Abstract
Information on outcome of pediatric pericardial diseases from India is limited. The aim of this study is to study the outcome of significant pericardial effusion of infectious etiology in children. This study is a retrospective analysis of significant pericardial effusion of infectious etiology in children admitted to a tertiary care hospital of northern India during the last 10 years. Of the 74 patients, 71.6% (53/74) had tuberculosis, most being “probable” tubercular effusion. Pyogenic cases (17/74) usually had a pleura-pulmonary focus. Pericardial fluid adenosine deaminase (ADA) and contrast enhanced computer tomography (CECT) chest were useful diagnostic aids in tubercular effusions. Pericardiocentesis and surgery were done in 72.9% (54/74) and 12.1% (9/74), respectively. On median follow-up of 18 months, death or chronic constrictive pericarditis was seen in 2 patients each, both had tubercular effusions. Tuberculosis is still the commonest infectious cause of pericardial effusion in children from this part of the world. Introduction of early intervention with pericaridiocentesis or surgery may improve the outcome of this once deadly disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Significant pericardial effusion is uncommon in pediatric population. Etiologies include infectious (bacterial, tubercular, and viral), noninfectious (autoimmune, malignancy, and postoperative), and idiopathic [1]. Most of the available publications are from west where infectious pericardial effusions are uncommon [2,3,4,5]. In Africa and Asia, infections constitute the usual cause of pediatric pericardial effusion [6,7,8,9,10,11,12,13].
There are a handful of studies on pediatric pericardial effusion of infectious origin, especially examining the outcome [9, 11, 13]. Therefore, we decided to do this retrospective observational study with an objective of describing the etiology and outcome of significant pericardial effusion of infectious etiology in children.
Methods
This retrospective analysis was undertaken in a tertiary care hospital in New Delhi, India. Records of children under 18 years, admitted from January 2010 to September 2019 with significant pericardial effusion of an infectious etiology, were reviewed, from hospital register and follow-up clinic files. Seventy-four patients were included in this study (STROBE flow chart Fig. 1). Twenty-two out of the total 74 patients were a part of a previous publication in which we described the clinical features, etiology, and outcome of these patients [11]. In this study, we have included 52 additional patients and analyzed their outcome for a longer time period in follow-up clinic as well.
Tubercular pericardial effusion (TBPE) was labelled as “definite” or “probable.” “Definite” tuberculous pericarditis was considered with evidence of tubercle bacilli in pericardial fluid (smear/polymerase chain reaction/cartridge-based nucleic acid amplification test). Diagnosis of “probable” disease was made on the proof of tuberculosis elsewhere in the body, or on the basis of indirect tests in pericardial fluid (e.g., adenosine deaminase level ( ADA) ≥ 35 IU/l) and/or an appropriate clinical response to a trial of anti-tuberculosis chemotherapy. Significant Mantoux positivity was defined as value of 10 mm or more induration within 72 h of injecting intradermal 1 Tuberculin Unit Purified Protein Derivative (PPD RT 23.). Interferon gamma release assay (IGRA) was not done in our study.
Purulent pericardial effusion (PPE) was diagnosed in the presence of a consistent history, clinical or lab findings, and/or an exudative effusion or frank pus on pericardial fluid aspiration. Culture reports of pericardial fluid, blood, or from any other focus were also collected.
Viral pericarditis was diagnosed when clinical history and pericardial fluid analysis were suggestive of the same.
“Significant pericardial effusion” was defined as echo-free space more than 1cm in front of the right/left ventricle, and/or pericardial tamponade confirmed in the presence of right ventricular diastolic collapse [5].
Surgical intervention was done in cases with thick organized effusion not amenable to catheter drainage. Contrast enhanced computer tomography (CECT) chest had been performed in cases where there was difficulty in ascribing a diagnosis. PPE received a third generation cephalosporin and vancomycin until a positive culture report, while TBPE cases received standard four drug ATT with 4–6 weeks of steroids. Outcome variables analyzed were pericardiocentesis, surgical intervention, constrictive pericarditis, or mortality.
Missing data were excluded from the analysis. Follow-up data were analyzed for only those patients where data was available. Data was analyzed using SPSS version 19. Baseline characteristics of subject in both the groups were compared using Students t test or Wilcoxon rank sum test (for quantitative variables), or Chi square test or Fischer exact test (for categorical variables).
