Abstract
To compare the efficacy and tolerability of heme-iron Optifer (HIO) versus intravenous (IV) iron saccharate/Ferosac in treatment of iron deficiency anemia (IDA) during pregnancy. Two hundred and thirty-six (236) women with moderate IDA (hemoglobin > 7 and < 10 gm/dl) were included in this comparative multicenter study: 117 women in HIO/Optifer group and 119 women in IV/Ferosac group. Women in HIO/Optifer group received Optifer tablets twice daily till hemoglobin level of 11–12 gm/dl then once daily (maintenance dose). Total IV iron dose calculated for the studied women in the IV/Ferosac group according to the manufacturer instructions. The pre-treatment ferritin, hemoglobin (Hb), RBCs-mean corpuscular volume (MCV), and mean corpuscular hemoglobin (MCH) were compared by post-treatment values in the two studied groups. The mean post-treatment Hb and ferritin were similar with no significant difference between HIO/Optifer group and IV/Ferosac group (11.7 ± 5.5 gm/dl and 118.8 ± 66.9 μg/l, respectively, versus 12.4 ± 6.1 and 132.9 ± 75.3, respectively), (P = 0.87 and 0.89, respectively). The mean post-treatment MCV and MCH were similar with no significant difference between HIO/Optifer group and IV/Ferosac group (94.0 ± 7.2 fl and 29.4 ± 2.9 pg, respectively, versus 97.7 ± 6.6 and 31.7 ± 4.2, respectively), (P = 0.17 and 0.99, respectively). The HIO/Optifer is an effective, well tolerable oral iron for treatment of moderate IDA during pregnancy with similar efficacy to IV iron saccharate/Ferosac. The IV iron saccharate/Ferosac is safe and an effective alternative to heme-iron for treatment of moderate IDA in women presented with IDA at later gestation when rapid replacement of iron stores required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anemia affects 1.5 billion worldwide [1]. About 52% of pregnant women are suffering from anemia in developing countries [2]. Iron deficiency (ID) is the commonest cause of anemia compared to other nutritional deficiencies (B12 and folic acid) [1, 3]. The iron requirement is high during pregnancy, and it increases furthermore during the second and third trimesters [4]. The daily requirement of iron for non-pregnant women is about 1–8 mg. However, more external iron is required in pregnancy for fetal, and placental development, and increased mother’s blood volume. The daily recommended amount of iron for pregnant women is about 27 mg [5].
In addition, 7% of the vaginal deliveries and 23% of cesarean sections are associated with ≥ 1000 ml blood loss [5, 6].
Maternal anemia is a risk of adverse maternal and perinatal outcomes [7,8,9,10]. Froessler et al. found the ID and iron deficiency anemia (IDA) were associated with adverse maternal outcome as reduced cognitive activities and increased depressive disorders. In addition, they reported the preterm labor (PTL), IUGR (intra-uterine growth retardation), IUFD (intra-uterine fetal death), and neonatal infection as adverse neonatal outcomes related to ID and IDA [11].
Peri-partum anemia increases the need for red blood cells (RBCs) transfusion [12, 13]. The RBCs transfusion corrects the hemoglobin (Hb) temporarily, and not the underlying cause [14].
Adequate iron supplementation is crucial during pregnancy to reduce the perinatal morbidity related to IDA [15].
Oral iron is safe and effective option for treatment of IDA during pregnancy. The conventional oral iron salts are associated with gastric discomfort/upset, constipation, and intolerability, which adversely affect the compliance and treatment outcome [16, 17].
The heme-iron is an effective, tolerable oral iron preparation, improves the compliance, and ensures continuous iron intake during pregnancy [18].
Hoppe et al. found the dietary-based treatment containing heme-iron has few side effects and can be used efficiently to improve the iron status of reproductive age women [19].
Nissenson et al. found the heme-iron an effective treatment option for IDA in hemodialysis patients and replaced the intravenous (IV) iron preparations [20].
Nagaraju et al. trial found the heme-iron polypeptide (HIP) has similar efficacy to IV iron sucrose in maintaining Hb in non-dialysis chronic kidney patients [21].
