Abstract
Rare but severe central nervous system complications associated with vitamin B deficiency, such as Wernicke encephalopathy, Korsakoff syndrome, and Marchiafava–Bignami disease, have been reported as a result of neurotoxicity related to alcohol use. Their pathophysiologic mechanisms remain unclear, particularly for Marchiafava–Bignami disease. These conditions remain underdiagnosed due to the non-specificity of their presenting symptoms and a lack of specific tests or diagnostic criteria. From a prognostic perspective, it is beneficial to start vitamin B complex supplementation before a definitive diagnosis. The mechanisms of thiamine deficiency–induced cell damage in Wernicke encephalopathy have been elucidated but remain unclear in Marchiafava–Bignami disease. We hypothesize that the mechanisms of Wernicke encephalopathy and Marchiafava–Bignami disease are different. Cytokine-mediated cytotoxic edema is a pathophysiological mechanism in Marchiafava–Bignami disease, in addition to vitamin B1 deficiency–related mechanisms. Although the amnesia of Korsakoff syndrome has generally been considered irreversible, recent studies have shown an improvement of cognitive function after memory rehabilitation. Alcohol-related central nervous system disorders associated with vitamin B deficiency occur in alcoholics. Rapid diagnosis and treatment of Wernicke encephalopathy and Marchiafava–Bignami disease have a profound effect on patient prognosis and prevent progression to Korsakoff syndrome. The newly proposed pathophysiological hypothesis of Marchiafava–Bignami disease may be useful for disease management in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The effects of alcohol on the central nervous system are classified as acute (those associated with increased blood levels of alcohol) or chronic (those associated with prolonged alcohol exposure). Similarly, the onset of alcoholism is classified either as acute or as chronic (Table 1). Alcohol easily crosses the blood–brain barrier and causes damage to neurons and glial cells, resulting in central nervous system pathology. The pathophysiologic mechanisms of alcohol-induced neuronal damage involve cellular energy metabolism, neurotransmission, and synaptogenesis in a complex fashion. Among the central nervous system disorders that occur in alcoholics, rare but severe complications associated with vitamin B deficiency, such as Wernicke encephalopathy (WE), Korsakoff syndrome (KS), and Marchiafava–Bignami disease (MBD), have been reported. These are thought to result from neurotoxicity due to chronic alcohol use. Vitamin B deficiencies, primarily thiamine (also known as vitamin B1), and malnutrition are common in chronic alcoholics [1]. Thiamine is an essential nutrient required by all tissues, including the brain. Because the human body cannot synthesize thiamine, it must be ingested. Alcohol can impair thiamine ingestion, metabolism, absorption, storage, and utilization: alcohol is high in calories, which reduces hunger and promotes malnutrition; alcohol metabolism requires thiamine pyrophosphate (a coenzyme in energy metabolism); alcoholic gastroenteritis impairs the absorption of thiamine; alcoholic liver disease reduces thiamine storage in the liver; and alcohol may impair thiamine utilization [2]. However, the pathophysiologic role of alcohol in these processes remains unclear. This article focuses on the diagnosis, pathophysiology, clinical examination, and treatment of WE, MBD, KS, and dementia, which frequently occur in elderly alcoholics.
Wernicke Encephalopathy
In an alcoholic patient who presents with impaired consciousness, WE should be in the differential diagnosis [3]. Alcoholics may become afflicted by Wernicke–Korsakoff syndrome, which is characterized by a transition to chronic dementia and KS. The cause of WE is thiamine deficiency due to impaired thiamine intake, storage, and consumption, which is common among alcoholics. The meal itself is often small among alcoholics, or the intake is unbalanced, such as a small snack of alcohol. When combined with alcohol-associated malabsorption, such as chronic pancreatitis, thiamine is not properly absorbed. Thiamine is stored in the liver, but alcohol-associated hepatic dysfunction reduces its storage capacity. In addition, since thiamine is required for alcohol metabolism, metabolic consumption of thiamine is relatively increased in alcoholics. The amount of thiamine required depends on the quantity of alcohol, glucose, and calories consumed. Typically, the stored amount of thiamine is small and quickly depleted. Thiamine is stored in the liver as the metabolically active form, thiamine pyrophosphate, in an amount ranging between 20 and 30 mg. It is depleted within approximately 18–20 days. In addition, magnesium is necessary to convert thiamine to thiamine pyrophosphate and serves as a cofactor for thiamine-dependent enzymes and neurochemical transmission [4]. In alcoholics, magnesium may be deficient due to diarrhea. Furthermore, alcohol itself suppresses thiamine pyrophosphate production, resulting in thiamine deficiency. In some alcoholics, the activity of transketolase, an enzyme involved in thiamine metabolism, is reduced; approximately 20% of heavy alcohol drinkers have an abnormal decrease in transketolase activity, which is thought to be involved in the development of Wernicke–Korsakoff syndrome [5].
Polymorphisms related to the gamma-aminobutyric acid (GABA) receptor are also associated with alcoholism and KS [6]. In addition, mutations of the thiamine transporter (SLC19A3) and thiamine pyrophosphokinase (L28S) genes have been associated with WE [7, 8]. Furthermore, genetic variations in alcohol metabolism, although not a direct cause, may be a risk factor for WE.