Results
Out of 74 patients, a majority, 53/74 (71.6%) had tubercular effusion, 17 had pyogenic, and 4 had viral effusions. Clinical features and selected investigations are shown in Table 1. The mean age was 7.7 (4.1) years for the whole group. Cases with pyogenic effusions were younger to those with tubercular etiology (P<0.04), 5.9 (3.5) and 8.3 (4.2) years, respectively. Pyogenic effusion was associated with more pleural effusion/empyema (P<0.05), respiratory distress (P=0.00), and higher leukocyte count (P=0.00).
Echocardiographic findings at admission and pericardial fluid analysis are tabulated in Table 2. Tamponade was more commonly associated with PPE. Apart from ADA, other parameters were not useful in differentiating TBE from PPE.
Among those with tubercular effusion, history of contact and Mantoux positivity was observed in 26.5% (n=13/49) and 59.0% (n=26/44), respectively. Acid fast bacilli (AFB) stain was not positive in the pericardial fluid in any case. Physical appearance of the pericardial aspirate (hemorrhagic/serosanguinous, straw, and pus) had no correlation with the etiological diagnosis.
In pyogenic effusions, Staphylococcus aureus was the commonest agent isolated; however, culture positivity yield was low (35.2%, 6/17). Pericardial fluid culture was positive in two (Staphylococcus aureus and Pseudomonas), whereas blood culture was positive in 4 patients (2 Staph aureus, 1 each Streptococcus pneumonia and Klebsiella).
Duration of hospitalization was longer for children with PPE. Pericardiocentesis and surgical intervention were required in 72.9% (54/74) and 12.1% (9/74), respectively. Post-discharge medium-term outcome was available for 70.2% of the patients (Table 3). Median overall follow-up duration was 18 (6.5–37) months. Development of chronic constrictive pericarditis 2.7% (2/74) and mortality 2.7% (2/74) was low, for the entire group, and was seen only in those with tubercular effusion.
Discussion
This study shows that tuberculosis is the commonest cause of infectious pericardial effusion in this part of the world. Importantly the outcome of pericardial effusion of infectious etiology in the current era appears favorable with low mortality and low occurrence of constrictive pericarditis. The etiology of pericardial disease varies in different regions of the world. Publications from high-income countries have limited information on infectious pericardial effusion in children. This is one of the largest single-center studies on infectious pericardial effusion in children and exemplifies the difficulties in diagnosing definite tuberculosis in the pediatric population [11]. TBPE in children is a pauci-bacillary disease. It is considered to be an inflammatory response to the low concentration of tubercle bacilli in the pericardium accounting for the low isolation rate. Definite tubercular effusion was seen in only 21% (4/19) in our study. Low yield of tubercle bacilli from pericardial fluid and biopsy of pericardium has been reported in children [7, 8].
Elevated pericardial ADA levels (>35 U/L sensitivity and specificity of 90% and 74%, respectively) is a useful test for the diagnosis of tubercular effusion [14]; it was positive in 86.9% (n=20/23) of our cases. High ADA in tuberculosis appears to be indirectly related to the subsets of activated T cell lymphocytes involved in the antigenic response to tuberculous bacilli [15].
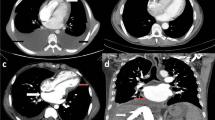
CECT chest was particularly helpful in doubtful cases. Twenty patients underwent CECT chest as a part of diagnostic evaluation, especially when it was difficult to differentiate between tubercular and pyogenic effusion. Nineteen out of 20 had features suggestive of tuberculosis in the form of necrotic mediastinal lymph nodes or lung involvement suggestive of tuberculosis. In 6/20, there was no other evidence of tuberculosis apart from CECT chest. In additional 6 patients, Mantoux positivity was the only additional clue for tuberculosis apart from CECT chest. Thus in 12/20 (60%) cases when there was doubt about the etiology, CECT chest was helpful in establishing diagnosis. In a previous study, Cherian et al reported the presence of enlarged mediastinal lymph nodes >10 mm in all 22 patients of tubercular pericardial effusion and in none of the patients of a control group with large viral/idiopathic or postoperative pericardial effusion [16]. We believe there is a role of CECT chest in the diagnosis of tubercular pericardial effusion especially where microbiological diagnosis has not been made and pericardial fluid ADA is not elevated.
Pyogenic effusions occurred at a lower age with more respiratory distress, more pleura-pulmonary involvement, and more tamponade, and they had a longer hospital stay compared to tuberculous effusions. Similar to that reported previously, we also found pleural/pulmonary infection to be the focus in the majority of our patients; other foci were bone, soft tissue, and liver [9, 10, 13]. Most patients had received iv antibiotics prior to presentation which may be the cause of low culture positivity.
