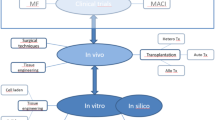
Abstract
Articular cartilage defects tend to have silent progress, leading to joint incongruency, secondary osteoarthritis, and subsequently pain, restricted mobility, and quality of life impairments in patients worldwide. Yet, functional articular cartilage repair has remained a big challenge in the orthopedics and tissue engineering field. In this mini-review, the clinical efficiency of the currently used methods and procedures for articular cartilage repair and promising emerging technologies that would possibly replace or be implemented in the currently used techniques were highlighted. The future directions in articular cartilage repair methods and materials were also critically addressed by elaborating on current limitations hindering the evolution of the emerging technologies supporting articular cartilage regeneration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cartilage lesions are one of the most common causes of joint functional impotence, which impair the overall quality of life and work performance of individuals. As stated in the European Injury Database, an average of 6.1 million people are hospitalized annually in the European Union due to sports injuries, leading to chondral defects of any degree [1]. The etiology of cartilage lesions may be traumatic, hereditary, or vascular. Arthroscopic procedures are the mainstay of surgical treatment when conservative treatment is impossible or has failed. The lesions are classified and treated according to their arthroscopic appearance as follows:
-
Debridement and chondroplasty are currently recommended for symptomatic lesions.
-
Displaced osteochondral fragments can sometimes be replaced and secured with small, recessed screws or absorbable pins.
-
For discrete, isolated, full-thickness cartilage injuries, several treatment options are in clinical use.
Age, lesion size, patient’s desired activity level, alignment, meniscal integrity, and ligamentous stability must all be taken into consideration in selecting the appropriate treatment option. On the other hand, several studies demonstrated that the incidence of chondral defects increases by up to 67% even after arthroscopy surgeries [2, 3]. In this mini-review, we discussed commonly used surgical methods and procedures for cartilage repair in clinics. Moreover, a critical perspective of the need for implementing potential novel treatment approaches into these currently used methods and procedures was elaborated.
Microfracture Technique
The microfracture (MF) technique is the most widely used restoration procedure for small defects (< 4 cm2). It involves perforation of the subchondral bone after removal of the tidemark cartilage with eventual clot formation and fibrocartilaginous repair tissue. However, the main disadvantage of this method is fibrocartilage formation at the operated site, which degenerates after a while due to a mechanical mismatch between the newly formed tissue and the native cartilage tissue. Several studies showed that the efficiency of this technique might be further improved with the use of engineered scaffolds [4,5,6]. In the study of Tahoun et al., followed to a standard microfracture surgery, a chitosan-based material, BST-CarGel®, was used as supporting material in patients (n = 21) of age 18–55 years diagnosed with femoroacetabular impingement. The results showed that the addition of BST-CarGel® scaffolds after microfracture surgery triggered native cartilage-like functional tissue formation as compared to the groups, which did not receive any scaffolds [7]. Biological implants are also under development as a support material applied during microfracture surgeries. A recent study showed that a biological scaffold, composed of collagen type I, could increase GAGs production in the chondral defect area. In this study, a collagen type I scaffold was implanted in combination with microfractures in twenty-five patients [8]. MRI measurements confirmed that GAGs production within the chondral defect area accelerated in patients treated with microfracture combined with collagen type I scaffold 12 months after the surgery. Interestingly, this trend was opposite 6 months after the surgery, GAGs’ content was the greatest within the defect area following the microfracture technique without collagen scaffold.
Autograft, Allograft, Autologous Chondrocyte Implantation (ACI), and Matrix-Associated Autologous Chondrocyte Implantation (MACI) for Knee Cartilage Regeneration
Autografting restores chondral lesions by transplanting the patient’s cartilage into the lesion site to treat simultaneously both cartilage and subchondral bone, using either an arthroscopic or a mini-open procedure. Osteochondral autografts (OA) have been shown a promising alternative in many cases for younger patients with relatively smaller lesions (< 4 cm2) [9, 10]. Generally, the transplant overlying the bony part usually integrates with the surrounding bone properly while the cartilage surface may not fully heal in many patients. In the study of Zamborsky et al., they evaluated 21 articles that included 891 patients for a meta-analysis study where they compare the efficiency of the different surgical techniques for the repair of knee articular cartilage defects in randomized clinical trials [11]. According to the analysis, when the reoperation rates of the patients were compared, OA showed significantly lower rates in patients at the > 3-year follow-up period as compared to MF [11]. In another clinical trial study, the patients having 80–165 mm2 of lesions operated on using either OA or microfracture (MF) technique. In the study, the MF technique was even reinforced using extracellular matrix and bone marrow aspirate concentrate. Yet, the overall modified magnetic resonance observation of cartilage repair tissue scores significantly higher in patients treated with OA (71 ± 15.60) versus MF (55.67 ± 24.11) over 15 months [12]. On the other hand, donor site morbidity due to native tissue harvest and scar tissue formation in the surgery area are two common problems associated with osteochondral autograft procedures.