In addition, Abdelazim et al. found the HIP well tolerable with similar efficacy to IV iron for treatment of IDA during pregnancy [22]. Therefore, this study is designed to compare the efficacy and tolerability of the heme-iron Optifer (HIO/Optifer) versus iron saccharate/Ferosac in treatment of IDA during pregnancy.
Materials and Methods
This multicentric comparative study was conducted during the years 2019 and 2020, after approval of the study by the Obstetrics Departments of Ahmadi Hospital, Kuwait and West Kazakhstan Marat Ospanov Medical University, Kazakhstan (Approval Number: OB_09012_18).
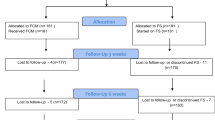
Two hundred and fifty (250) pregnant women with moderate IDA during pregnancy (Hb > 7 and < 10 gm/dl) were included in the beginning of this study after informed consents in accordance with the Declaration of Helsinki, and trial registration (ACTRN12618001483246) [23].
Studied pregnant women received either HIO/Optifer tablets twice daily (HIO/Optifer group = 125 women) or IV iron saccharate/Ferosac (IV/Ferosac group = 125 women) for correction of their IDA.
Eight (8) women in HIO/Optifer group (incomplete ante-natal records (4) and PTL (4)) and six (6) women in IV/Ferosac group (incomplete ante-natal records (2) and travelling (4)) were excluded from this study.
Finally, this study completed with two hundred and thirty-six (236) women with moderate IDA (117 women in HIO/Optifer group and 119 women in the IV/Ferosac group).
The diagnosis of moderate IDA was based on serum ferritin < 15 μg/l (normal 15–150 μg/l), Hb (> 7 and < 10 gm/dl), RBCs-mean corpuscular volume (MCV) < 80 fl (normal 80–100 fl), and hemoglobin (MCH) < 28 pg (normal 28–32 pg) [24,25,26,27].
Inclusion criteria include pregnant women ≥ 20 years old, 14–26 weeks’ gestation, with serum ferritin < 15 μg/l, Hb > 7, and < 10 gm/dl, MCV < 80 fl, and MCH < 28 pg.
Women with intolerance or hypersensitivity to iron, anemia other than IDA, Hb < 7 gm/dl, and received blood transfusion were excluded from this study.
The HIO (Optifer) tablets (L’Avenir Med., MediTec FerroCare, Sweden) contain 18 mg heme-iron. The heme-iron of the Optifer tablets has a specific heme carrier protein-1 receptor in the small intestine. The serum peak of iron reached within 2–4 h after the oral intake of HIO/Optifer tablets. Each HIO/Optifer tablet increases the blood iron by > 3 mg (average 3.15 mg) [18].
Women in HIO/Optifer group received Optifer tablets twice daily, till Hb level of 11–12 gm/dl then once daily (maintenance dose) [18].
Total IV iron dose calculated for the studied women in the IV group according to the manufacturer’s formula: IV iron dose in mg = 2.4 × pre-pregnancy weight (kg) × (target Hb − actual Hb) gm/dl + 500 mg.
Twelve (12) is the target Hb, and 500 is the amount of stored iron in adults, while 2.4 is a correction factor [22].
Women in IV/Ferosac group received the calculated IV iron dose over 6–8 sessions. In every session, 200 mg of iron saccharate diluted in saline and given as an IV infusion/1 h every other day [22].
Participants were monitored during the IV iron infusion for signs of intolerance, and/or anaphylaxis [22]. Iron sucrose (iron saccharate/Ferosac) is cleared from serum within 5–6 h and used for erythropoiesis [22].
Studied pregnant women in both groups received oral folic acid to avoid folate deficiency. Participants were asked during ante-natal visits about their compliance and side effects related to oral iron (metallic taste, gastrointestinal (GIT) intolerance/upset, and/or constipation) or IV iron (skin eruption, headache, tachycardia, hypotension, abdominal, or chest pain).
The pre-treatment ferritin, Hb, RBCs-MCV, and MCH were compared by post-treatment values in the two studied groups to compare the efficacy and tolerability of HIO/Optifer versus IV iron saccharate/Ferosac in treatment of IDA during pregnancy as a primary outcome [24,25,26,27]. Secondary outcome measures, the tolerability, and side effects are related to studied iron preparations.
