively lytic and responsible for bi-cortical destruction (Fig. 1a). The brain MRI (Fig. 1b–d) depicted a poly-lobulated extra-axial mass centered on the left frontal bone with homogeneous and intense contrast enhancement. Although the bulk was located within the bone, a small component of the tumor seemed to have breached through the dura mater and came in contact with the brain parenchyma (Fig. 1c). Dynamic susceptibility-weighted contrast (DSC) perfusion shows a high rCBV (Fig. 1d). Four-vessel digital subtraction angiography (DSA) confirmed the highly vascular nature of the lesion that was fed by hypertrophic branches of the left superficial temporal artery (STA) and middle meningeal artery (MMA). The feeding arteries were divided into numerous radially arranged branches inside the tumor (Fig. 2). The full-body CT scan did not detect any other malignancies.
CT scan coronal view (a) showing an expansive mass located in the left frontal bone highly destructive with bi-cortical disruption (yellow arrows). The margins were ill-defined, and there were no intra-tumoral calcifications. Brain MRI with gadolinium enhancement, coronal view (b) sections showing an extra-axial mass with significant and homogenous contrast enhancement. On the MRI T2-weighted image, coronal view (c) the mass appeared to be completely extradural except for a small part that seemed to have breached the dura (red arrows). Dynamic susceptibility-weighted contrast (DSC) perfusion shows a high rCBV (d, yellow arrowheads)
The initial clinic and radiologic diagnostic hypotheses were a sarcomatous lesion, a plasmacytoma, an osseous metastatic lesion, an aggressive bone haemangioma, and, although very unlikely, an atypical meningioma.
Due to the highly vascular profile of the tumor, an onyx embolization of the two feeding vessels (STA and MMA) was initially performed that achieved a reduction of the tumor blush (Fig. 2c). The embolization procedure was followed by the surgical intervention 24 h later.
Surgical Procedure
The surgery was carried out with the patient under general anesthesia and endotracheal intubation. After careful dissection of the subcutaneous tissue, we came upon a bulky lobulated and encapsulated purple mass destroying the calvaria. The periosteum covering the mass was infiltrated.
With the help of the navigation system, a large frontal craniectomy was performed within the healthy bone surrounding the osseous lesion using a fluted and diamond high-speed drill. The cancellous bone in the vicinity of the lesion was infiltrated, hemorrhagic, and friable. The cleavage plan between the dura and the tumor was easily obtained. After progressive debulking of the lesion and a careful dissection of the dura mater, we came upon a small dural defect. Except for a small portion in the periphery of the trans-dural contingent, the dura mater did not seem macroscopically infiltrated. The small intradural part of the tumor came in contact with the frontal cortex. The intradural contingent was easily dissected due to the presence of a preserved extra-arachnoid cleavage plane. The cortex did not show any macroscopic signs of infiltration. We removed the whole dura that came in direct contact with the bony lesion extending far beyond the portion breached by the tumor. The resected dura was sent for proper histopathological analysis with the resected tumor. An artificial dura substitute was used to close the dural defect (Neuro-patch® Brown). Cranioplasty with the aid of fashioned bone cement (Palacos ® Heraeus) was performed in order to cover the bone defect (Fig. 3). The preoperative embolization resulted in minimal blood loss during tumor resection, which in turn led to a shorter surgical time of 2 h.
The post-operatory period was uneventful. The post-contrast CT scan and MRI performed 2 months following the surgical removal showed no residual tumor (Fig. 3a).
Postoperative Course
The histopathological investigation showed moderate-to-high tumor cell proliferation (8%) without any necrosis. The tumor exhibited a vascular component with a branching, staghorn configuration. The immunohistochemistry analysis showed positive nuclear STAT6 expression and focal CD34 positivity and was negative for epithelial membrane antigen (EMA). The Ki67 proliferation index was high, reaching 8%. The macroscopical infiltration of the dura mater near the intradural part was confirmed by the histopathological result. The rest of the dura mater did not reveal any signs of tumor infiltration. The lesion was classified as a grade III SFT/HPC according to the last WHO 2016 classification [7].
While discussing the case in a multidisciplinary meeting, given the histopathological features (high Ki67 level), postoperative radiation was proposed. A dose of 54 Gy in 27 fractions of 2Gy was delivered to the resection cavity using a Linac-based 3D conformal technique with 3 coplanar beams (right anterior oblique: 325°, left anterior oblique: 40°, and left posterior oblique: 140°). A clinical target volume (CTV) was created using a 5-mm margin around the gross tumor volume (GTV) represented by the resection cavity. A 5-mm isotropic expansion from the CTV was used to generate the planning target volume.