Thiamine deficiency is not unique to alcoholics. Non-alcoholic Wernicke–Korsakoff syndrome may occur in the setting of gastrointestinal disorder, repeated vomiting and diarrhea, leukemia, pregnancy, anorexia due to mental illness, other eating disorders, malnutrition, excessive nutritional supplementation, limited eating preferences, acquired immunodeficiency syndrome, dialysis, and starvation. Thiamine deficiency may also be iatrogenic in a hospital setting if thiamine is not administered with fluid replacement.
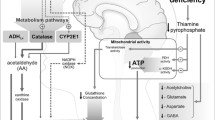
Thiamine is an important coenzyme for carbohydrate metabolism; its deficiency significantly reduces pyruvate dehydrogenase and α-ketoglutarate dehydrogenase activity in the tricarboxylic acid cycle as well as transketolase activity in the pentose phosphate pathway [9]. Pyruvate dehydrogenase and α-ketoglutarate dehydrogenase are thiamine-dependent enzymes that generate adenosine triphosphate (ATP); a reduced activity of these enzymes causes a decrease in ATP production, resulting in neuronal damage and lactic acidosis due to accumulation of pyruvate, which is converted to lactate. In addition, pyruvate dehydrogenase is necessary to generate myelin. Because α-ketoglutarate dehydrogenase influences the level of the neurotransmitters glutamate, GABA, and aspartate, inhibition of α-ketoglutarate dehydrogenase leads to glutamate accumulation and decreased GABA and aspartic acid (Fig. 1). The thiamine-dependent enzyme transketolase is responsible for the degradation of glucose via the pentose phosphate pathway. Glucose-6-phosphate is modified by transketolase and divided into two intermediates: ribose-5-phosphate and nicotinamide adenine dinucleotide phosphate (NADPH). Ribose 5-phosphate serves as a precursor to nucleotide and histidine biomolecules, which are essential for cell composition [10]. NADPH plays a role in protecting against reactive oxygen species toxicity and functions in the biosynthesis of fatty acids and steroids [11]. Thus, reduced transketolase activity interferes with these essential biochemical processes (Fig. 1). N-Methyl-d-aspartate (NMDA) receptor–mediated excitotoxicity has also been implicated in WE [12]. In summary, disruption of thiamine-mediated energy metabolism in the brain leads to encephalopathy, resulting from glutamate accumulation, decreased GABA and aspartic acid, NMDA receptor–mediated abnormal neuronal excitation, and oxidative stress (neuronal damage due to active oxygen) (Fig. 1). A recent study has reported that serum growth differentiation factor 15 (GDF15) levels are elevated in patients with thiamine deficiency as with mitochondrial disorders [13]. GDF15 is a cytokine released in response to various stressors. As abovementioned, WE is primarily caused by pathogenic effects of thiamine deficiency, which lead to mitochondrial dysfunction [12]. The mitochondrial dysfunction and other concomitant cellular responses, such as increased reactive oxygen species generation and increased lactate production, might induce intracellular GDF15 expression, which result in increased blood GDF15 levels in patients with WE as with mitochondrial disorders (Fig. 1) [14, 15].
The four thiamine-dependent enzymes and the role of thiamine in various enzymatic pathways. The thiamine-dependent enzyme transketolase is an important enzyme in the breakdown of glucose via the pentose phosphate pathway. Glucose-6-phosphate is cleaved into ribose-5-phosphate and nicotinamide adenine dinucleotide phosphate (NADPH) by transketolase. The thiamine-dependent enzymes pyruvate dehydrogenase and α-ketoglutarate dehydrogenase contribute to the metabolism of glucose and the tricarboxylic acid cycle; their main role is to generate adenosine triphosphate (ATP). The reduced activity of these enzymes from thiamine deficiency causes a decrease in ATP production resulting in neuronal damage. The tricarboxylic acid cycle and α-ketoglutarate dehydrogenase influence the maintenance of the levels of the neurotransmitters glutamate, gamma-aminobutyric acid (GABA), and aspartate. This sort of pathological pathways might lead to the mitochondrial dysfunction and neuronal damage, resulting in increased blood growth differentiation factor 15 (GDF15) levels in patients with thiamine deficiency. TTP, thiamine pyrophosphate
Although alcohol use is not essential, based on these mechanisms, alcoholics are predisposed to develop Wernicke encephalopathy due to the following: (1) thiamine deficiency due to poor food intake, (2) thiamine malabsorption due to gastrointestinal disorders related to alcohol use, (3) suppression of thiamine pyrophosphate production, (4) a relative increase in thiamine requirement due to alcohol metabolism, and (5) impaired thiamine storage capacity and metabolic breakdown [16].
Pathologically, symmetric neuronal damage occurs in the third periventricular gray matter (particularly the medial thalamic nucleus), periaqueductal gray matter, fourth periventricular gray matter, mammillary body, tetrahedral body, and middle cerebellum. The main lesion of WE is observed in the mammillary bodies, which are involved in up to 80% of cases and where vascular stasis and bleeding are observed on magnetic resonance imaging (MRI) (Fig. 2) [17]. Histologically, capillary endothelial cell proliferation, vascular hyperplasia, and gliosis are visualized.