Allografting is a widely accepted procedure, particularly for older patients, to avoid potential post-operational complications concerning tissue harvest sites. The 10-year success rate of allografts reported above 80% post-operatively according to several clinical studies [13, 14]. The chondrocyte viability ensures the quality of the allografts after transplantation. There is a direct link between the chondrocyte viability and the implant integrity to the articular surface because viable cells assure the presence of appropriate extracellular matrix environment components, such as collagen and sulfated glycosaminoglycans. Numerous studies have shown that chondrocyte viability was drastically reduced after 28 days of storage at 4 °C [15,16,17]. In a recent clinical paper, a novel allograft preservation method (MOPS), described as the preservation of osteochondral allografts in a proprietary solution at room temperature (~ 25 °C), promoted the patient-reported outcome measures (PROMs), such as rehabilitation, revisions, and failures, in a total of 194 patients after 1 year of surgery. Moreover, MOPS prolonged the graft storage time (up to 56 days) and provided substantially greater viable chondrocyte density at the time of implantation (98.8%), addressing a critical problem of the allograft storage in clinics [18]. On the other hand, according to 3 and 4 years of follow-up reports, there was no significant difference in the pain scores and functional outcomes between the patients who received MOPS grafts versus patients who underwent osteochondral allograft transplantation with standard preservation methods. Nevertheless, the problems resulted from long-term storage of the fresh grafts, chondrocyte apoptosis, and graft rejection are still under discussion in clinical practice.
ACI is the first cell-based regenerative procedure to treat patients having substantial defects. In ACI, autologous chondrocytes are derived from adjacent healthy cartilage using arthroscopy. After several days of expansion in vitro culture, the harvested chondrocyte populations are injected into the lesion site followed by periosteal flap support. The fibrocartilage-like tissue formation is one of the biggest concerns in the augmented areas, which is often associated with the phenotypic instability of in vitro-expanded chondrocytes [19, 20]. This unwanted phenotypic change can deteriorate the quality of regenerated tissue. The significant barriers of cell-based cartilage repair methods are the choice of appropriate cell source and the instability of in vitro cell expansion method, which might result in the loss of differentiated phenotype of isolated chondrocytes at the injection site [21, 22]. Autologous periosteal patches that are highly elastic tissue sheaths on the bone surfaces can be also used to seal the implanted autologous chondrocytes. These patches contain precursor cells that promote chondrogenesis during tissue healing [23]. The technique is based on the use of the inner cambium layer, harvested the medial proximal tibia, which is rotated so as that is facing outward. The patch is carefully sewn in place and cartilage-like tissue may grow out from the undifferentiated cambium layer of the graft. Numerous studies showed that the functional outcome of ACI treatment is significantly better than other surgical techniques commonly used to restore defected cartilage [24,25,26].
On the other hand, the periosteal flap isolation for ACI may lead to cartilage hypertrophy, even the enchondral bone formation at the implant area, resulting in the chondral delamination and mechanical mismatch between the tissues [27, 28]. The ACI method can be refined using either a collagen-based matrix or a composite scaffold, pre-seeded with chondrocytes before implantation (MACI). In MACI, the cellular implant is fixed using fibrin glue, rather than suturing the implant over the damaged area. Several reports have been confirmed that long-term knee functionality has been established in the patients treated with MACI, compared to ACI tissues [29, 30]. This can result from using fibrin glue, which is a gentle application to the native tissue when compared to stitching [31]. In the study of Murphy et al., a matrix-associated stem cell transplantation (MAST) was shown as one promising alternative for patients with larger lesions > 15 mm2 or failed microfracture therapies [32]. In the study, matrix-associated stem cells, which were harvested from the cancellous (spongiosa) bone of the patient’s iliac crest, were used to treat osteochondral lesions. The bone marrow aspirate concentrate was embedded into a collagen-based matrix before implantation. The study demonstrated that 83% of patients were able to resume their previous sport activities after an average of 36.7 months. Additionally, the patients with previous failed attempts at microfracture were also included in this clinical trial. Thus, the study showed that the MAST technique could be safely applied to the patients previously operated on with the microfracture technique. Nevertheless, potential donor site morbidity after autologous cell harvest and availability are the two main drawbacks associated with these techniques [33, 34]. One exceptional study confirmed the safety and the potential of functional tissue formation of human nasal septum chondrocytes in a limited number of patients with post-traumatic full-thickness cartilage lesions [35]. The phase II study has been initiated in 2018. This study showed the potential use of different cell sources to reconstruct cartilage tissue. In brief, effective treatment for articular cartilage defects remains a clinical challenge due to the low risk of disease transmission when the contaminated allografts are used, chondrocyte viability, and the limited intrinsic healing capacity of this particular tissue [36]. Although there have been significant advances in cartilage repair research and development to discover novel approaches, the existing materials and surgical techniques are still lacking long-term efficiency.
