Abstract
The purpose of this review is to summarize the advances and discoveries in the potential treatment with hormone analogs and some challenges still pending, beyond the classic treatment with corticosteroids. We conduct a review of the functions of hormones on hearing, in human and animal models, the presence of their most relevant receptors, and the desirable and undesirable effects for their therapeutic uses. Different hormones play a regulatory role in the development and maintenance of hearing. Hormone receptors in the ear have been identified over the years, which can be a support to use them as new therapeutic targets Moreover, their mediators, that include cells, neurotrophic factors, or other hormones, could be also useful for treating various hearing impairments The use of synthetic analogs could exert a therapeutic effect on hearing. Hormone therapy, in fact, can contribute positively to the treatment and prevention of various auditory pathologies, through the regeneration or protection. Considering that an optimal result has to be a garantee, it is a must to know their unwanted effects and contraindications. The use of hormones may protect, regenerate, and modulate multiple pathological conditions of the ear, but it would be necessary to standardize drug dosages, find alternative routes, and conduct prospective studies in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From the beginning of evolution, it seems clear that hormones were the first messengers, before a nervous system was established, responsible for the communication between cells or acting intracellularly modulating cellular functions. Therefore, it is logic that hormones play a key role in the development and functioning of many different structures of the human body, acting both endocrine as in auto/paracrine way. This is the case, for instance, of the ear. Although it is still little known, the ear is sensitive to the actions of various hormones, such as melatonin (MT) [1], somatostatin (SS) [2], or estradiol (E2) [3], which have a direct effect on the ear after binding to their specific receptors in the cochlea. Other hormones, such as progesterone (P), act indirectly on the ear functioning, modulating it [4].
The function of different hormones on the ear appears early, during the embryonic development, therefore playing a prominent role in the genesis of hearing, leading to a correct processing of the auditory function [5,6,7]. This is the reason by which some hormonal dysfunctions lead to auditory alterations. Similarly, metabolic disorders occurring as a consequence of hormonal alterations; hyperglycemia or hypothyroidism are clear examples, mainly when they appear in the early stages of neurodevelopment, damaging the cochlea because of the loss of hair cells (HC), the auditory receptors, and transducers [8, 9].
The cochlea has been considered as a gland that has an endocrine function, because it expressed a peptide similar to the corticotropin-releasing factor (CRF) responsible, among other functions, as a central neurotransmitter, of the induction of the expression and release of the pituitary adrenocorticotropic hormone (ACTH). As it is well known, ACTH induces the synthesis and release of corticosteroids in the adrenal gland, and it has been hypothesized that the cochlear release of the CRF-like factor would allow the cochlea to respond to cellular stress (noise, ototoxic drugs) [10, 11]. In fact, the use of corticosteroids for treating both middle and inner ear pathology is a standard procedure, with satisfactory results, for instance, in controlling sudden hearing loss or Menière’s disease (MD) [12].
According to these and other evidences, it is likely that hormonal therapies could be developed, aiming to protect or restoring a deficient or loss hearing function. Particularly in the case of sensorineural hearing loss (SNHL), the aim has to be not only to achieve the regeneration of cochlear HC, but also to make them functionally efficient [13]. In this review, we will analyze the current scientific evidence and future prospects of hormone therapy on hearing, beyond corticosteroid treatments.
Hormone Delivery Approaches in the Ear
The main disadvantage of a systemic treatment (either by oral, intramuscular, or intravenous administration) might be the appearance of side effects on other structures of the body, particularly when using hormones or drugs that exert a pleiotropic effect. To avoid the possibility of the appearance of these side effects, topical vehicles would allow a direct and effective delivery of the products of interest. The simplest form of medication delivery is through intratympanic injections, with or without tympanostomy tubes. An interesting option would be the use of osmotic mini-pumps, although in this case, doses are not controlled and like the conventional intratympanic treatment, the duration of its effect is too short. To avoid this inconvenience, hydrogels might be used, allowing the medication to remain in the ear and to be gradually released. Other potential vehicles would include the use of viral vectors, specifically targeted, although their use is still under development, with promising results in animal models despite anatomical difficulties [14].