Wernicke encephalopathy. a Axial fluid-attenuated inversion recovery (FLAIR) imaging of the brain shows symmetric, high-intensity signal abnormalities in the mammillary bodies and midbrain. b Axial FLAIR imaging shows symmetric, high-intensity signal abnormality around the third ventricle. c Axial FLAIR imaging shows symmetric, high-intensity signal abnormalities in the thalami. d Coronal FLAIR imaging shows symmetric, high-intensity signal abnormalities in the mammillary bodies
Approximately 80% of WE cases will transition to KS, but the number of severe cases has been reduced by a better understanding of the disease and early treatment [18]. However, only approximately 15% are diagnosed before death [19]. The frequency of WE is not very common in the general emergency setting, but if suspected, appropriate treatment should be administered early. The differential diagnosis of acute impaired consciousness disorder is wide and includes head trauma, hepatic encephalopathy, complicated cerebrovascular disorders, heavy alcohol consumption (drunkenness, dehydration, electrolyte abnormalities), hypoglycemia, ketosis, alcohol withdrawal, and zinc, folate, and magnesium deficiency (Table 1). In particular, magnesium deficiency directly affects the condition of WE because magnesium is a cofactor for thiamine-dependent enzymes and neurochemical transmission [4]. First, computed tomography should be performed to evaluate the possibility of head trauma. Traumatic subarachnoid hemorrhage and the presence of a subdural hematoma are urgent diagnoses and require a specialized neurosurgical treatment. The classic triad of WE consists of mental status change, oculomotor dysfunction, and gait ataxia (Table 2). In addition to the classic triad, stupor or coma, hypotension, and hypothermia are prominent findings in unsuspected patients [20]. Absence of one or more of the classic symptoms likely leads to underdiagnosis. In one series, WE was diagnosed premortem in only 26 of 131 patients whose brains revealed chronic WE lesions, yet all signs of the classic triad were recorded in 17%; none was recorded in 19% [20]. Currently, the clinical diagnosis of WE is based on the Caine criteria, which specify that one of the following two conditions must be satisfied: malnutrition, eye movement disorder, cerebellar ataxia, and consciousness disorder [21]. Caine et al. reported that in 106 patients with pathologically confirmed WE, the sensitivity of these criteria was 85%, as compared to a sensitivity of only 22% in those who presented with the classic triad [21]. However, in an actual clinical setting, it can be difficult to accurately determine the presence of an eye movement disorder or cerebellar ataxia. Patients have a variety of acute symptoms and are unlikely to exhibit typical symptoms of WE. Moreover, they may not be able to follow instructions. The most common clinical feature of WE is a change in consciousness, which may range from apathy and mild cognitive impairment to coma [22]. Thus, it is important to be suspicious and notice malnutrition. Blood thiamine level is essential for a definitive diagnosis of WE, but clinicians should not hesitate to treat before the result is obtained. In addition, alcoholics may develop WE even if the thiamine level is normal; alcohol suppresses the production of thiamine pyrophosphate, the activated form of thiamine (Fig. 1). In other words, alcoholics are at risk for developing WE even if there is no significant decrease in the blood thiamine level due to the effect of alcohol itself.
Brain MRI is useful for the diagnosis of WE. In approximately two-thirds of cases, it shows an area of high signal intensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) images in the mammillary bodies, thalamus, hypothalamus, and the area around the fourth ventricle [22]. In atypical cases, lesions may be found in the cerebellum, cranial nerve nuclei, dentate nuclei, caudate, red nuclei, splenium, and cerebral cortex [23]. Hyperintense lesions in the thalamus and mammillary bodies on FLAIR images are specific for WE, probably reflecting thalamic and mammillary body vascular stasis and bleeding; however, these findings have low sensitivity pathologically (Fig. 2). Some MRI studies have reported frontal lobe atrophy and enlargement of the lateral, third, and fourth ventricles in WE, but these findings are commonly found in alcoholics and have low specificity. In addition, MRI contrast enhancement of the mammillary bodies was reported to be more typical in patients with the alcohol abuse group than in patients with the no alcohol abuse group, and there was a statistically positive correlation between the contrast-enhanced MRI of the mammillary bodies and the alcohol abuse group [24].