Overall 10% (7/70) (5 tubercular and 2 pyogenic) had thrombus formation. Thrombosis was seen in jugular vein, inferior vena cava, lung, and left ventricle. Infection associated with venous stasis could be the contributory factor. This has not been reported previously. Does it entail a chance association or a complication needs to be looked into and is worth observing in future studies.
Procedure-related complication occurred in four patients. Myocardial perforation by needle, bradycardia and vasovagal syncope while removing pigtail, difficulty in removing pigtail due to knot in the pigtail and pneumothorax occurred in one patient each. Two patients had dry tap.
During the first hospital stay, there was no mortality. Follow-up information after discharge was available for 70.2% (53/74) patients. Mortality occurred in 3.7% (2/53), both occurring in follow-up in those with tubercular effusion. Both patients had undergone successful drainage and came back within a week after discharge, with re-accumulation of effusion and succumbed to tamponade.
Low mortality rates have been observed with the use of modern anti-tubercular treatment in pediatric patients [7, 8]. This is in contrast to adult studies where higher mortality has been observed (17–40%) despite 6 months of anti-tubercular therapy (ATT) [17]. Higher mortality in adult studies is probably due to human immunodeficiency virus (HIV), older age, and concomitant pulmonary tuberculosis. It is also probably related to the higher bacillary load in adults compared to children, where it has been seen that bacillary load determines mortality [18].
Chronic constrictive pericarditis (CCP) occurred in 3.7% (2/53) of the patients. Both cases were seen in those with tubercular effusion. This low rate of constrictive pericarditis is consistent with that seen in the current era in both adults and children [8, 19].
Steroid may have a role in decreasing the incidence of CCP compared to historical cohort. Hugo-Hamman et al reported that 14% children with pericardial effusion developed constrictive pericarditis [7]. None of these patients who developed CCP were given steroids, whereas all our patients were given steroids. The large IMPI trial also suggested that there is a role of steroids in preventing CCP [19]. However, the effect of steroid on development of CCP is confounded by fact that previous studies have varied on HIV positivity rates, time of starting therapy, and most importantly timing and effectiveness of drainage of pericardial fluid [16, 20].
In our study, 13/54 (2 pyogenic and 11 tubercular) patients had organized/partially organized effusion with features of subacute constriction at presentation. Besides medical management, eight underwent pericardial tap and 2 underwent immediate surgery. CCP developed in the remaining 2 out of 3 patients in whom neither tap nor surgical intervention was done. None of the patients who underwent drainage developed CCP. This may indicate that effusion needs to be drained especially where it is organized, in order to prevent CCP.
Strang et al in a factorial design randomized trial allocated patients with tuberculous effusion to open drainage vs percutaneous drainage and prednisolone vs placebo and followed them for over 10 years. In their study, lowest mortality and adverse events occurred in those with open drainage and steroids [20]. Similar observations were made by Cakir et al in children with pyogenic effusions, wherein pericardiocentesis and sub-xiphoid tube placement followed by pericardial window and or primary pericardiocentesis in patients with thick exudates resulted in no constrictive pericarditis in their series [10]. Future studies should study the effect and timing of surgical drainage in patients with organized effusions which cannot be drained percutaneously.
Limitations of the Study
The major limitation of our study is that it is a retrospective review of data and a single-institution study. Definite tubercular, pyogenic, or viral pericardial effusion was present in a minority of patients. There was incomplete data and lack of follow-up data in many cases.
References
Rheuban KS. Pericardial Diseases. In: Allen HD, Gutgesell HP, editors. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult. 7th ed. Philadelphia:Lippincott Williams & Wilkins; 2008 1290-9.
Kühn B, Peters J, Marx GR, Breitbart RE. Etiology, management, and outcome of pediatric pericardial effusions. Pediatr Cardiol. 2008;29(1):90–4.
Mok G, Mehnahem S. Large pericardial effusions of inflammatory origin in childhood. Cardiol Young. 2003;13(2):131–6.
Shakti D, Hehn R, Gauvreau K, Sundel RP, Newburger JW. Idiopathic pericarditis and pericardial effusion in children: contemporary epidemiology and management. J Am Heart Assoc. 2014;3(6):e001483.
Guven H, Bakiler AR, Ulger Z, et al. Evaluation of children with a large pericardial effusion and cardiac tamponade. ActaCardiol. 2007;62:129–33.