Emerging Biotechnologies
At present, there is no long-term, optimized, and efficient articular cartilage injury treatment in clinics. The existing methods and procedures are unable to generate fully effective clinical results in articular cartilage lesion management and treatment. The use of bioactive molecules, such as growth factors and platelet-rich plasma (PRP), is currently under discussion as an option to promote articular cartilage lesions and even alter early degenerative arthritis [37,38,39,40,41,42]. The PRP application, which is typically composed of several concentrated growth factors and platelets derived from patients’ blood, has been growing attention in clinics for treating large cartilage defects in the knee. Hyaluronic acid (HA) derivatives, which is one of the components of healthy joint fluid, have often been injected into the knee joint to support lubrication and shock absorption. For example, a meta-analysis review based on several clinical studies on the effectiveness of the PRP technique showed that PRP application ameliorated functional outcomes in operated many patients with osteoarthritic knees, compared to only hyaluronic acid and saline injections [43]. Another promising approach is the local injection of growth factors to the site of injury. The regenerative potential of several growth factors, such as bone morphogenetic proteins (BMP), fibroblast growth factors (FGF), platelet-derived growth factors (PDGF), vascular endothelial growth factor (VEGF), and insulin-like growth factors (IGF), has been demonstrated in vitro and in vivo models [44,45,46,47,48]. For example, FGF-2 has been one of the extensively investigated growth factors, known as promoting the synthesis of the hyaline cartilage matrix. In a recent study by Wang et. al, FGF-2 was tested in a rabbit model to investigate its repair mechanism for articular cartilage defects [49]. They showed that local delivery FGF-2 to the subchondral bone within a collagen-based membrane significantly improved articular cartilage defects in rabbits. In another interesting study, a single injection of a recombinant human IGF-1 effectively promoted cartilage healing in rabbits, based on Mankin scoring system results when it was compared to hyaluronic acid injections [50]. Therefore, the use of bioactive molecules holds tremendous promise for the treatment of articular cartilage defects by triggering necessary signals for healing at sites of tissue injury. On the other hand, despite these encouraging results, there have been no long-term randomized clinical studies, and there have been no undergoing clinical trials showing the efficacy of these growth factors in a larger group of patients. On the other hand, combination approaches, including the conjugation of bioactive molecules with the tissue-engineered biomaterials, are one of the most promising strategies to regenerate articular cartilage. Depending on the need of patients and the disease progression, scaffolding approaches can be advised as a treatment modality for patients with articular cartilage defects. The development of new tissue-engineered products might facilitate the success rate of the current repair techniques. In the study of Nie et al., a novel sponge-like decellularized tissue engineered hyaline cartilage graft consisted of chondrocytes, which were initially grown within alginate gels, was implanted into knee articular chondral defects of pigs. At 60 months, a superior alignment of cartilaginous neo-tissue was observed in tissue-engineered scaffolds groups compared to untreated defect and ACI groups [51]. Furthermore, tissue engineering approaches have the potential in lowering the risk of donor site morbidity, providing shorter recovery time, and triggering functional tissue regeneration when the selected methods display appropriate host tissue integration and response [52, 53]. Therefore, the physical and biological designs of engineered biomaterials (e.g., porosity, architecture, and the presence of either endogenous or exogenous signals biological signals) have an impact on articular cartilage regeneration.
Encouraging advances for the future clinical management of cartilage defects have been shown in the gene therapy field, where particular target genes are transferred to the injury site to regulate the expression of specific growth factors or anti-inflammatory agents to accelerate the treatment of several diseases effectively [54,55,56,57]. The only gene therapy product, which completed the phase III clinical trials in the USA in 2018, is Invossa™ (TissueGene-C) [58]. Invossa acts as a disease-modifying agent, consists of human allogeneic chondrocytes and irradiated GP2-293 cells transduced to overexpress TGFβ1, and possibly hinders inflammation around the osteoarthritic environment by the upregulation of transforming growth factor β1 (TGFβ1) and M2 macrophage differentiation [59]. According to the clinical reports at 3 years obtained from 163 patients, the efficacy endpoints demonstrated that OA disease progression was hindered in Invossa™-treated patients compared to placebo groups Invossa™ was associated with statistically significant improvement in function and pain in patients with OA [58]. At this point, it is worth mentioning the advances in CRISPR/Cas technology, which has been providing powerful tools for gene therapy approaches and gene manipulation in the last 10 years. Once these remarkable genome editing systems are optimized, they seem as feasible alternatives to the current treatment approaches in the future due to their accuracy and low-cost production. Thus cartilage regeneration research could indeed benefit from this technology [60,61,62]. On the other hand, the short-term stability behavior of the gene therapy products, allowing temporary expression of a therapeutic protein, is the most challenging barrier for their successful transition into the clinics [63,64,65]. Therefore, the development of appropriate carrier materials, ensuring the retention of gene therapy products at the defect site, should be taken into consideration in the design of new gene therapy products. In the study of Gao et al., in vitro chondrogenesis ability of a lenti-BMP2/GFP transduced human muscle-derived stem cells (hMDSCs) was evaluated [55]. 3D pellet culture assays showed that LBMP2/GFP-transduced hMDSC pellets exhibited stronger chondrogenic matrix deposition as compared to non-induced cells after 24 days of in vitro culture. Moreover, the cartilage repair in osteoarthritic rats was scored, comparing their hMDSC-LBMP2/GFP groups with a slow-release system group, containing BMP2 and hMDSCs. The articular cartilage regeneration scores were found to be similar for these two groups. On the other hand, when a soluble FMS-like tyrosine kinase-1 (sFLT-1) protein was added to their hMDSC-LBMP2/GFP gene therapy, a better articular cartilage structure was observed as compared to the other groups tested. Thus, the combined approaches, such as gene therapy and bioactive biomaterials, should be taken into consideration to have the most beneficial outcomes in cartilage tissue regeneration.