The treatment of WE is high-dose thiamine [2]. Dramatic improvement can be seen if treated early; if not, the disease can become severe. Prompt administration of thiamine has been reported to prevent progression of WE to irreversible KS [25]. Mainly, thiamine hydrochloride (500 mg, 3 times a day for 5 days), other vitamins (vitamins B2, B6, and B12, nicotinic acid, and folate), an electrolyte/sugar infusion, and anticonvulsants are administered, given over 30 min [2, 26]. The plasma half-life of thiamine is only 96 min; thus, thiamine appears to be more effective when administered three times a day compared to a single daily dose [27]. However, although there are several randomized controlled trial (RCT) studies in the past, the evidences of thiamine dose, frequency, route, and duration of administration for the treatment of WE are still insufficient [28]. As an expert opinion, thiamine is recommended to be given slowly and intravenously [2, 26]. Therefore, treatment recommendations for WE may change depending on future RCTs. In addition, because alcoholics often have malabsorption due to a gastrointestinal disorder, the initial treatment with thiamine should be given intravenously instead of orally. Glucose administration before thiamine administration is contraindicated because this increases the demand for thiamine in the brain. Since the safety of thiamine administration is high, it should be administered before results of thiamine blood concentration are known; initial treatment should be aggressive in patients with suspected WE. Delay could result in KS. Symptoms of WE often improve in a few weeks after early appropriate treatment and cerebral oculomotor palsy may remit within hours; ataxia may improve within a few days and mental symptoms within a few weeks. However, residual deficits in many patients were observed in the largest cohort study as follows [18]. While gaze palsies recovered completely in most cases, permanent horizontal nystagmus was found in 60% of WE patients. Although approximately 40% of WE patients recovered from ataxia, other patients had deficits ranged from inability to walk at all to a wide-based, slow, shuffling gait. As the symptoms of acute encephalopathy improved, deficits in learning and memory became more apparent. Only approximately 20% of WE patients recovered completely or substantively, and the remainder had a permanent amnestic syndrome (KS).
Korsakoff Syndrome
KS is an alcohol-related dementia characterized by anterograde amnesia, disorientation, and speech. Patients not treated with thiamine typically transition to chronic KS after onset of WE [29]. However, only approximately 18% of patients diagnosed with KS by autopsy had a diagnosis of WE [30]. This implies that WE is difficult to diagnose. The cause of WE progression to KS is not well understood. Progression is thought to be influenced by the amount of drinking, duration of drinking, and patient age. Pathologically, an impaired Papez circuit, which is an important neural circuit in memory, is thought to be involved in the appearance of amnesia. This circuit is composed of the following neural pathway: hippocampal formation (subiculum) → fornix → mammillary bodies → mammillothalamic tract → anterior thalamic nucleus → cingulum → entorhinal cortex → hippocampal formation. Tissue damage is found at a high frequency in the mediodorsal thalamic nucleus, which is associated with the frontal lobe and amygdala and thought to be involved in the pathology of KS [31]. However, evidence also supports that the anterior thalamic nucleus, a component of the Papez circuit, is the critical lesion site for memory disorder in KS [32]. Histologically, bleeding and spongy changes are observed in the acute phase; in the chronic phase, astrocyte swelling, reduction of myelin, an increased number of reactive astrocytes, and blood vessel hyperplasia are seen.
Symptoms of KS include cognitive and behavioral abnormalities, disorientation, impaired memory, and speech disorder, but there are no clear diagnostic criteria. The memory impairment includes both anterograde and retrograde amnesia. Recent memory is significantly impaired, whereas memory of more remote time periods (such as childhood) is better preserved [33]; semantic and procedural memories also remain relatively preserved. Cognitive functioning other than memory may be impaired, including executive function, visual perception, and working memory [34]. Executive deficits have been recognized in 80% of KS patients and involve various executive processes, including higher-order organization assessment, response inhibition, response generation, working memory updating, planning, cognitive flexibility, and concept shifting [35, 36]. In addition, patients exhibit a complete lack of insight and reduced spontaneous activity, or apathy. Two types of verbal utterances are frequently observed in KS patients: fantastic confabulations and expressions of embarrassment of their impaired memory. Both types are recognized throughout the course of the disease, but the expressions of embarrassment may be more common in the early stages.
Cognitive function tests and MRI of the brain are used to diagnose KS. Frontal lobe atrophy and increased ventricle size (lateral ventricles, third and fourth ventricles) are frequently observed, but this is a general finding in alcoholics of low specificity to KS (Fig. 2). Brain MRI in many alcoholic patients with KS shows atrophy of both gray and white matter, which is thought to be indicative of the widespread neurotoxic effect of chronic alcohol consumption; however, this is also observed in those without KS [37]. More specific MRI findings of Wernicke–Korsakoff syndrome include changes in the mammillary bodies, thalami, tectal plate, and periaqueductal area, but their sensitivity is only 53% [38]. Neuropsychological testing of KS patients may show impaired memorization with little disability of intellectual function; on the other hand, the Wechsler Adult Intelligence Scale may not show a significant impairment. Tests that reflect frontal lobe function, such as the Wisconsin Card Sorting Test, may point out an impediment [39]. The frequency of transition from WE to KS is reportedly high (approximately 80%) [18]. After transition to KS, the symptoms typically do not improve, but they may improve to some extent [40]. At present, there is no effective pharmacological treatment for KS after acute phase treatment with vitamin and electrolyte replacement other than alcohol abstinence [41]. However, there is increasing evidence that memory rehabilitation may be beneficial [42].