At the last follow-up, almost 5 years following the surgery, the brain MRI and the whole-body PET scan did not show any signs of local recurrence nor distant metastasis.
Discussion and Review of Literature
This article illustrates the case of a 64-year-old man with a primary bone SFT originating from the frontal bone. While bone is frequently affected by extra-neural metastases, the occurrence of a primary osseous SFT is rare. A study conducted at the Mayo Clinic, which reviewed 8452 primary bone tumors, revealed that bone SFT is an exceedingly uncommon condition, accounting for only 0.08% of all primary bone tumors and 0.1% of primary malignant bone tumors [5].
Due to the limited number of cases available, determining a risk assessment model for patients with primary bone SFT was extremely challenging. Demicco et al. proposed a risk assessment score for soft tissue SFT that took into account the patient’s age, tumor size, mitotic index, and the presence of necrosis. In their studies, larger tumors, a mitotic index of ≥4/10 high-power fields, the presence of necrosis, and age ≥55 were associated with a higher risk of metastasis. [8, 9] However, a recent study by Bianchi et al. reported on a series of 24 patients with primary bone SFT and found that, unlike SFT of soft tissues, the aggressive behavior of primary SFT of the bone appeared to be independent of the mitotic count or any other clinicopathological and molecular features, and therefore, the risk stratification model proposed by Demicco was not applicable [10]. In their series, localized lesions and surgically treated patients (16 out of 24, 66%) had a better 5-year disease-specific survival than those with metastatic disease (74% vs. 33%) [10].
It is challenging, if not impossible, to determine the optimal management of primary bone SFT based on the limited cases reported in the literature. Treatment has primarily relied on experiences with soft tissue SFT, with no established treatment algorithm or prospective trials available for these tumors. Surgery appears to play an undisputed role in the majority of studies. Pasquali et al. conducted a study on a series of extra-pleural, extra-meningeal SFTs surgically resected, which showed that patients had a 5-year disease-free survival and overall survival of 78% and 83%, respectively [11]. The role of radiotherapy (RT) as a primary or adjuvant treatment is yet to be determined. Most series report on postoperative RT. The majority of authors agree that RT should be considered as an alternative treatment option to surgery when the lesion is inaccessible or complete excision is not achievable. Adjuvant RT has been proposed when high-risk histopathological features are present in the surgical specimen and/or complete resection was not achieved [12,13,14,15,16,17].
In a retrospective observational study by Hass et al., involving 549 patients with extra-meningeal SFT, the authors compared the outcomes of surgery alone versus perioperative radiation therapy. The study found that the addition of radiation therapy to surgery may improve local control and reduce the risk of local recurrence without affecting overall survival [18]. However, it is unclear whether these results can be applied to primary bone SFT, given the rarity of this disease. Unfortunately, conducting prospective randomized phase III trials may not be feasible due to the limited number of cases.
The previous literature has reported ten cases of primary osseous SFT of the skull, as shown in Table 1 [5, 19,20,21,22,23,24]. In seven of the reported cases, the tumors were located in the petrous part of the temporal bone, while the remaining three were located in the calvaria. Seven out of the ten cases had a significant intradural component. However, it can be challenging to distinguish between a soft tissue SFT with secondary bone erosion and a primary bone SFT with secondary meningeal and intradural invasion in most cases. Only three cases had radiological and intraoperative characteristics such as a small or no intradural component and no dural invasion, which allowed them to be classified as primary bone SFTs [5, 24, 25].
Although extremely rare, the possibility of this lesion being a bone tumor should be considered. Due to their highly vascular nature and potential for significant bleeding, preoperative preparation is highly advisable, especially in cases of very large tumors. MR imaging with perfusion sequences and DSA are helpful in evaluating the vascularity of these lesions.
Preoperative embolization can be considered as a means to decrease intraoperative bleeding, which can facilitate a safer and more effective tumor resection. Hanak et al. conducted a study on preoperative embolization of intracranial SFT, which revealed a negative correlation between the extent of angiographic devascularization and intraoperative blood loss. They also observed that SFTs supplied dominantly by the external carotid artery (ECA) were more successfully embolized than those supplied by pial vascularization [26]. Therefore, calvarial SFTs, which are exclusively supplied by the ECA, are promising candidates for preoperative embolization.
Long-term follow-up is widely recognized as crucial for detecting local recurrence or distant metastasis in patients with SFT. According to Ghose et al., the average time for the development of distant metastases was approximately 91.33 (±16.44) months [27]. Some studies have proposed using whole-body CT/PET, which provides metabolic information, to regularly monitor patients with SFT. However, there are no current reports on the use of PET MR for this purpose [4, 28].