Cartilage repair materials should adapt to the dynamic inflammatory environment of the injury site after their implantation [66]. Otherwise, the interaction between the implanted material and the host tissue can be detrimental, which eventually hinders implant performance. Immunomodulatory materials have great potential to tune the best host environment if their design is appropriate [67]. Rheumatoid arthritis (RA) is a chronic inflammatory disease resulting in serious defects in cartilage, swelling, stiffness, and pain due to the inflammation at the joints. Currently, there is no effective treatment for RA. The adaptation of cartilage repair materials to the defect site becomes even more important to promote cartilage regeneration. A study published in 2018 showed that the dexamethasone-loaded liposome injection to arthritic rats significantly downregulated the blood concentration of pro-inflammatory cytokines including tumor necrosis factor-α and interleukin-1β, resulting in suppressing the joint swelling in arthritic rats [68]. Moreover, the dexamethasone, which was loaded to the liposomes, was effectively retained at the injection site for an extended time compared to free dexamethasone. An increasing number of studies, based on the modulation of the phenotypic polarization of macrophages, have focused on the design of immunomodulatory biomaterials for cartilage regeneration. The macrophages are recruited at the injury site in the early stages of the repair. The phenotypic change of macrophages (M0) from inflammatory (M1) to regenerative (M2) can direct cartilage regeneration via reducing or activating the immune response. In the study of Ji et al., a thermosensitive hydroxypropyl chitin hydrogel was functionalized using a transforming growth factor-β1 (TGFβ1) to study cartilage repair in a rat osteochondral defect model. They reported that TGFβ1 addition to hydroxypropyl chitin hydrogels prevented the newly formed cartilage from degradation 6 weeks after the surgery [69]. The articular stability of the newly formed tissue was associated with the potential immunomodulatory effect of macrophages while tissue healing. They also showed that TGFβ1 addition to chitin composite hydrogels significantly hindered the expression of specific genes responsible for M1 polarization, such as iNOS, IL-1, TNF-α, IL-6, and CD86 [64]. Hence, the activation of M0 macrophages towards the M2 phenotype could lead to a functional cartilage repair at the site of the injury. Thus far, despite years of advances in cartilage repair research, there is no clinically approved bioactive product for orthopedic use.
Overall, the future of effective cartilage repair is likely to be built over the discovery of novel therapeutic proteins, tissue-engineered biomaterials, and gene therapy products. It is essential to modify the existing cellular models, allowing a more accurate protein production, such as developing relevant cell lines that are capable of expressing genes and proteins stimulating chondrogenesis. Therefore, the industry should also consider investing more extra in large-scale protein production platforms.
Conclusion
The two main obstacles standing in future novel products are, particularly concerning the bioactive molecules and gene therapy products, the high cost of the manufacturing phase while a decent reimbursement mechanism is still lacking, as well as the burdensome regulatory pathway. Therefore, the promotion of direct contact with the authorities, who need to provide clear and coherent guidance both in the EU and in the USA for the translation of smart-active materials, is one of the key points that would accelerate the clinical testing of novel materials. It is further critical to analyze and interpret the most recent changes in EU regulation on medical devices and medicinal products. The power of a dynamic interaction between the academy and industry should not be underestimated. The synergy between these two communities helps to overcome the obstacles in the new product development and its safe transition. The industry should be supportive and cooperative to develop advanced bioengineering techniques and novel platforms to address current limitations in the product design, addressing stability, adverse effects, and low potency.
Availability of Data and Material (Data Transparency)
Not applicable.
Code Availability (Software Application or Custom Code)
Not applicable.
References
Urbanek O, Kołbuk D, Wróbel M. Articular cartilage: new directions and barriers of scaffolds development – review. Int J Polym Mater. 2019;68(7):396–410.
Curl WW, Krome J, Gordon ES. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–60.
Southworth TM, Naveen NB, Nwachukwu BU, Cole BJ, Frank RM. Orthobiologics for focal articular cartilage defects. Clin Sports Med. 2019;38(1):109–22.
Medvedeva EV, Grebenik EA, Gornostaeva SN, Telpuhov VI, Lychagin AV, Timashev PS, Chagin AS. Repair of damaged articular cartilage: current approaches and future directions. Int J Mol Sci. 2018;19(8):2366.