Marchiafava–Bignami Disease
MBD is a rare neurological disease usually associated with chronic alcoholism that is characterized by neurodegeneration of the corpus callosum and may involve the adjacent subcortical white matter. MBD is occasionally observed in patients with chronic malnutrition even in the absence of alcohol addiction [43, 44]. Its etiology remains unclear, but a combination of alcohol-induced neurotoxicity and vitamin B complex deficiency has been hypothesized [45]. The possible mechanisms involved include cytotoxic edema, breakdown of the blood–brain barrier, demyelination, and necrosis. In the early stage of the disease, cytotoxic edema may play a dominant role, as evidenced by hyperintense regions on diffusion-weighted imaging (DWI) with reduced diffusivity on apparent diffusion coefficient (ADC) mapping in the splenium of the corpus callosum on brain MRI; these regions are often reversible (Fig. 3). Interestingly, similar cytotoxic edema is found on brain MRI in a wide variety of diseases and conditions [46]. In addition, the reversal of MRI changes, particularly on DWI, can be a prognostic indicator of clinical recovery [47]. Recently, these lesions have been called “cytotoxic lesions of the corpus callosum” [48]. Cytokine-mediated cytotoxic edema in the splenium of the corpus callosum has been suggested as the pathophysiological basis for this radiologic finding, which may be secondary to several disorders, including MBD. Furthermore, since thiamine has been reported to reduce the production of cytokines, such as TNF-α and IL-6, its deficiency results in enhanced cytokine release [49]. Recent studies have reported that patients who overconsume alcohol have a higher level of pro-inflammatory cytokines, such as IL-6, IFN-γ, and MCP-1, which suggests increased systemic inflammatory activity. In addition, the microglia expression of IL-1β, which induces activation of aquaporin 4 (AQP4) in animal experiments, progressively increases with the duration of alcohol consumption [50, 51]. Viewing these results together, a pathophysiological hypothesis for MBD has been formulated [46]. First, a combination of alcohol-induced neurotoxicity and vitamin B complex deficiency induces a release of several cytokines associated with inflammation. Endothelial damage causes activation of microglia that further accelerates the release of these cytokines. IL-1 can induce glutamate uptake in astrocytes, where the glutamate reacts with ammonia to form glutamine, which is transported to the extracellular fluid and taken up by neurons; this process continues, thus increasing extracellular glutamine. Intracellular ATP depletion, which results in mitochondrial dysfunction and oxidative stress, is caused by activation of the glutamate–glutamine cycle. The excitotoxic action of NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), and AQP4 receptors is triggered through a complex cell–cytokine mechanism that results in an influx of water into both astrocytes and neurons, which leads to cytotoxic edema, characterized by cellular swelling due to intracellular accumulation of fluid and Na+ (Fig. 4). In addition, thiamine deficiency itself can cause accumulation of α-ketoglutarate by inhibiting α-ketoglutarate dehydrogenase, leading to activation of the glutamate–glutamine cycle. The lesions in MBD may be primarily observed in the splenium of the corpus callosum due to the abundant blood flow in this region, causing increased susceptibility to cytotoxic edema from cytokines released into the bloodstream; the splenium of the corpus callosum is supplied by both the anterior circulation and the posterior circulation, unlike the remainder of the corpus callosum, which is solely supplied by the internal carotid artery of the anterior circulation [52, 53].
MRI studies of Marchiafava–Bignami disease (a, b, and c). MRI studies at onset: diffusion-weighted imaging (DWI) show hyperintensity in the splenium of the corpus callosum (arrowhead) (a) with corresponding hypointensity on apparent diffusion coefficient (ADC) imaging (b). Axial fluid-attenuated inversion recovery (FLAIR) imaging shows no signal abnormalities (c). MRI studies at 2-week follow-up (d, e, and f). DWI shows a normal signal in the splenium of the corpus callosum (d) with corresponding isointensity on ADC imaging (e). There is no abnormal signal on FLAIR imaging (f). The photographs of brain MRI were reprinted and modified from the reference with permission from the author [[44], Fig. 1, Creative Commons CC BY]
Pathophysiological mechanism of Marchiafava–Bignami disease (cytokine-mediated cytotoxic edema). A combination of alcohol-induced neurotoxicity and vitamin B complex deficiency induces the release of several cytokines associated with inflammation. Endothelial damage causes the activation of microglia that further accelerates the release of these cytokines that can also induce astrocytes to take up glutamate that reacts with ammonia to form glutamine; glutamine is then transported to the extracellular fluid where it is taken up by neurons and then the process restarts, thus increasing extracellular glutamine. The excitotoxic action of receptor activation is triggered through a complex cell–cytokine mechanism that results in an influx of water into both astrocytes and neurons, which leads to cytokine-mediated cytotoxic edema. In addition, thiamine deficiency itself can cause accumulation of α-ketoglutarate by inhibiting α-ketoglutarate dehydrogenase, leading to activation of the glutamate–glutamine cycle. The figure is cited by modifying from the reference with permission from the author [[46], Fig. 4, Creative Commons CC BY]
Although the clinical features may be quite variable and non-specific, MBD should be suspected in patients with chronic alcohol abuse or malnutrition who exhibit neurological symptoms. Symptoms of MBD include dementia, cognitive impairment, altered mental status, limb hypertonia, delirium/coma, spasticity, dysarthria, pharyngalgia, ataxia, gait abnormalities, seizures, confusion, headache, somnolence, dizziness, desynchronization of bilateral symmetric movements, agraphia, apraxia, dyspraxia, and anomia lasting for several weeks [54, 55]. The course of the disease may be acute, subacute, or chronic and is marked by dementia, spasticity, dysarthria, and inability to walk. Patients may fall into coma and die, survive for many years in a demented condition, or occasionally recover [56]. Establishing the definitive diagnosis of MBD at an early stage is essential; thiamine administration within 2 weeks after symptom onset results in improved outcome compared to delayed treatment. In addition, timely treatment with thiamine and vitamin B complex can reverse the disease [56]. Serial MRI has demonstrated complete disappearance of lesions after early diagnosis and treatment (Fig. 3) [57].