Peter ID, Asani MO, Aliyu I. Pericardial effusion and outcome in children at a Tertiary Hospital in Northwestern Nigeria: a 2 year retrospective review. Res Cardiovasx Med. 2019;8:14–8.
Hugo-Hamman CT, Scher H, De Moor MM. Tuberculous pericarditis in children: a review of 44 cases. Pediatr Infect Dis J. 1994;13(1):13–8.
Obihara NJ, Walters E, Lawrenson J, Garcia-Prats AJ, Hesseling AC, Schaaf HS. Tuberculous pericardial effusions in children. J Pediatric Infect Dis Soc. 2018;7(4):346–9.
Jayashree M, Singhi SC, Singh RS, Singh M. Purulent pericarditis: clinical profile and outcome following surgical drainage and intensive care in children in Chandigarh. Ann Trop Paediatr. 1999;19(4):377–81.
Çakir Ö, Gurkan F, Balci A, Eren N, Dikici B. Purulent pericarditis in childhood: ten years of experience. J PediatrSurg. 2002;37:1404–8.
Bagri N, Yadav D, Agarwal S, Aier T, Gupta V. Pericardial effusion in children: experience from tertiary care center in Northern India. Indian Pediatr. 2014;51:211–3.
Nessa L, Rouf A, Sen SS, Munshi YA, Shahriar A. Pericardial effusion in children and outcome: experience from a tertiary care hospital, Noakhali, Bangladesh. Chest Heart J. 2015;39:75–8.
Agrawal A, Jhamb U, Nigam A, Agrwal S, Saxena R. Purulent pericardial effusion in children: experience from a tertiary care center in North India. Anals of Pediatric Cardiol. 2020;13:289–93.
Burgess LJ, Reuter H, Taljaard JJ, et al. Role of biochemical tests in the diagnosis oflarge pericardial effusions. Chest. 2002;121:495–9.
Lee YC, Rogers JT, Rodriguez RM, et al. Adenosine deaminase levels in nontuberculous lymphocytic pleural effusions. Chest. 2001;120:356–61.
Cherian G, Habashy AG, Uthaman B, Cherian JM, Salama A, Anim JT. Detection and follow-up of mediastinal lymph node enlargement in tuberculous effusions using computed tomography. Am J Med. 2003;114:319–22.
Mayosi BM, Wiysonge CS, Ntsekhe M, Gumedze F, Volmink JA, Maartens G, et al. Mortality in patients treated for tuberculous pericarditis in sub-Saharan Africa. S Afr Med J. 2008 Jan;98(1):36–40.
Pasipanodya JG, Mubanga M, Ntsekhe M, Pandie S, Magazi BT, Gumedze F, et al. Tuberculous pericarditis is multibacillary and bacterial burden drives high mortality. EBioMedicine. 2015;2(11):1634–9.
Mayosi BM, Ntsekhe M, Bosch J, Pandie S, Jung H. Gumedze F et al IMPI trial investigators prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med. 2014 Sep 1;371(12):1121–30.
Strang J, Nunn A, Johnson D, Casbard A, Gibson DG, Girling DJ. Management of tuberculous constrictive pericarditis and tuberculous pericardial effusion in Transkei: results at 10 years follow-up. QJM. 2004;97:525–35.
Author information
Authors and Affiliations
Contributions
All authors contributed to conception, data acquisition, analysis and interpretation of data, and drafting and reviewing the paper. All approve the final version and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethical Standards
Institutional ethics committee of ABVIMS and Dr RML Hospital has approved the study 13/8/2020, No. 146(15/2016) IEC/ABVIMS/RMLH.
Conflict of Interest
The authors declare no competing interests.
Additional information
What Is Already Known?
• Tuberculosis is the most common cause of pericardial effusion in low- and middle-income countries.
What This Study Adds?
• Current therapy with drainage (surgical and percutaneous) and antibacterial therapy has decreased mortality and occurrence of constrictive pericarditis in children with infectious pericardial effusion.
• Diagnosis of definite tubercular pericardial effusion is difficult.
• CECT chest is a useful adjunct in cases where diagnosis is difficult.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Kumar, D., Bhatt, .D., Verma, A. et al. Outcome of Significant Pericardial Effusion of Infectious Etiology in Children: an Observational Retrospective Cohort Study. SN Compr. Clin. Med. 3, 2251–2255 (2021). https://doi.org/10.1007/s42399-021-01026-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-021-01026-8