Pipino G, Risitano S, Alviano F, Wu EJ, Bonsi L, Vaccarisi DC, Indelli PF. Microfractures and hydrogel scaffolds in the treatment of osteochondral knee defects: a clinical and histological evaluation. J Clin Orthop Trauma. 2019;10(1):67–75.
Han Y, Lian M, Sun B, Jia B, Wu Q, Qiao Z, Dai K. Preparation of high precision multilayer scaffolds based on Melt Electro-Writing to repair cartilage injury. Theranostics. 2020;10(22):10214–30.
Tahoun MF, Tey M, Ormazabal I, Elsayed AS, Said HG, Monllau JC. Promising radiological outcome after repair of acetabular chondral defects by microfracture augmented with chitosan-based scaffold: mid-term T2 mapping evaluation. Knee Surg Sports Traumatol Arthrosc. 2021;29(1):324–8.
Šprláková-Puková A, Štouračová A, Repko M, Koriťáková E, Tintěra J, Dostál M, Otaševič T. Prospective multiparametric magnetic resonance monitoring of changes in lesions of hyaline cartilage of the knee joint after treatment by microfractures and implantation of biological collagen type i matrix implants. Acad Radiol. 2020;30 S1076-6332(20):30337–8.
Ishimatsu T, Yoshimura I, Kanazawa K, Hagio T, Yamamoto T. Return to sporting activity after osteochondral autograft transplantation for Freiberg disease in young athletes. Arc Orthop Trauma Surg. 2017;137(7):959–65.
Wang D, Chang B, Coxe FR, Pais MD, Wickiewicz TL, Warren RF, Rodeo SA, Williams RJ. Clinically meaningful improvement after treatment of cartilage defects of the knee with osteochondral grafts. J Sports Med. 2019;47(1):71–81.
Zamborsky R, Danisovic L. Surgical techniques for knee cartilage repair: an updated large-scale systematic review and network meta-analysis of randomized controlled trials, arthroscopy. Arthroscopy. 2020;36(3):845–58.
Sofka C, Cabe T, Deland J, Drakos M, Patel K. A comparison of functional and radiographic outcomes following microfracture with extracellular matrix augmentation versus osteochondral autograft transplantation for the treatment of medium-sized osteochondral lesions of the talus. Orthop J Sports Med. 2020;8(7 suppl6):2325967120S00387.
León SA, Mei XY, Safir OA, Gross AE, Kuzyk PR. Long-term results of fresh osteochondral allografts and realignment osteotomy for cartilage repair in the knee. Bone Joint J. 2019;101-B(1_ Supple_A):46–52.
Bloch B, Asplin L, Smith N, Thompson P, Spalding T. Higher survivorship following meniscal allograft transplantation in less worn knees justifies earlier referral for symptomatic patients: experience from 240 patients. Sports Traumatol Arthrosc. 2019;27(6):1891–9.
Merkely G, Ackermann J, Farina EM, VanArsdale C, Lattermann C, Gomoll AH. Shorter storage time is strongly associated with improved graft survivorship at 5 years after osteochondral allograft transplantation. Am J Sports Med. 2020;17:363546520956311.
Han Y, Qu P, Zhang K, Bi Y, Zhou L, Xie D, Song H, Dong J, Jianhong Q. Storage solution containing hydrogen improves the preservation effect of osteochondral allograft. Cell Tissue Bank. 2019;20(2):201–8.
Calvo R, Espinosa M, Figueroa D, Pozo LM, Cong P. Assessment of cell viability of fresh osteochondral allografts in N-acetylcysteine-enriched medium. Cartilage. 2020;11(1):117–21.
Stannard JP, Cook JL. Prospective assessment of outcomes after primary unipolar, multisurface, and bipolar osteochondral allograft transplantations in the knee: a comparison of 2 preservation methods. Sports Med. 2020;48(6):1356–64.
Sun M, Lu Z, Cai P, Zheng L, Zhao J. Salidroside enhances proliferation and maintains phenotype of articular chondrocytes for autologous chondrocyte implantation (ACI) via TGF-β/Smad3 Signal. Biomed Pharmacother. 2020;122:109388.
Eschen C, Kaps C, Widuchowski W, Fickert S, Zinser W, Niemeyer Ph, Roël G. Clinical outcome is significantly better with spheroid-based autologous chondrocyte implantation manufactured with more stringent cell culture criteria. Osteoarthr Cartil Open. 2020;2(1):100033.
Kisiday JD. Expansion of chondrocytes for cartilage tissue engineering: a review of chondrocyte dedifferentiation and redifferentiation as a function of growth in expansion culture. Regen Med Front. 2020;2(1):e200002.
Elkhenany HA, Szojka ARA, Mulet-Sierra A, Liang Y, Kunze M, Lan X, Sommerfeldt M, Jomha NM, Adesida AB. Bone marrow mesenchymal stem cell-derived tissues are mechanically superior to meniscus cells. Tissue Eng Part A. 2021;27:13–4.