Conclusion
In this review, we have summarized the current knowledge of three alcohol-related central nervous system disorders associated with vitamin B deficiency: WE, KS, and MBD. The understanding of these diseases has been advancing as increased data from humans and animal models has become available. However, they remain underdiagnosed, mainly due to the non-specificity of their symptoms. The lack of specific tests and clear diagnostic criteria leads to difficulty in making their diagnosis. From a prognostic perspective, it is beneficial to start vitamin B complex supplementation before a definitive diagnosis. Although the mechanisms of thiamine deficiency–induced cell damage in WE have been elucidated, they remain unclear in MBD. We hypothesize that the mechanisms of WE and MBD are different: cytokine-mediated cytotoxic edema appears to be a pathophysiological mechanism involved in MBD, in addition to vitamin B1 deficiency–related mechanisms. Due to the complex and often overlapping clinical features of these diseases, thorough knowledge of their pathophysiology and individual clinical presenting symptoms is necessary to make a correct diagnosis. The newly proposed hypothesis of MBD pathophysiology may be useful for disease management in the future. Rapid diagnosis and treatment of WE and MBD have a profound beneficial effect on patient prognosis and can prevent progression of WE to KS. Clinicians must keep this in mind; a high level of suspicion is key to improving patient prognosis. Classically, amnesia was generally considered irreversible after progression to KS. However, more recent studies have shown that memory rehabilitation, including memory compensation techniques and error-free learning strategies, may improve cognitive function.
Data Availability
The data used and analyzed during the current article are available from the corresponding author on reasonable request.
Abbreviations
- WE:
-
Wernicke encephalopathy
- KS:
-
Korsakoff syndrome
- MBD:
-
Marchiafava–Bignami disease
- GABA:
-
gamma-aminobutyric acid
- ATP:
-
adenosine triphosphate
- NADPH:
-
nicotinamide adenine dinucleotide phosphate
- NMDA:
-
N-methyl-d-aspartate
- GDF15:
-
growth differentiation factor 15
- MRI:
-
magnetic resonance imaging
- FLAIR:
-
fluid-attenuated inversion recovery
- RCT:
-
randomized controlled trial
- DWI:
-
diffusion-weighted imaging
- ADC:
-
apparent diffusion coefficient
- AQP4:
-
aquaporin 4
- AMPA:
-
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
References
Keil VC, Greschus S, Schneider C, Hadizadeh DR, Schild HH. The whole spectrum of alcohol-related changes in the CNS: practical MR and CT imaging guidelines for daily clinical use. Rofo. 2015;187(12):1073–83.
Sechi G, Serra A. Wernicke’s encephalopathy: new clinical settings and recent advances in diagnosis and management. Lancet Neurol. 2007;6(5):442–55.
Chandrakumar A, Bhardwaj A, ’t Jong GW. Review of thiamine deficiency disorders: Wernicke encephalopathy and Korsakoff psychosis. J Basic Clin Physiol Pharmacol. 2018;30(2):153–62.
Lonsdale D. Thiamine and magnesium deficiencies: keys to disease. Med Hypotheses. 2015;84(2):129–34.
Alexander-Kaufman K, Harper C. Transketolase: observations in alcohol-related brain damage research. Int J Biochem Cell Biol. 2009;41(4):717–20.
Koulentaki M, Kouroumalis E. GABA(A) receptor polymorphisms in alcohol use disorder in the GWAS era. Psychopharmacology. 2018;235(6):1845–65.
Zeng WQ, Al-Yamani E, Acierno JS Jr, Slaugenhaupt S, Gillis T, MacDonald ME, et al. Biotin-responsive basal ganglia disease maps to 2q36.3 and is due to mutations in SLC19A3. Am J Hum Genet. 2005;77(1):16–26.
Huang W, Qin J, Liu D, Wang Y, Shen X, Yang N, et al. Reduced thiamine binding is a novel mechanism for TPK deficiency disorder. Mol Gen Genomics. 2019;294(2):409–16.
Harrigan GG, Maguire G, Boros L. Metabolomics in alcohol research and drug development. Alcohol Res Health. 2008;31(1):26–35.
Coleman JP, Smith CJ. X Pharm: the comprehensive pharmacology reference. 2007:1–6. https://doi.org/10.1016/b978-008055232-3.60227-2.
Jiang F, Guo N, Dusting GJ. Modulation of nicotinamide adenine dinucleotide phosphate oxidase expression and function by 3′,4′-dihydroxyflavonol in phagocytic and vascular cells. J Pharmacol Exp Ther. 2008;324(1):261–9.