Hearing and Sex Hormones
Androgen and alpha and beta estrogen receptors have been detected in animals and humans. Interestingly, the expression of these receptors has been found to be regulated in the sensory epithelium of the adult cochlea, suggesting that they have to play a physiological role [3, 15]. However, no progesterone receptors have been found at this level, although their effect (usually negative) on hearing has been demonstrated [16]. The presence of estrogen receptors in the spiral ganglion (SG) and HC suggests that E2 may influence hearing transmission, while receptors in the stria vascularis (SV) may affect the electrolyte balance of cochlear fluids [17]. In addition, estrogen receptors in cochlear blood vessels can influence auditory function by modulating blood flow [18]. E2 receptors (ER) α and β are located in different central auditory structures in mice, and their differential location suggests distinct roles in auditory processing [19], as it occurs with testosterone in other animal models [20]. Particularly, E2Rβ is an important factor for preventing age-related hearing loss (ARHL). E2Rβ knock-out mice (E2R-β-/-) present hearing loss at 1 year of age with absence of HC and loss of the entire spiral organ (SO), initiated in the basal gyrus of the cochlea, with a loss of many neurons. KO mice were deaf, compared with the wild type, which had severe hearing loss [21].
The question is, what might be the effects of sex hormone treatments on hearing? Animal models may guide us thereon. Estrogens seem to play a clear repairing role, both directly and by interacting with mediators such as epidermal growth factor (EGF) and transforming growth factor ß (TGFß) [22]. For instance, chronic treatment with E2 in ovariectomized mice produces molecular changes in central (inferior colliculus) and peripheral (cochlea) auditory pathway [23]. Downregulation of the mRNA from ERα in the cochlea and inferior colliculus may exert a direct effect on inhibiting estrogen-induced feedback from the transcription of ERα. The use of anti-estrogens in ovariectomized rats, on the other hand, generates a downregulation of the E2Rα in the SV marginal cells [24]. Separating mice into three groups that received estrogen/progesterone, estrogen alone, or placebo revealed significant hearing loss in those treated with combined therapy. This loss was observed in the high and medium frequency ranges (15–29 and 30–45 kHz) as soon as 2 months after starting treatment, evidenced by distortion product otoacoustic emissions (DPOAE) and auditory brainstem responses (ABR) [25]. Despite these data, Horner et al. (2007) [26] demonstrated in guinea pigs that long-term high-dose estrogens developed severe hearing loss, related to otosclerosis, in some animals. Histological examination revealed a thinning SV and loss of stereocilia bundles in the middle and apical cochlear portions. The explanation for this phenomenon can be attributed to the interaction of E2 with other regulatory hormones.
Several reports evaluate the role of sex steroids in human hearing, but confounding factors such as ARHL, noise-induced hearing loss (NIHL), and hormone replacement combination therapies limit the quality of the results. Estrogens and progesterone play an important role in the development of tinnitus and hyperacusis, which explains the greater predisposition of this symptom in women [27]. Cyclical changes in hearing thresholds depend on menstrual period, pregnancy, and menopause, related to estrogen and progesterone levels [28]. Older men have a 10–25 dB worse hearing loss at high frequencies than women of the same age, due to the protective role of estrogens in hearing [29]. Testosterone, on the other hand, is responsible for the worse otoacoustic emission (OAE) thresholds in males of any age; consequently, it decreases hearing sensitivity [30].