Sample Size
The required sample size is calculated using data from previous studies [10, 22], and G Power software version 3.17 for sample size calculation (Heinrich Heine Universität; Düsseldorf; Germany), setting α-error probability at 0.05, power (1-β error probability) at 0.95%, and effective sample size (w) at 0.5. An effective sample includes ≥ 220 women in two groups needed to produce a statistically acceptable figure.
Statistical Analysis
Collected data were analyzed using Statistical Package for Social Sciences (SPSS) version 20 (Chicago, IL, USA). The mean and standard deviation (±SD) used to present the numerical values, while the number (n) and percentage (%) used to present the categorical values. Chi-square (χ2) test was used for analysis of qualitative data. Student’s (t) test was used to compare the pre-treatment ferritin, Hb, RBCs-MCV, and MCH by post-treatment values to compare the efficacy of HIO/Optifer versus IV/Ferosac in treatment of IDA during pregnancy. P-value < 0.05 is considered significant.
Results
Two hundred and thirty-six (236) women with moderate IDA (Hb > 7 and < 10 gm/dl) were included in this comparative multicenter study: 117 women in HIO/Optifer group and 119 women in the IV/Ferosac group to compare the efficacy and tolerability of HIO/Optifer versus IV iron saccharate/Ferosac in treatment of moderate IDA during pregnancy.
There was no significant difference between the two studied groups regarding the mean age, BMI (body mass index), and parity (P = 0.06, 0.8, and 0.9, respectively).
There was no significant difference between the HIO/Optifer group and IV/Ferosac group regarding the gestational age at diagnosis of IDA (22.5 ± 3.6 weeks versus 23.3 ± 4.1, respectively; P = 0.9), pre-treatment Hb (7.7 ± 3.1 gm/dl versus 7.6 ± 4.3, respectively; P = 0.9), pre-treatment ferritin (12.3 ± 6.4 μg/l versus 14.9 ± 6.7, respectively; P = 0.6), pre-treatment MCV (77.2 ± 8.1 fl versus 74.9 ± 7.2, respectively; P = 0.1), and pre-treatment MCH (25.7 ± 5.7 pg versus 25.1 ± 6.8, respectively; P = 0.9) (Table 1).
In addition, there was no significant difference between the HIO/Optifer group and IV/Ferosac group regarding the mean post-treatment Hb and ferritin (11.7 ± 5.5 gm/dl and 118.8 ± 66.9 μg/l, respectively, versus 12.4 ± 6.1 and 132.9 ± 75.3, respectively), (P = 0.87 and 0.89, respectively). There was no significant difference between the HIO/Optifer group and IV/Ferosac group regarding the mean post-treatment MCV and MCH (94.0 ± 7.2 fl and 29.4 ± 2.9 pg, respectively, versus 97.7 ± 6.6 and 31.7 ± 4.2, respectively), (P = 0.17 and 0.99, respectively) (Table 2).
The rates of poor compliance/treatment interruption and GIT intolerance were reported in 3.4% (4/117) and 1.7% (2/117), respectively, of HIO/Optifer group versus 0.84% (1/119) and 0% (0/119), respectively, in IV/Ferosac group (P = 0.1 and 0.8, respectively, in-significant difference). No other side effects or adverse reactions were recorded in both studied groups.
Discussion
IDA is commonly treated with oral iron preparations. However, the conventional oral iron salts are associated with poor compliance and treatment interruption which adversely affect the treatment outcome [1]. The heme-iron is an effective and tolerable oral iron preparation for treatment of IDA [18, 20].
Therefore, two hundred and thirty-six (236) women moderate with IDA (Hb > 7 and < 10 gm/dl) were included in this comparative multicenter study (117 women in HIO/Optifer group and 119 women in IV/Ferosac group), to compare the efficacy and tolerability of the HIO/Optifer versus IV/Ferosac in treatment of moderate IDA during pregnancy.
There was no significant difference between the two studied groups regarding the gestational age at diagnosis of IDA (P = 0.9), pre-treatment Hb (P = 0.9), pre-treatment ferritin (P = 0.6), pre-treatment MCV (P = 0.1), and pre-treatment MCH (P = 0.9).