Hsiao HY, Cheng CM, Kao SW, Liu JK, Chang CS, Harhaus L, Huang JJ. The effect of bone inhibitors on periosteum-guided cartilage regeneration. Sci Rep. 2020;10:8372.
Schrock JB, Kraeutler JM, Houck DA, McQueen MB, McCarty EC. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sports Med. 2017;5(5):2325967117704634.
Na Y, Shi Y, Liu W, Jia Y, Kong L, Zhang T, Han C, Ren Y. Is implantation of autologous chondrocytes superior to microfracture for articular-cartilage defects of the knee? A systematic review of 5-year follow-up data. Int J Surg. 2019;68:56–62.
Niemeyer P, Laute V, Zinser W, Becher C, Kolombe T, Fay J, Pietsch S, Kuźma T, Widuchowski W, Fickert S. A prospective, randomized, open-label, multicenter, phase III noninferiority trial to compare the clinical efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology versus arthroscopic microfracture for cartilage defects of the knee. Orthop J Sports Med. 2019;7(7):2325967119854442.
Niethammer TR, Loitzsch A, Horng A, Baur-Melnyk A, Bendiks M, Gülecyüz MF, Müller PE, Pietschmann MF. Graft hypertrophy after third-generation autologous chondrocyte implantation has no correlation with reduced cartilage quality: matched-pair analysis using T2-weighted mapping. Am J Sports Med. 2018;46(10):2414–21.
Barié A, Kruck P, Sorbi R, Rehnitz C, Oberle D, Walker T, Zeifang F, Moradi B. Prospective long-term follow-up of autologous chondrocyte implantation with periosteum versus matrix-associated autologous chondrocyte implantation: a randomized clinical trial. Am J Sports Med. 2020;48(9):2230–41.
Niemeyer P, Schubert T, Grebe M, Hoburg A. Matrix-associated chondrocyte implantation is associated with fewer reoperations than microfracture: results of a population-representative, matched-pair claims data analysis for cartilage defects of the knee. Orthop J Sports Med. 2019;7(10):2325967119877847.
Riboh JC, Cvetanovich GL, Cole BJ, Yanke AB. Comparative efficacy of cartilage repair procedures in the knee: a network meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25:3786–99.
Kreuz PC, Kalkreuth RH, Niemeyer P, Uhl M, Erggelet C. Long-term clinical and MRI results of matrix-assisted autologous chondrocyte implantation for articular cartilage defects of the knee. Cartilage. 2019;10(3):305–13.
Murphy EP, Fenelon C, Egan C, Kearns SR. Matrix-associated stem cell transplantation is successful in treating talar osteochondral lesions. Knee Surg Sport Traumatol Arthrosc. 2019;27:2737–43.
Liou JJ, Rothrauff BB, Alexander PG, Tuan RS. Effect of platelet-rich plasma on chondrogenic differentiation of adipose- and bone marrow-derived mesenchymal stem cells. Tissue Eng Part A. 2018;24(19–20):1432–43.
Zellner J, Pattappa G, Koch M, Lang S, Weber J, Pfeifer CG, Mueller MB, Kujat R, Nerlich M, Angele P. Autologous mesenchymal stem cells or meniscal cells: what is the best cell source for regenerative meniscus treatment in an early osteoarthritis situation? Stem Cell Res Ther. 2017;8(1):225.
Mumme M, Barbero A, Miot S, Wixmerten A, Feliciano S, Wolf F, Asnaghi AM, Baumhoer D, Bieri O, Kretzschmar M, Pagenstert G, Haug M, Schaefer DJ, Martin I, Jakob M. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 2016;388(10055):1985–94.
Hulet C, Sonnery-Cottet B, Stevenson C, Samuelsson K, Laver L, Zdanowicz U, Stufkens S, Curado J, Verdonk P, SpaldingT. The use of allograft tendons in primary ACL reconstruction. Knee Surg Sports Tr A. 2019;27:1754–70.
Hede K, Christensen BB, Jensen J, Foldager CB, Lind M. Combined bone marrow aspirate and platelet-rich plasma for cartilage repair: two-year clinical results. Cartilage. 2019;20:1947603519876329.
Irmak G, Gümüşderelioğlu M. Photo-activated platelet-rich plasma (PRP)-based patient-specific bio-ink for cartilage tissue engineering. Biomed Mater. 2020;15(6):065010.
Yan W, Xu X, Xu Q, Sun Z, Jiang Q, Shi D. Platelet-rich plasma combined with injectable hyaluronic acid hydrogel for porcine cartilage regeneration: a 6-month follow-up. Reg Biomaterials. 2020;7(1):77–90.
Danieli MV, FernandesGuerreiro JP, Queiroz AO, Pereira HR, Cataneo DC. Leucocyte-poor-platelet-rich plasma intra-operative injection in chondral knee injuries improve patients outcomes. A prospective randomized trial. International Int Orthop. 2021;45(2):463–71.
