Abstract
Intestinal obstruction in pregnancy is an uncommon event. It is a high-risk emergency for both the mother and fetus. Diagnosis of obstruction in pregnancy is often delayed due a multitude of reasons including the nonspecific presentation of symptoms, normal laboratory values, and hesitancy to perform necessary imaging studies. Closed loop bowel obstruction (CLBO) is a rare type of intestinal obstruction that compromises blood flow, and it is a surgical emergency that requires prompt intervention. We present of case of CLBO in a pregnant patient at 27 weeks of gestation. Unique to her situation was that this diagnosis was the second time in the same pregnancy. Our patient had a necrotic bowel segment removed via an open laparotomy. The remainder of her pregnancy was uncomplicated, and she delivered at term a viable male via planned cesarean delivery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intestinal obstruction in pregnancy is a rare but high-risk event with incidence rates varying from 1:1500 to 1:16,000 [1]. The maternal and fetal mortality rate can reach as high as 30 and 50%, respectively [2, 3]. In particular, closed loop bowel obstruction (CLBO) carries a high risk of bowel necrosis and strangulation [4]. Diagnosis of bowel obstruction in pregnancy is often a challenge due an overlap of symptoms with normal pregnancy such as abdominal pain and meteorism, delay in imaging, and inconclusive lab values [3]. Surgical intervention is required for CLBO, and an open laparotomy is the choice of intervention due to high risk of bowel ischemia and necrosis [3]. In this report, we present a unique case of a pregnant patient at 27 weeks of gestation with a history of previous episode of closed loop bowel obstruction in the first trimester of the current pregnancy requiring an enterectomy who presents with recurrent abdominal pain. The first case occurred at 7 weeks of gestation and was the result of adhesive disease. The patient’s postoperative course was uncomplicated.
Case Presentation
A 37-year-old G4 P1021 at 27 weeks 3 days dated by IVF presented for an acute onset epigastric abdominal pain. Her pain had been ongoing for 2 h and was a 10 on the numeric pain scale. Patient also reported one episode of non-bilious non-bloody emesis but reported normal bowel movements. Her last oral intake was 3 h prior to the onset of pain. She reported good fetal movement. She described her pain as consistent in presentation to her prior small bowel obstruction she had earlier in the current pregnancy. She denied vaginal bleeding, discharge, dysuria, or hematuria. She had no fever, chills, or contact with sick individuals.
Pregnancy complications were advanced maternal age, IVF pregnancy, and a low-lying placenta on anatomy scan. She previously had two spontaneous abortions at 9 and 7 weeks, respectively, and a cesarean delivery at 34 weeks for complete placenta previa. In addition, she had a laparotomy for ruptured appendicitis with bowel resection, laparoscopic bilateral salpingectomy for hydrosalpinx, and enterectomy for small bowel obstruction (SBO) at 7 weeks of gestation in her current pregnancy. At the first episode of obstruction, the patient presented to the emergency department with acute onset abdominal pain with nausea and vomiting, and the patient noted she has not had a bowel movement or passed flatus for 1 day. Imaging revealed obstruction, and an exploratory laparotomy revealed intestinal obstruction from internal hernia, adhesions with gangrene of small intestine and pelvic, and peritoneal and subdiaphragmatic abscesses. A small bowel resection of the necrotic segment was performed. Her past medical history is significant for infertility, and family history is negative for gynecological cancers or bleeding/clotting disorders. Patient denied use of alcohol, drugs, or smoking during her pregnancy. Her only medication was prenatal vitamins, and she had no known drug allergies.
During physical examination, the patient was in acute distress, vomiting, and in severe pain. Abdominal examination was significant for a gravid uterus and epigastric tenderness to palpation without peritonitis. Vital signs were normal throughout the encounter. Sterile vaginal exam revealed a closed, long, and high cervix. Fetal heart rate was 120 beats per minute with moderate variability with accelerations and no decelerations. No contractions were observed on tocodynamometry, and ultrasound revealed a cephalic fetus with anterior placenta.
The differential diagnosis included acute cholecystitis, pancreatitis, diverticulitis, small bowel obstruction (SBO), bowel perforation, and volvulus. Laboratory results revealed an elevated white blood cell (WBC) count of 13.2 × 109/L, high neutrophil count of 69%, and lactate of 1.9 mmol/L. Other laboratory values including lipase and amylase were normal. An ultrasound done at the emergency department did not reveal signs of cholecystitis or gallstones. However, bidirectional peristalsis and free fluid in the left upper quadrant were concerning for SBO.