Women in HIO/Optifer group received Optifer tablets twice daily till hemoglobin level of 11–12 gm/dl then one tablet daily (maintenance dose) [18].
Women in IV/Ferosac group received the calculated IV iron dose over 6–8 sessions. In every session, 200 mg of iron saccharate diluted in saline and given as an IV infusion/1 h every other day [22].
The mean post-treatment Hb and ferritin were similar with no significant difference between HIO/Optifer group and IV/Ferosac group (11.7 ± 5.5 gm/dl and 118.8 ± 66.9 μg/l, respectively, versus 12.4 ± 6.1 and 132.9 ± 75.3, respectively), (P = 0.87 and 0.89, respectively). In addition, the mean post-treatment MCV and MCH were similar with no significant difference between HIO/Optifer group and IV/Ferosac group (94.0 ± 7.2 fl and 29.4 ± 2.9 pg, respectively, versus 97.7 ± 6.6 and 31.7 ± 4.2, respectively), (P = 0.17 and 0.99, respectively).
Kriplani et al. and Kochhar et al. found the IV iron sucrose was effective in treatment of moderate IDA with pregnancy without serious side effects [28, 29].
When the IV iron sucrose is compared to heme-iron preparation, Nissenson et al. found the heme-iron an effective treatment option for IDA in hemodialysis patients and replaced the IV iron preparations [20].
Nagaraju et al. trial found the HIP has similar efficacy to IV iron sucrose in maintaining hemoglobin in non-dialysis chronic kidney patients [21].
Abdelazim et al. found the HIP well tolerable with similar efficacy to IV iron for treatment of IDA during pregnancy [22].
Moreover, an intrinsically labelled 58Fe-heme and non-heme 57Fe (ferrous sulfate) were given to pregnant women in the 3rd trimester and blood samples (maternal and cord) were collected at delivery to assess the 58Fe and 57Fe levels. The maternally absorbed 58Fe present in neonates were significantly higher compared to 57Fe (2.7 ± 1.3 versus 2.2 ± 1.4, respectively). This finding suggests that heme-iron may be favorably transported across the placenta to fetus [30].
The rates of poor compliance/treatment interruption and GIT intolerance were reported in 3.4% (4/117) and 1.7% (2/117), respectively, of HIO/Optifer group versus 0.84% (1/119) and 0% (0/119), respectively, in IV/Ferosac group (P = 0.1 and 0.8, respectively, in-significant difference). No other side effects or adverse reactions were recorded in both studied groups.
The iron sucrose (IS) was approved in the States and Europe in November 2000 [31]. The incidence of serious life-threatening anaphylaxis with IS is 0.002%, and the hypersensitivity reactions have not been reported with IS [31]. IS showed high safety profile during pregnancy in the largest published trial [31].
In addition, Kriplani et al. and Kochhar et al. found the IV iron sucrose was effective in treatment of moderate IDA with pregnancy without serious side effects [28, 29].
Abdelazim et al. reported GIT intolerance/upset in 1.7% (2/117) with heme-iron during treatment of IDA with pregnancy [18].
Pal et al. found the rate of poor compliance was 4% in heme-iron treated group compared to 12% in iron salts treated group [30].
al-Momen et al. reported high rates of poor compliance (30%) and GIT symptoms (30%) with oral iron preparations [32].
The higher poor compliance/treatment interruption and side effects with iron salts can be explained by the fact that only 1–8% of iron is absorbed from the available oral iron salts. The iron absorption increases with increasing oral iron doses only up to 160 mg/day. Accordingly, the recommended dose of elemental iron for treating IDA in pregnancy is 100–200 mg/day. Increasing iron dose beyond this recommended dose leads to GIT side effects without improving the efficacy [33].
Habib et al. studied the Hb outcome in pregnant women with IDA in relation to their compliance to iron supplement and they found that the Hb levels improved significantly only among strictly compliant women. Anemia was significantly associated with non-compliant women (odds ratio (OR) 6.19 (95% CI: 2.55–15.02; P < 0.0001)) [34].