Cugat R, Alentorn-Geli E, Navarro J, Cuscó X, Steinbacher G, Seijas R, Álvarez-Díaz P, Barastegui D, Laiz P, Samitier G, García-Balletbó M. A novel autologous-made matrix using hyaline cartilage chips and platelet-rich growth factors for the treatment of full-thickness cartilage or osteochondral defects: Preliminary results. J Orthop Surg. 2019;28(1):1–7.
Pérez JMD, Fernández-Sarmiento JA, García DA, Machuca MMG, Rodríguez JM, Calvo RN, Arévalo JP, Poveda JMC, Alentorn-Geli E, Boada PL, Bertomeu RC. Cartilage regeneration using a novel autologous growth factors-based matrix for full-thickness defects in sheep. Knee Surg Sports Traumatol Arthrosc. 2019;27(3):950–61.
Chen P, Huang L, Ma Y, Zhang D, Zhang X, Zhou J, Ruan A, Wang Q. Intra-articular platelet-rich plasma injection for knee osteoarthritis: a summary of meta-analyses. J Orthop Surg Res. 2019;14:385.
Deng S, Zhou JL, Peng H, Fang HS, Liu F, Weng JQ. Local intra-articular injection of vascular endothelial growth factor accelerates articular cartilage degeneration in rat osteoarthritis model. Mol Med Rep. 2018;17(5):56311–8.
Lui JC, Colbert M, Cheung CSF, Ad M, Lee A, Zhu Z, Barnes KM, Dimitrov DS, Baron J. Cartilage-targeted IGF-1 treatment to promote longitudinal bone growth. Mol Ther. 2019;27(3):673–80.
Murahashi Y, Yano F, Nakamoto H, Maenohara Y, Iba K, Yamashita T, Tanaka S, Ishihara K, Okamura Y, Moro T, Saito T. Multi-layered PLLA-nanosheets loaded with FGF-2 induce robust bone regeneration with controlled release in critical-sized mouse femoral defects. Acta Biomater. 2019;85:172–9.
Kanga S, Yoo JS, Lee JY, Kim HJ, Park K, Kim SE. Long-term local PDGF delivery using porous microspheres modified with heparin for tendon healing of rotator cuff tendinitis in a rabbit model. Carbohydr Polym. 2019;209:372–81.
Vayas R, Reyes R, Arnau MR, Évora C, Delgado A. Injectable scaffold for bone marrow stem cells and bone morphogenetic protein-2 to repair cartilage. Cartilage. 2019;11:19476035198416822019.
Yang W, Cao Y, Zhang Z, Du F, Shi Y, Li X, Zhang Q. Targeted delivery of FGF2 to subchondral bone enhanced the repair of articular cartilage defect. Acta Biomater. 2018;69:170–82.
Lubis AMT, Wonggokusuma E, Marsetio AF. Intra-articular recombinant human growth hormone injection compared with hyaluronic acid and placebo for an osteoarthritis model of New Zealand rabbits. Knee Surg Relat Res. 2019;31(1):44–53.
Nie X, Chuah YJ, Zhu W, He P, Peck Y, Wang D. Decellularized tissue engineered hyaline cartilage graft for articular T cartilage repair. Biomaterials. 2020;235:119821.
Schüttler KF, Götschenberg A, Klasan A, Stein T, Pehl A, Roessler PP, Figiel J, Heyse TJ, Efe TM. Cell-free cartilage repair in large defects of the knee: increased failure rate 5 years after implantation of a collagen type I scaffold. Arch Orthop Trauma Surg. 2019;139:99–106.
Ding H, Cheng Y, Niu X, Hu Y. Application of electrospun nanofibers in bone, cartilage and osteochondral tissue engineering. J Biomater Sci Polym Ed. 2021;32(4):536–61.
Liang C, Liu Z, Song M, Li W, Wu Z, Wang Z, Wang Q, Wang S, Yan K, Sun L, Hishida T, Cai Y, Belmonte JCI, Guillen P, Chan P, Zhou Q, Zhang W, Qu J, Liu GH. Stabilization of heterochromatin by CLOCK promotes stem cell rejuvenation and cartilage regeneration. Cell Res. 2021;31(2):187–205.
Gao X, Cheng H, Awada H, Tang Y, Amra S, Lu A, Sun X, Lv G, Huard C, Wang B, Bi X, Wang Y, Huard J. A comparison of BMP2 delivery by coacervate and gene therapy for promoting human muscle-derived stem cell-mediated articular cartilage repair. Stem Cell Res Ther. 2019;10(1):346.
Venkatesan JK, Rey-Rico A, Cucchiarini M. Current trends in viral gene therapy for human orthopaedic regenerative medicine. Tissue Eng Regen Med. 2019;16:345–55.
Lee H, Kim H, Seo J, Choi K, Lee Y, Park K, Kim S, Mobasheri A, Choi H. TissueGene-C promotes an anti-inflammatory micro-environment in a rat monoiodoacetate model of osteoarthritis via polarization of M2 macrophages leading to pain relief and structural improvement. Inflammopharmacology. 2020;28:1237–52.