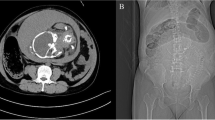
High clinical suspicion of SBO was an indication for computed tomography imaging with intravenous (IV) contrast. Imaging revealed multiple fluid filled, dilated, and thickened loops of small bowel within the left upper quadrant and two transition points on imaging. There was also evidence of small bowel thickening, mesenteric edema with vascular engorgement but no evidence of pneumatosis or free air. The radiological findings are shown in Figs. 1, 2, and 3.
Post contrast CT coronal image through the abdomen and pelvis. Within the left upper quadrant, there are several dilated and fluid filled loops of small bowel, additionally, mesenteric edema and venous engorgement (astrix). These findings are of highly suspicious for high grade small bowel obstruction. A transition point is noted within the left upper quadrant (arrow). Note, it is made of a gravid uterus
Post contrast CT coronal image through the abdomen and pelvis. Within the left upper quadrant, there are several dilated and fluid filled loops of small bowel. Additionally, there is associated wall thickening (arrow head), mesenteric edema, and venous engorgement (astrix). A second transition point is noted within the left upper quadrant (arrow). These findings are of highly suspicious for a closed loop small bowel obstruction
Post contrast CT axial image through the abdomen within the left upper quadrant, there are several dilated and fluid filled loops of small bowel (arrows). Again noted bowel wall thickening (arrow head). There is significant venous engorgement (astrix) within the left upper quadrant. The “misty mesentery” noted by higher attenuation of mesenteric fat as compared to the contralateral side, consistent with reactive mesenteric edema
General surgery was consulted and at that time the patient had developed peritonitis. Therefore, the patient was taken to the operating room for laparotomy. Intraoperative findings included serosanguinous fluid in the abdomen with 30 cm of intestine with a closed loop obstruction. The obstructed segment was necrotic, and enterectomy with primary anastomosis was performed. The mesentery of the closed-loop obstruction was taken using ligasure, and a side-to-side functional end-to-end anastomosis was performed. The abdomen was irrigated and fascia was closed. The patient had no immediate complications. Serial non-stress tests (NSTs) were performed daily. Bedside ultrasound revealed a cephalic fetus with normal movement and an anterior lying placenta.
Betamethasone 6 mg/mL was administered during the first 48 h postoperatively. The nasogastric tube was removed postoperative day 4, and the patient was discharged home postoperative day 6. NSTs and serial ultrasounds performed daily throughout her stay yielded normal results. Patient was admitted at 37 weeks and 5 days for repeat low-transverse cesarean section. A viable male infant weighing 2565 g was delivered in the cephalic position with Apgar scores of 8 and 9 at 1 and 5 min, respectively. The uterus was cleared of adhesions. She was discharged on postoperative day 3.
Discussion and Conclusion
Non-obstetric surgery during pregnancy has an incidence of 2% per year, with abdominal surgery being the most common [5]. Adhesive disease is the most common reason of obstruction in pregnancy and is the leading cause of acute abdomen during the third trimester of pregnancy [3]. Obstruction commonly occurs in the second and third trimester, especially between 16 to 20 weeks and 36 weeks [6]. In our case, the first episode occurred in the first trimester, which is similar to only one reported case of recurrent obstruction [7].
Diagnosis of bowel obstruction in pregnancy is often delayed for a multitude of reasons. First, there is a hesitancy of exposing the fetus to ionizing radiation [8]. However, the effects of radiation on the fetal nervous system and growth are significantly reduced in later stages of pregnancy [8]. In addition, the radiation dose affecting fetal neurodevelopment ranges between 60 and 310 mGy [9], while the risk of leukemia is increased at a dose of 10–20 mGy [10]. Abdominal and pelvic CT with IV contrast in pregnant patients can be performed at a dose as low as 2.5 mGy [11], thus fetal risk from radiation exposure can be minimized consistent with the As Little As Reasonably Acheivable (ALARA) principle [12]. The preferred imaging modality to observe obstruction in pregnancy is multidetector CT (MDCT) since it captures the gut, vasculature, mesenteries, omentum, peritoneum, retroperitoneum, and subperitoneum. Common CT findings of CLBO include C-shaped or U-shaped configuration of the bowel loop, “beak” sign of the obstructed segment, merging of mesenteric vessels as well as the afferent and efferent segments [13]. Second, laboratory tests in pregnant patients with CLBO can be confounded. Leukocytosis, which may be an indicator of obstruction and strangulation, is a common finding in normal pregnancies [14]. A serial elevation in WBC count over a short period of time, however, is a pathological sign and warrants further workup [3]. Our case was similar, and the patient had a slightly elevated WBC count while other indices were normal. Electrolyte changes may be better indicators of obstruction and ischemia. O’Leary et al. found that among factors such as tachycardia, fever, acidosis, leukocytosis, and hyponatremia, the latter was the only statistically significant predictor of ischemia [15]. These electrolyte abnormalities are not always present, however, such as the case of our patient. Third, symptoms of nausea, vomiting, and abdominal pain which are usually indicative of obstruction may be confused with normal pregnancy symptoms, further complicating the diagnosis [3].