Radhika et al. meta-analysis found the IV iron an effective alternative to address ID in women require rapid replacement of stores [35].
Gupta et al. trial concluded that IV iron sucrose was well tolerated and beneficial for pregnant women presenting with anemia at later gestation [36].
This study concluded that the HIO/Optifer is an effective, well tolerable oral iron for treatment of moderate IDA during pregnancy with similar efficacy to IV iron saccharate/Ferosac. The IV iron saccharate/Ferosac is safe and an effective alternative to heme-iron for treatment of moderate IDA in women presented with IDA at later gestation when rapid replacement of iron stores required.
The tolerability of HIO/Optifer is an important advantage because the compliance to oral iron is the main obstacle toward effective treatment of IDA during pregnancy.
The current study was the first registered study, comparative, multicenter study, conducted to compare the efficacy and tolerability of HIO/Optifer versus IV iron saccharate/Ferosac in treatment of IDA during pregnancy.
Incomplete patients’ records because of PTD and travelling was the only limitation faced during this study. The efficacy of HIO/Optifer in treatment of IDA during pregnancy should be compared to other heme-iron preparations or to the new IV iron (Ferric Carboxymaltose) in future studies.
Conclusion
The HIO/Optifer is an effective, well tolerable oral iron for treatment of moderate IDA during pregnancy with similar efficacy to IV iron saccharate/Ferosac. The IV iron saccharate/Ferosac is safe and an effective alternative to heme-iron for treatment of moderate IDA in women presented with IDA at later gestation when rapid replacement of iron stores required.
Availability of Data
The datasets analyzed during the current study are not publicly available due to the concern for patient safety and confidentiality but are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Api O, Breyman C, Çetiner M, Demir C, Ecder T. Diagnosis and treatment of iron deficiency anemia during pregnancy and the postpartum period: iron deficiency anemia working group consensus report. Turk J Obstet Gynecol. 2015;12(3):173–81. https://doi.org/10.4274/tjod.01700.
Güleç ÜK, Özgünen FT, Evrüke İC, Demir SC. Anemia in pregnancy. Arch Med Rev J. 2013;22(3):300–16. [scopemed.org].
World Health Organization. Iron and folate supplementation: standards for maternal and neonatal care. Integrated Management of Pregnancy and Childbirth (IMPAC). Department of Making Pregnancy Safer. WHO; 2007. [Google Scholar]
Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72(1 Suppl):257S–64S. https://doi.org/10.1093/ajcn/72.1.257S.
Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol. 2008;199(5):519.e1–7. https://doi.org/10.1016/j.ajog.2008.04.049.
Breymann C, Bian XM, Blanco-Capito LR, Chong C, Mahmud G, Rehman R. Expert recommendations for the diagnosis and treatment of iron-deficiency anemia during pregnancy and the postpartum period in the Asia-Pacific region. J Perinat Med. 2011;39(2):113–21. https://doi.org/10.1515/JPM.2010.132.
Kalaivani K. Prevalence & consequences of anaemia in pregnancy. Indian J Med Res. 2009;130(5):627–33.
Shafi D, Purandare SV, Sathe AV. Iron deficiency anemia in pregnancy: intravenous versus oral route. J Obstet Gynaecol India. 2012;62(3):317–21. https://doi.org/10.1007/s13224-012-0222-0.
American College of Obstetricians and Gynecologists. ACOG practice bulletin no. 95: anemia in pregnancy. Obstet Gynecol. 2008;112(1):201–7. https://doi.org/10.1097/AOG.0b013e3181809c0d.
Abdelazim IA, Abu-Faza M, Bou Hamdan S. Intravenous iron saccharate infusion for treatment of iron deficiency anemia before labor. ARC J Gynecol Obstet. 2016;1(3):16–20. https://doi.org/10.20431/2456-0561.0103004.
Froessler B, Gajic T, Dekker G, Hodyl NA. Treatment of iron deficiency and iron deficiency anemia with intravenous ferric carboxymaltose in pregnancy. Arch Gynecol Obstet. 2018;298:75–82. https://doi.org/10.1007/s00404-018-4782-9.