Conclusions
Primary osseous SFT is an extremely rare form of hypervascular and highly destructive bone tumor. Calvaria SFT seems to fill in perfectly all the criteria of a candidate for a complete and safe resection (well-defined tumors, easily accessible due to their superficial localization, vascularization exclusively from the external carotid artery). Although many of the authors are in favor of postoperative stereotactic radiotherapy in inoperable cases, in cases with incomplete resection or in lesions with a high mitotic index, the question of the role of adjuvant RT in the recurrence-free survival or overall survival is unlikely to be answered due to the rarity of this disease. We emphasize the fact that patients treated for primary osseous SFT should be closely monitored for prolonged periods of time in order to detect local recurrence or distant metastasis, which can occur even after a long period
Patient’s Perspective
The patient was initially anxious due to the rarity of this lesion, the potential aggressive behavior, and the absence of a well-established protocol of treatment. He was, however, reassured by the fact that surgery managed to remove the whole tumor and that radiotherapy was rapidly performed after the diagnostic. Throughout the years of close follow-up, he grew increasingly confident in the therapeutic protocol due to the reassuring results of the radiologic follow-up.
Data Availability
N/A
Code Availability
N/A
References
Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23(8):1231–51. https://doi.org/10.1093/NEUONC/NOAB106.
Stout AP, Murray MR. Hemangiopericytoma: a vascular tumor featuring Zimmermann’s pericytes. Ann Surg. 1942;116(1):26-33. http://www.ncbi.nlm.nih.gov/pubmed/17858068. Accessed January 10, 2019.
Craven JP, Quigley TM, Bolen JW, Raker EJ. Current management and clinical outcome of hemangiopericytomas. Am J Surg. 1992;163(5):490–3. https://doi.org/10.1016/0002-9610(92)90394-7.
Ratneswaren T, Hogg FRA, Gallagher MJ, Ashkan K. Surveillance for metastatic hemangiopericytoma-solitary fibrous tumors-systematic literature review on incidence, predictors and diagnosis of extra-cranial disease. J Neurooncol. 2018;138(3):447-467. http://www.ncbi.nlm.nih.gov/pubmed/29551003. Accessed December 26, 2018.
Tang JS, Gold RH, Mirra JM, Eckardt J. Hemangiopericytoma of bone. Cancer. 1988;62(4):848-859. http://www.ncbi.nlm.nih.gov/pubmed/3293766. Accessed January 2, 2019.
AP STOUT, MR MURRAY. Hemangiopericytoma a vascular tumor featuring Zimmermannʼs pericytes. Ann Surg. 1942;116(1):26–33. https://doi.org/10.1097/00000658-194207000-00004.
Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–20. https://doi.org/10.1007/s00401-016-1545-1.
Demicco EG, Park MS, Araujo DM, et al. Solitary fibrous tumor: a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25(9):1298–306. https://doi.org/10.1038/modpathol.2012.83.
Demicco EG, Wagner MJ, Maki RG, et al. Risk assessment in solitary fibrous tumors: validation and refinement of a risk stratification model. Mod Pathol. 2017;30(10):1433–42. https://doi.org/10.1038/modpathol.2017.54.
Bianchi G, Lana D, Gambarotti M, et al. Clinical, histological, and molecular features of solitary fibrous tumor of bone: a single institution retrospective review. Cancers (Basel). 2021;13(10) https://doi.org/10.3390/CANCERS13102470.
Pasquali S, Gronchi A, Strauss D, et al. Resectable extra-pleural and extra-meningeal solitary fibrous tumours: a multi-centre prognostic study. Eur J Surg Oncol. 2016;42(7):1064–70. https://doi.org/10.1016/J.EJSO.2016.01.023.
Lin YJ, Tu YK, Lin SM, Shun CT. Primary hemangiopericytoma in the axis bone: case report and review of literature. Neurosurgery. 1996;39(2):397–400. https://doi.org/10.1097/00006123-199608000-00036.
Olson C, Yen CP, Schlesinger DD, Sheehan J. Radiosurgery for intracranial hemangiopericytomas: outcomes after initial and repeat Gamma Knife surgery: Clinical article. J Neurosurg. 2010;112(1):133–9. https://doi.org/10.3171/2009.3.JNS0923.
Ren K, Zhou X, Wu SJ, Sun X. Primary osseous hemangiopericytoma in the thoracic spine. Clin Neuropathol. 2014;33(5):364–70. https://doi.org/10.5414/NP300741.
Spagnolo R, Torelli L, Brusamolino R, Bono A, Rossi N, Zurrida S. Primary haemangiopericytoma of bone: a case report and literature review. Int J Oncol. 1993;2(4):601–5. https://doi.org/10.3892/ijo.2.4.601.