The unique physiological changes in pregnancy pose a challenge to surgeons [16]. A general consensus is to perform emergent surgeries such as CLBO during pregnancy and postpone elective surgeries to postpartum [17]. The general guidelines for CLBO surgery in pregnancy including pre-operative care, anesthesia considerations, and optimal positioning during surgery are well-documented [17]. In clinically stable patients with no signs of ischemia or necrosis, bowel obstruction can be managed with the gastrografin protocol [18]. Decompression using a nasogastric tube should be initiated, and gastrografin is administered through the tube. Serial abdominal X-rays are performed, and the patient is reassessed in 24 h. If the patient has bowel movement or if contrast is seen in the colon, the NG tube is removed, and clear liquid diet is initiated. Surgical intervention is the option in patients who fail gastrografin challenge or in suspected CLBO [18].
The risk for preterm labor is the highest for surgeries during the third trimester [5]. The first postoperative week is the most critical, after which the risk for preterm labor is the same as the general population [19]. Routine tocolytic use postoperatively is not standard, as it has not shown to improve surgical outcomes [20]. Fetal monitoring depends on the gestational age. Before the 24 weeks of gestation, a Doppler ultrasound detecting fetal heart rate is sufficient before and after the procedure is done. Tocodynamometry is added in cases with > 24 weeks of gestation [17].
Table 1 lists the cases of recurrent bowel obstruction in pregnancy reported in the literature. To our knowledge, this is the first case of two CLBOs requiring laparotomy with bowel resection in the same pregnancy. Cumming et al. reported a recurrent case of bowel obstruction in the same pregnancy requiring laparotomy, but the obstruction was relieved with no bowel resection [21].
Even though recurrence of obstruction in pregnancy is possible, the rate remains unclear. Thus, counseling patients with a history of obstruction can be challenging. Given the difficulty of diagnosing bowel obstruction in the setting of pregnancy, we recommend a low threshold for imaging especially in the second and third trimester for pregnant patients with a history of bowel obstruction. The benefits of imaging in this scenario outweigh its risks; the most important of which is early diagnosis, especially that delayed intervention has been shown to be deleterious to both the mother and the fetus [3]. Imaging is also crucial as it dictates management: simple obstruction may be managed conservatively or through laparoscopy if needed, while laparotomy remains the standard option for CLBO. In addition, we recommend a high clinical suspicion of bowel obstruction in a pregnant patient with a history of obstruction presenting for abdominal pain. The symptoms of pregnancy such as abdominal pain, nausea, and vomiting might delay the diagnosis of bowel obstruction in pregnancy. Therefore, history of obstruction puts mesenteric ischemia high on the differential diagnosis. Even though the mortality rate for bowel obstruction is higher in the pregnant population compared to the general public, as outlined in Table 1 and our case, the outcomes can be successful for both the mother and the fetus. Term delivery is also possible for these patients unless a deterioration in fetal status is observed.
Clinical trials on CLBO in pregnancy are not feasible, so reports and studies such as our case are crucial to our understanding of this rare entity for both the mother and the fetus. Since obstruction can be life-threatening for both the mother and the fetus, such reports can help guide the management of bowel obstruction in pregnancy. In case of a similar presentation with a different patient, establishing a case history can guide obstetric and surgical teams to reduce unnecessary delay in treatment and improve the overall prognosis. The patient was satisfied with the overall treatment she received. She was made aware that her case is quite unique, especially that she had two cases of obstruction in different trimesters. She was also informed that her description of her abdominal pain during the second episode as being similar to the first episode helped guide the diagnosis and treatment plan. The patient was happy that the fetus survived both episodes and was delivered at term. She had a smooth postoperative course as well.
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Parangi S, et al. Surgical gastrointestinal disorders during pregnancy. Am J Surg. 2007;193(2):223–32.
Heiken J, Smithuis R. Closed loop in small bowel obstruction. 2012 [cited 2022 March 2]; Available from: https://radiologyassistant.nl/abdomen/bowel/closed-loop-in-small-bowel-obstruction.
Connolly MM, Unti JA, Nora PF. Bowel obstruction in pregnancy. Surg Clin North Am. 1995;75(1):101–13.