Lee B. INVOSSA, a first-in-class of cell and gene therapy for osteoarthritis treatment: the phase III trial. Osteoarthr Cartil. 2018;26(1):S43–4.
Choi K, Lee H, Kim D, Lee H, Kim M, Lim CL, Lee YJ, Lee B, Kim S, Lee M, Choi H. InvossaTM (TISSUEGENE-C) induces an anti-inflammatory environment in the arthritic knee joints via macrophage polarization. Osteoarthr Cartil. 2017;25:S76–444.
Seidl CI, Fulga TA, Murphy CL. CRISPR-Cas9 targeting of MMP13 in human chondrocytes leads to significantly reduced levels of the metalloproteinase and enhanced type II collagen accumulation. Osteoarthritis Cartilage. 2019;27(1):140–7.
Lu Y, Xue J, Deng T, Zhou X, Yu K, Deng L, Huang M, Yi X, Liang M, Wang Y, Shen H, Tong R, Wang W, Li L, Song J, Li J, Su X, Ding Z, Gong Y, Zhu J, Wang Y, Zou B, Zhang Y, Li Y, Zhou L, Liu Y, Yu M, Wang Y, Zhang X, Yin L, Xia X, Zeng Y, Zhou Q, Ying B, Chen C, Wei Y, Li W, Mok T. Safety and feasibility of CRISPR-edited T cells in patients with refractory non-small-cell lung cancer. Nat Med. 2020;26:732–40.
Gayet RV, Puig H, English MA, Soenksen LR, Nguyen PQ, Mao AS, Angenent-Mari NM, Collins JJ. Creating CRISPR-responsive smart materials for diagnostics and programmable cargo release. Nat Protoc. 2020;15(9):3030–63.
Karimian A, Azizian K, Parsian H, Rafieian S, Shafiei-Irannejad V, Kheyrollah M, Yousefi M, Majidinia M, Yousefi B. CRISPR/Cas9 technology as a potent molecular tool for gene therapy. J Cell Physiol. 2019;234(8):12267–77.
Wang XY, Yi DD, Wang TY, Wu YF, Chai YR, Xu DH, Zhao CP, Song C. Enhancing expression level and stability of transgene mediated by episomal vector via buffering DNA methyltransferase in transfected CHO cells. J Cell Biochem. 2019;120(9):15661–70.
Kohn DB, Booth C, Kang EM, Pai SY, Shaw KL, Santilli G, Armant M, Buckland KF, Choi U, De Ravin SS, Dorsey MJ, Kuo CY, Leon-Rico D, Rivat C, Izotova N, Gilmour K, Snell K, Xu-Bayford Dip J, Darwish J, Morris EC, Terrazas D, Wang LD, Bauser CA, Paprotka T, Kuhns DB, Gregg J, Raymond HE, Everett JK, Honnet G, Biasco L, Newburger PE, Bushman FD, Grez M, Gaspar HB, Williams DA, Malech HL, Galy A, Adrian J. Lentiviral gene therapy for X-linked chronic granulomatous disease, Thrasher, the Net4CGD consortium16. Nat Med. 2020;26(2):200–6.
Bauza G, Pasto A, Mcculloch P, Lintner D, Brozovich A, Niclot FB, Khan I, Francis LW, Tasciotti E, Taraballi F. Improving the immunosuppressive potential of articular chondroprogenitors in a three-dimensional culture setting. Sci Rep. 2020;10(1):16610.
Im GI. Biomaterials in orthopaedics: the past and future with immune modulation. Biomater Res. 2020;4(24):7.
Jia M, Deng C, Luo J, Zhang P, Sun X, Zhang Z, Gong T. A novel dexamethasone-loaded liposome alleviates rheumatoid arthritis in rats. Int J Pharm. 2018;540(1–2):57–64.
Ji X, Lei Z, Yuan M, Zhu H, Yuan X, Liu W, Pu H, Jiang J, Zhang Y, Jiang X, Xiao J. Cartilage repair mediated by thermosensitive photocrosslinkable TGFβ1-loaded GM-HPCH via immunomodulating macrophages recruiting MSCs and promoting chondrogenesis. Theranostics. 2020;10(6):2872–87.
Author information
Authors and Affiliations
Contributions
E.V.: conceptualization, methodology, design, investigation, writing — original draft preparation.
E.S.: reviewing, original draft editing.
P.Y.Z.: reviewing, original draft editing.
Corresponding author
Ethics declarations
Ethics Approval (Include Appropriate Approvals or Waivers)
Not applicable.
Consent to Participate (Include Appropriate Statements)
Not applicable.
Consent for Publication (Include Appropriate Statements)
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Surgery
Rights and permissions
About this article
Cite this article
Vardar, E., Samara, E. & Zambelli, PY. A Mini-review of Current Methods and Emerging Technologies in Articular Cartilage Repair. SN Compr. Clin. Med. 3, 2278–2284 (2021). https://doi.org/10.1007/s42399-021-01044-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-021-01044-6