Vang PS, Falk E. Haemangiopericytoma of bone: review of the literature and report of a case. Acta Orthop. 1980;51(1-6):903–7. https://doi.org/10.3109/17453678008990892.
Wold LE, Unni KK, Sim FH. Hemangiopericytoma of the bone. Am J Surg Pathol. 1982;6(1):53–8. https://doi.org/10.1097/00000478-198201000-00005.
Haas RL, Walraven I, Lecointe-Artzner E, et al. Management of meningeal solitary fibrous tumors/hemangiopericytoma; surgery alone or surgery plus postoperative radiotherapy? Acta Oncol (Madr). 2020. https://doi.org/10.1080/0284186X.2020.1826574.
Birzgalis AR, Ramsden RT, Lye RH, Richardson PL. Haemangiopericytoma of the temporal bone. J Laryngol Otol. 1990;104(12):998-1003. http://www.ncbi.nlm.nih.gov/pubmed/2280162. Accessed December 27, 2018.
Bist S, Bisht M, Varshney S. Temporal bone haemangiopericytoma – diagnostic and therapeutic challenge. Research. 2014:1. https://doi.org/10.13070/rs.en.1.642.
Chang CC, Kuwana N, Tanabe Y, Kitamura H. Haemangiopericytoma of occipital bone with invasion of the transverse sinus. J Clin Neurosci. 1999;6(6):515-518. http://www.ncbi.nlm.nih.gov/pubmed/18639194. Accessed January 2, 2019.
Chin LS, Rabb CH, Hinton DR, Apuzzo ML. Hemangiopericytoma of the temporal bone presenting as a retroauricular mass. Neurosurgery. 1993;33(4):728-731. http://www.ncbi.nlm.nih.gov/pubmed/8232815. Accessed January 2, 2019.
Tewfik TL, Finlayson M, Attia EL. Hemangiopericytoma of the temporal bone. J Otolaryngol. 1981 10(1):72-77. http://www.ncbi.nlm.nih.gov/pubmed/7206032. Accessed January 2, 2019.
Cross DL, Mixon C. Temporal bone hemangiopericytoma. Otolaryngol Head Neck Surg. 1996;114(4):631. https://doi.org/10.1016/S0194-59989670258-9.
Sipal S, Demirci E, Calık M, Gundogdu B, Sengul G, Gundogdu C. Primary hemangiopericytoma of the parietal bone: a case report. Eurasian J Med. 2009;41(3):205-207. http://www.ncbi.nlm.nih.gov/pubmed/25610105. Accessed January 3, 2019.
Hanak BW, Haussen DC, Ambekar S, Ferreira M, Ghodke BV, Peterson EC. Preoperative embolization of intracranial hemangiopericytomas: case series and introduction of the transtumoral embolization technique. J Neurointerv Surg. 2016;8(10):1084–94. https://doi.org/10.1136/neurintsurg-2015-011980.
Ghose A, Guha G, Kundu R, Tew J, Chaudhary R. CNS Hemangiopericytoma. Am J Clin Oncol. 2017;40(3):223–7. https://doi.org/10.1097/COC.0000000000000146.
Purandare NC, Dua SG, Rekhi B, Shah S, Sharma AR, Rangarajan V. Metastatic recurrence of an intracranial hemangiopericytoma 8 years after treatment: report of a case with emphasis on the role of PET/CT in follow-up. Cancer Imaging. 2010;10(1):117. https://doi.org/10.1102/1470-7330.2010.0017.
Author information
Authors and Affiliations
Contributions
Tania Idriceanu collected the data, conceived and designed the article, analyzed the data, and wrote the paper. Wissem Lahiani conceived and designed the article. Erwah Kalsoum analyzed the data and wrote the paper. Gokoulakrichenane Loganadane analyzed the data and wrote the paper. Stephane Palfi conceived and designed the article, analyzed the data, and wrote the paper.
Corresponding author
Ethics declarations
Ethics Approval
N/A
Consent to Participate
The patient gave his informed consent for publication.
Consent for Publication
The patient gave his consent for images or other clinical information relating to my case to be reported in a medical publication. He understood that his name and initials will not be published and that efforts will be made to conceal his identity, but that anonymity cannot be guaranteed. He understood that the material may be published in a journal, website, or other form of publication. As a result, he understood that the material may be seen by the public. He understood that the material may be included in medical books.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Surgery
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Idriceanu, T., Lahiani, W., Kalsoum, E. et al. A Rare Case of Calvaria Solitary Fibrous Tumor: Case Report and Review of Literature. SN Compr. Clin. Med. 5, 150 (2023). https://doi.org/10.1007/s42399-023-01489-x
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-023-01489-x