Roberts CL, Nippita TA. International caesarean section rates: the rising tide. Lancet Glob Health. 2015;3(5):e241–2. https://doi.org/10.1016/S2214-109X(15)70111-7.
Patterson JA, Roberts CL, Isbister JP, Irving DO, Nicholl MC, Morris JM, et al. What factors contribute to hospital variation in obstetric transfusion rates? Vox Sang. 2015;108(1):37–45. https://doi.org/10.1111/vox.12186.
Froessler B, Palm P, Weber I, Hodyl NA, Singh R, Murphy EM. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Ann Surg. 2016;264(1):41–6. https://doi.org/10.1097/SLA.0000000000001646.
EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific opinion on the safety of heme iron (blood peptonates) for the proposed uses as a source of iron added for nutritional purposes to foods for the general population, including food supplements. EFSA J. 2010;8(4):1585. https://doi.org/10.2903/j.efsa.2010.1585www.efsa.europa.eu. Last Accessed 28/10/2020.
Pavord S, Myers B, Robinson S, Allard S, Strong J, Oppenheimer C. British Committee for Standards in Haematology. UK guidelines on the management of iron deficiency in pregnancy. Br J Haematol. 2012;156(5):588–600. https://doi.org/10.1111/j.1365-2141.2011.09012.x.
Johnson-Wimbley TD, Graham DY. Diagnosis and management of iron deficiency anemia in the 21st century. Ther Adv Gastroenterol. 2011;4(3):177–84. https://doi.org/10.1177/1756283X11398736.
Abdelazim IA, Abu-Faza M, Shikanova S, Zhurabekova G, Maghrabi MM. Heme-bound iron in treatment of pregnancy-associated iron deficiency anemia. J Family Med Prim Care. 2018;7(6):1434–8. https://doi.org/10.4103/jfmpc.jfmpc_271_18.
Hoppe M, Brün B, Larsson MP, Moraeus L, Hulthén L. Heme iron-based dietary intervention for improvement of iron status in young women. Nutrition. 2013;29(1):89–95. https://doi.org/10.1016/j.nut.2012.04.013.
Nissenson AR, Charytan C. Controversies in iron management. Kidney Int Suppl. 2003;87:S64–71. https://doi.org/10.1046/j.1523-1755.64.s87.10.x.
Nagaraju SP, Cohn A, Akbari A, Davis JL, Zimmerman DL. Heme iron polypeptide for the treatment of iron deficiency anemia in non-dialysis chronic kidney disease patients: a randomized controlled trial. BMC Nephrol. 2013;14:64. https://doi.org/10.1186/1471-2369-14-64.
Abdelazim IA, Abu-Faza M, Elbiaa AAM, Othman HS, Alsharif DA, Elsawah WF. Heme iron polypeptide (Proferrin®-ES) versus iron saccharate complex (Ferrosac) for treatment of iron deficiency anemia during pregnancy. Acta Med Int. 2017;4(1):55–60. https://doi.org/10.5530/ami.2017.4.11 [Google Scholar].
Heme-bound iron (Optifer) versus intravenous iron (Ferosac) in treatment of pregnancy associated iron deficiency anemia. http://www.ANZCTR.org.au/ACTRN12618001483246.aspx. Last Accessed 25/10/2020.
Short MW, Domagalski JE. Iron deficiency anemia: evaluation and management. Am Fam Physician. 2013;87(2):98–104.
Abdelazim IA, Nusair B, Svetlana S, Zhurabekova G. Treatment of iron deficiency and iron deficiency anemia with intravenous ferric carboxymaltose in pregnancy. Arch Gynecol Obstet. 2018;298(6):1231–2. https://doi.org/10.1007/s00404-018-4943-x.
Kanshaiym S, Abdelazim IA, Starchenko T, Mukhambetalyeva G. Effect of intravenous iron sucrose on hemoglobin level, when administered in a standard dose, to anemic pregnant women in rural Northern India. J Family Med Prim Care. 2019;8(2):769–70. https://doi.org/10.4103/jfmpc.jfmpc_438_18.
Kanshaiym S, Zhurabekova G, Abdelazim IA, Starchenko T. Intravenous ferriccarboxy maltose for the treatment of iron deficiency anemia: letter to editor. Hematol Transfus Cell Ther. 2020;42(1):98–9. https://doi.org/10.1016/j.htct.2019.01.008.