Desjardins P, Butterworth RF. Role of mitochondrial dysfunction and oxidative stress in the pathogenesis of selective neuronal loss in Wernicke’s encephalopathy. Mol Neurobiol. 2005;31(1–3):17–25.
Miyaue N, Yabe H, Nagai M. Serum growth differentiation factor 15 levels and clinical manifestations in patients with thiamine deficiency. Neurol Clin Neurosci. 2020;8(5):245–50. https://doi.org/10.1111/ncn3.12390.
Yatsuga S, Fujita Y, Ishii A, Fukumoto Y, Arahata H, Kakuma T, et al. Growth differentiation factor 15 as a useful biomarker for mitochondrial disorders. Ann Neurol. 2015;78(5):814–23.
Fujita Y, Taniguchi Y, Shinkai S, Tanaka M, Ito M. Secreted growth differentiation factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatr Gerontol Int. 2016;16(Suppl 1):17–29.
Sivolap YP. The current state of S. S. Korsakov's concept of alcoholic polyneuritic psychosis. Neurosci Behav Physiol. 2005;35(9):977–82.
Charness ME, DeLaPaz RL. Mamillary body atrophy in Wernicke’s encephalopathy: antemortem identification using magnetic resonance imaging. Ann Neurol. 1987;22(5):595–600.
Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome and related neurologic disorders due to alcoholism and malnutrition. 2nd ed. Philadelphia: FA Davis; 1989. https://doi.org/10.1192/S0007125000006243.
Thomson AD, Cook CC, Touquet R, Henry JA, Royal College of Physicians, London. The Royal College of Physicians report on alcohol: guidelines for managing Wernicke’s encephalopathy in the accident and Emergency Department. Alcohol Alcohol. 2002;37(6):513–21.
Harper CG, Giles M, Finlay-Jones R. Clinical signs in the Wernicke-Korsakoff complex: a retrospective analysis of 131 cases diagnosed at necropsy. J Neurol Neurosurg Psychiatry. 1986;49(4):341–5.
Caine D, Halliday GM, Kril JJ, Harper CG. Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry. 1997;62(1):51–60.
Galvin R, Bråthen G, Ivashynka A, Hillbom M, Tanasescu R, Leone MA, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17(12):1408–18.
Liou KC, Kuo SF, Chen LA. Wernicke encephalopathy with atypical magnetic resonance imaging. Am J Emerg Med. 2012;30(9):2086.e1–3.
Zuccoli G, Gallucci M, Capellades J, Regnicolo L, Tumiati B, Giadás TC, et al. Wernicke encephalopathy: MR findings at clinical presentation in twenty-six alcoholic and nonalcoholic patients. AJNR Am J Neuroradiol. 2007;28(7):1328–31.
Sinha S, Kataria A, Kolla BP, Thusius N, Loukianova LL. Wernicke encephalopathy-clinical pearls. Mayo Clin Proc. 2019;94(6):1065–72.
Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44(9):911–5.
Tallaksen CM, Sande A, Bøhmer T, Bell H, Karlsen J. Kinetics of thiamin and thiamin phosphate esters in human blood, plasma and urine after 50 mg intravenously or orally. Eur J Clin Pharmacol. 1993;44(1):73–8.
Ambrose ML, Bowden SC, Whelan G. Thiamin treatment and working memory function of alcohol-dependent people: preliminary findings. Alcohol Clin Exp Res. 2001;25(1):112–6.
Day E, Bentham PW, Callaghan R, Kuruvilla T, George S. Thiamine for prevention and treatment of Wernicke-Korsakoff syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;2013(7):CD004033. https://doi.org/10.1002/14651858.CD004033.pub3.
Blansjaar BA, Van Dijk JG. Korsakoff minus Wernicke syndrome. Alcohol Alcohol. 1992;27:435–7.
Mitchell AS, Chakraborty S. What does the mediodorsal thalamus do? Front Syst Neurosci. 2013;7:37.
Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123(Pt 1):141–54.
Fama R, Marsh L, Sullivan EV. Dissociation of remote and anterograde memory impairment and neural correlates in alcoholic Korsakoff syndrome. J Int Neuropsychol Soc. 2004;10(3):427–41.
Kopelman MD, Thomson AD, Guerrini I, Marshall EJ. The Korsakoff syndrome: clinical aspects, psychology and treatment. Alcohol Alcohol. 2009;44(2):148–54.
Van Oort R, Kessels RP. Executive dysfunction in Korsakoff’s syndrome: time to revise the DSM criteria for alcohol-induced persisting amnestic disorder? Int J Psychiatry Clin Pract. 2009;13(1):78–81.
Brion M, Pitel AL, Beaunieux H, Maurage P. Revisiting the continuum hypothesis: toward an in-depth exploration of executive functions in Korsakoff syndrome. Front Hum Neurosci. 2014;8:498.
Pitel AL, Chételat G, Le Berre AP, Desgranges B, Eustache F, Beaunieux H. Macrostructural abnormalities in Korsakoff syndrome compared with uncomplicated alcoholism. Neurology. 2012;78(17):1330–3.