Calame P, et al. Transmural bowel necrosis from acute mesenteric ischemia and strangulated small-bowel obstruction: distinctive CT features. AJR Am J Roentgenol. 2020;214(1):90–5.
Ravindra G, Madamangalam AS, Seetharamaiah S. Anaesthesia for non-obstetric surgery in obstetric patients. Indian J Anaesth. 2018;62(9):710–6.
Zachariah SK, Fenn MG. Acute intestinal obstruction complicating pregnancy: diagnosis and surgical management. BMJ Case Reports. 2014;2014:bcr2013203235.
Bajaj M, Gillespie C, Dale J. Recurrent sigmoid volvulus in pregnancy. ANZ J Surg. 2017;87(11):E226–7.
Khandelwal A, Fasih N, Kielar A. Imaging of acute abdomen in pregnancy. Radiol Clin North Am. 2013;51(6):1005–22.
Patel SJ, et al. Imaging the pregnant patient for nonobstetric conditions: algorithms and radiation dose considerations. Radiographics. 2007;27(6):1705–22.
Ray JG, et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA. 2016;316(9):952–61.
Committee Opinion No. 723: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol. 2017;130(4):e210–6.
Yoon I and Slesinger TL. Radiation exposure in pregnancy, in StatPearls. 2022, StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.: Treasure Island (FL).
Gore RM, et al. Bowel obstruction. Radiol Clin North Am. 2015;53(6):1225–40.
Ellsbury KE. Abdominal pain in pregnancy. J Fam Pract. 1986;22(4):365–71.
O’Leary MP, et al. Predictors of ischemic bowel in patients with small bowel obstruction. Am Surg. 2016;82(10):992–4.
NíMhuireachtaigh R, O’Gorman DA. Anesthesia in pregnant patients for nonobstetric surgery. J Clin Anest. 2006;18(1):60–6.
Okeagu CN, et al. Clinical management of the pregnant patient undergoing non-obstetric surgery: review of guidelines. Best Pract Res Clin Anaesthesiol. 2020;34(2):269–81.
D’Agostino R, et al. Small bowel obstruction and the gastrografin challenge. Abdom Radiol. 2018;43(11):2945–54.
Mazze RI, Källén B. Reproductive outcome after anesthesia and operation during pregnancy: a registry study of 5405 cases. Am J Obstet Gynecol. 1989;161(5):1178–85.
Berkman ND, et al. Tocolytic treatment for the management of preterm labor: a review of the evidence. Am J Obstet Gynecol. 2003;188(6):1648–59.
Cumming DC, Wren FD. Recurrent bowel obstruction during pregnancy after ileo-jejunal by-pass. Case report. Br J Obstet Gynaecol. 1979;86(9):745–6.
Simsek D, Ozgen G. Recurrent sigmoid volvulus: cause of colon perforation, sepsis, and fetal death. J Obstet Gynaecol Res. 2021;47(6):2230–33.
Cortez N, et al. Endoscopic decompression of recurrent sigmoid volvulus in pregnancy. J Investig Med High Impact Case Rep. 2020;8:2324709620975939.
Alrahmani L, Rivington J, Rose CH. Recurrent volvulus during pregnancy: case report and review of the literature. Case Rep Obstet Gynecol. 2018;2018:4510754.
Alshawi JS. Recurrent sigmoid volvulus in pregnancy: report of a case and review of the literature. Dis Colon Rectum. 2005;48(9):1811–3.
Phillips M, Curtis P, Karanjia N. An elemental diet for bowel obstruction in pregnancy: a case study. J Hum Nutr Diet. 2004;17(6):543–5.
Bandler M, Friedman S, Roberts M. Recurrent volvulus of the sigmoid colon during pregnancy complicated by toxemia of pregnancy. Am J Gastroenterol 1964;42:447–54.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by Dr. Araji and Dr. Wang. The first draft was written by Dr. Araji, Dr. Wang, and Dr. Kandalaft, and all authors commented on previous versions of the manuscript. The final draft was approved by all authors. Dr. Estroff was the primary surgeon during the second laparotomy.
Corresponding author
Ethics declarations
Ethics Approval
N/A. An informed consent was obtained from the patient.
Consent to Participate
A written informed consent was obtained from the patient to participate in our case report.
Consent for Publication
A written informed consent was obtained from the patient to publish our findings.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Surgery
Supplementary Information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Araji, T., Wang, S., Kandalaft, N. et al. Recurrent Closed Loop Bowel Obstruction in Third Trimester of Pregnancy: Case Report and Review of Literature. SN Compr. Clin. Med. 4, 178 (2022). https://doi.org/10.1007/s42399-022-01260-8
Accepted:
Published:
DOI: https://doi.org/10.1007/s42399-022-01260-8