Kriplani A, Mahey R, Dash BB, Kulshreshta V, Agarwal N, Bhatla N. Intravenous iron sucrose therapy for moderate to severe anaemia in pregnancy. Indian J Med Res. 2013;138(1):78–82.
Kochhar PK, Kaundal A, Ghosh P. Intravenous iron sucrose versus oral iron in treatment of iron deficiency anemia in pregnancy: a randomized clinical trial. J Obstet Gynaecol Res. 2013;39(2):504–10. https://doi.org/10.1111/j.1447-0756.2012.01982.x.
Pal B, Deshpande H, Sundari T, Biniwale P, Shah K, Goel S, et al. Heme iron polypeptide in iron deficiency anemia of pregnancy: current evidence. Open J Obstet Gynecol. 2017;7:420–31. https://doi.org/10.4236/ojog.2017.74044 [Google Scholar].
Khalafallah AA, Dennis AE. Iron deficiency anaemia in pregnancy and postpartum: pathophysiology and effect of oral versus intravenous iron therapy. J Pregnancy. 2012;2012:630519–0. https://doi.org/10.1155/2012/630519.
al-Momen AK, al-Meshari A, al-Nuaim L, Saddique A, Abotalib Z, Khashogji T, et al. Intravenous iron sucrose complex in the treatment of iron deficiency anemia during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;69(2):121–4.
Tandon R, Jain A, Malhotra P. Management of iron deficiency anemia in pregnancy in India. Indian J Hematol Blood Transfus. 2018;34(2):204–15. https://doi.org/10.1007/s12288-018-0949-6.
Habib F, Alabdin EH, Alenazy M, Nooh R. Compliance to iron supplementation during pregnancy. J Obstet Gynaecol. 2009;29(6):487–92. https://doi.org/10.1080/01443610902984961.
Radhika AG, Sharma AK, Perumal V, Sinha A, Sriganesh V, Kulshreshtha V, et al. Parenteral versus oral iron for treatment of iron deficiency anaemia during pregnancy and post-partum: a systematic review. J Obstet Gynaecol India. 2019;69(1):13–24. https://doi.org/10.1007/s13224-018-1191-8.
Gupta A, Manaktala U, Rathore AM. A randomized controlled trial to compare intravenous iron sucrose and oral iron in treatment of iron deficiency anemia in pregnancy. Indian J Hematol Blood Transfus. 2014;30(2):120–5. https://doi.org/10.1007/s12288-012-0224-1.
Author information
Authors and Affiliations
Contributions
Ibrahim A. Abdelazim (IAA) is responsible for the study concept and design, data collection and analysis, registration of the study as clinical trial, critical analysis of intellectual content, drafting and Microsoft editing of the manuscript, final revision before publication, and submission for publication.
Svetlana Shikanova (SS) and Bakyt Karimova (BK) are responsible for the study concept and design, data collection and analysis, Microsoft editing, revising the manuscript for important intellectual content, and final revision before publication.
Mukhit Sarsembayev (MS) and Gulmira Mukhambetalyeva (GM) are responsible for the study design, collection of data, Microsoft editing and drafting, update of references, and final revision before publication.
Corresponding author
Ethics declarations
Ethical Approval
The study was approved by the ethical committees of the Obstetrics and Gynecology Departments of Ahmadi Hospital, Kuwait and West Kazakhstan Marat Ospanov Medical University, Kazakhstan (Approval Number: OB_09012_18) and registered under the trial number ACTRN12618001483246.
Consent to Participate
Participants were included in this study after informed consents in accordance with the Declaration of Helsinki.
Consent for Publication
Informed consents were taken from participants to use their data for scientific activity and publication.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Abdelazim, I.A., Shikanova, S., Karimova, B. et al. Heme-Iron Optifer Versus Intravenous Iron/Ferosac in Treatment of Iron Efficiency Anemia During Pregnancy. SN Compr. Clin. Med. 3, 1344–1349 (2021). https://doi.org/10.1007/s42399-021-00891-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-021-00891-7