Covell T, Siddiqui W. Korsakoff Syndrome. 2019 May 5. StatPearls [Internet]. [cited 2020 May 16]. Treasure Island (FL): StatPearls Publishing; 2020. Available from http://www.ncbi.nlm.nih.gov/books/NBK539854/
Brokate B, Hildebrandt H, Eling P, Fichtner H, Runge K, Timm C. Frontal lobe dysfunctions in Korsakoff’s syndrome and chronic alcoholism: continuity or discontinuity? Neuropsychology. 2003;17(3):420–8.
Ridley NJ, Draper B, Withall A. Alcohol-related dementia: an update of the evidence. Alzheimers Res Ther. 2013;5(1):3. https://doi.org/10.1186/alzrt157 eCollection 2013.
Arts NJ, Walvoort SJ, Kessels RP. Korsakoff’s syndrome: a critical review. Neuropsychiatr Dis Treat. 2017;13:2875–90.
Svanberg J, Evans JJ. Neuropsychological rehabilitation in alcohol-related brain damage: a systematic review. Alcohol Alcohol. 2013;48(6):704–11.
Arbelaez A, Pajon A, Castillo M. Acute Marchiafava-Bignami disease: MR findings in two patients. AJNR Am J Neuroradiol. 2003;24(10):1955–7.
Tetsuka S, Kamimura T, Ohki G, Hashimoto R. Reversible lesion in the splenium of the corpus callosum in a patient with chronic alcoholism. J Gen Fam Med. 2020;21(3). https://doi.org/10.1002/jgf2.308.
Fernandes LMP, Bezerra FR, Monteiro MC, Silva ML, de Oliveira FR, Lima RR, et al. Thiamine deficiency, oxidative metabolic pathways and ethanol-induced neurotoxicity: how poor nutrition contributes to the alcoholic syndrome, as Marchiafava-Bignami disease. Eur J Clin Nutr. 2017;71(5):580–6.
Tetsuka S. Reversible lesion in the splenium of the corpus callosum. Brain Behav. 2019;9(11):e01440.
Oster J, Doherty C, Grant PE, Simon M, Cole AJ. Diffusion-weighted imaging abnormalities in the splenium after seizures. Epilepsia. 2003;44(6):852–4.
Galnares-Olalde JA, Vázquez-Mézquita AJ, Gómez-Garza G, Reyes-Vázquez D, Higuera-Ortiz V, Alegría-Loyola MA, et al. Cytotoxic lesions of the corpus callosum caused by thermogenic dietary supplements. AJNR Am J Neuroradiol. 2019;40(8).
Menezes RR, Godin AM, Rodrigues FF, Coura GME, Melo ISF, Brito AMS, et al. Thiamine and riboflavin inhibit production of cytokines and increase the anti-inflammatory activity of a corticosteroid in a chronic model of inflammation induced by complete Freund's adjuvant. Pharmacol Rep. 2017;69(5):1036–43.
Bjørkhaug ST, Neupane SP, Bramness JG, Aanes H, Skar V, Medhus AW, et al. Plasma cytokine levels in patients with chronic alcohol overconsumption: relations to gut microbiota markers and clinical correlates. Alcohol. 2019.
Pradier B, Erxlebe E, Markert A, Rácz I. Microglial IL-1β progressively increases with duration of alcohol consumption. Naunyn Schmiedeberg's Arch Pharmacol. 2018;391(4):455–61.
Kakou M, Velut S, Destrieux C. Arterial and venous vascularization of the corpus callosum. Neurochirurgie. 1998;44(1 Suppl):31–7.
Kahilogullari G, Comert A, Ozdemir M, Brohi RA, Ozgural O, Esmer AF, et al. Arterial vascularization patterns of the splenium: an anatomical study. Clin Anat. 2013;26(6):675–81.
Heinrich A, Runge U, Khaw AV. Clinicoradiologic subtypes of Marchiafava-Bignami disease. J Neurol. 2004;251(9):1050–9.
Rosa A, Demiati M, Cartz L, Mizon JP. Marchiafava-Bignami disease, syndrome of interhemispheric disconnection, and right-handed agraphia in a left-hander. Arch Neurol. 1991;48(9):986–8.
Hillbom M, Saloheimo P, Fujioka S, Wszolek ZK, Juvela S, Leone MA. Diagnosis and management of Marchiafava-Bignami disease: a review of CT/MRI confirmed cases. J Neurol Neurosurg Psychiatry. 2014;85(2):168–73.
Dong X, Bai C, Nao J. Clinical and radiological features of Marchiafava-Bignami disease. Medicine (Baltimore). 2018;97(5):e9626.
Acknowledgments
The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding author
Ethics declarations
Ethics Approval
This manuscript is a review article and does not contain any studies with human participants or animals performed by any of the authors and has included unidentifiable information. An informed consent was waived.
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Tetsuka, S., Hashimoto, R. Alcohol-Related Central Nervous System Disorders Associated with Vitamin B Deficiency. SN Compr. Clin. Med. 3, 528–537 (2021). https://doi.org/10.1007/s42399-021-00741-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-021-00741-6