Currently, no treatments aimed specifically for improving hearing using sex hormones have been reported. In the premenopausal and peri-menopausal period, women undergoing hormone replacement therapy (HRT) of any type (estrogen alone or estrogen with progesterone) show an improvement in tinnitus [31] and a discreet improvement at low frequency hearing thresholds (3–5 dB) compared with those who did not receive HRT [32]. However, as we have described previously, some authors question this protective effect [25, 33]. Other studies point to an improvement only in those patients treated only with estrogen; however, this exclusive treatment is not out of threats, increasing the endometrial cancer risk [34, 35]. Endogenous alterations in estrogen and progesterone in situations such as premenstrual syndrome, pregnancy, or oral contraceptive use may cause worsening on the hearing in patients with Meniere’s disease (MD). The effect is directly due either to alterations in the hydroelectrolytic balance of these hormones or indirectly through the induction of hyperprolactinemia [36]. In Turner syndrome (45,X0) patients, estrogen levels decrease due to ovarian dysgenesis. In these patients, high-frequency hearing loss is subsequently added to sensorineural decline at the age of 35, leading to rapid hearing loss and therefore communication impairments. If estrogen replacement begins after 13 years of age, or if women are deficient in estrogen for more than 2 years, the risk of SNHL is also increased. These data, however, are not independent of age [37]. As we can see, results about sex hormones and hearing appear to be contradictory. The duration of treatment, basal levels of sex hormones, and their mediators, along with other factors, should be considered to validate the use of these sex steroids.
Concerning androgens, immune-mediated sensorineural hearing loss (IMSNHL) has a particularly higher incidence rate in women, and testosterone exerts beneficial effects on the modulation of the expression of this autoimmunity [38]. Chang et al. (2001) [39] showed that a group of mice treated with several subcutaneous injections of testosterone had better results than the IMSNHL control animals in relation to the degree of hearing loss (10 to 20 dB vs. 10 to 40 dB) and the incidence of hearing loss greater than 20 dB in the ABR results.
Tinnitus: Otoprotective Properties of Melatonin
Different hormones have been used to manage tinnitus. Among them, many reports describe the beneficious effect of melatonin (MT). However, it is inherently limited due to the subjectivity of this symptomatology. For its evaluation and therapeutic response, questionnaires might be useful, such as Tinnitus Handicap Inventory (THI), Tinnitus Severity Index (TSI), or Self-Rated Tinnitus (SRT). Several authors found that patients with high scores on these tests, with/without difficulty sleeping, benefit from MT treatment. In this regard, low doses of MT (3 mg, at night) have been shown to be useful for the treatment of tinnitus [40]. In addition, combination with some drugs appears to exert a synergistic effect. In a study conducted in patients divided into 4 treatment groups including placebo, tinnitus perception decreased by up to 81% in patients treated with sulpiride plus MT [41]. It also appears that the combination of MT and sulodexide, a highly purified mixture of glycosaminoglycans composed of low molecular weight heparin (80%) and dermatan sulphate (20%), has better results in central and peripheral tinnitus, according to the results on THI test and acufenometry thresholds [42]).
But what is the explanation for melatonin’s effectiveness in controlling tinnitus? Probably, because tinnitus is perceived worse in quiet environments, the role of MT in sleep regulation is the most feasible explanation [43]. In addition, MT has an anxiolytic effect and regulates microcirculation, reduces muscle tone, and has antioxidant and immunomodulatory effects, conditions that can potentially validate its effectiveness [44]. Moreover, endogenous MT plays an important role in the development of hearing. N-acetyltransferase (NAT) and hydroxy-indole-O-methyltransferase (HIOMT), melatonin-forming enzymes, are synthesized in the guinea pig cochlea [45]. The organ of Corti (OC) and the basilar membrane, as well as other minor structures such as the cochlear nerve, the spiral ligament, and the SV, express this hormone [45]. MT has been identified in the cochlear epithelium and vestibulocochlear nerve in the fetal sheep on the 40th day of gestation [46]. Other studies demonstrated MT expression in the cochlear nuclei of the human fetal brain through in vitro auto-radiography and in situ hybridization [1]. In addition, there is a relationship between the hearing threshold and serum MT, being low plasma levels of this hormone a significant factor in the development of high frequency (HL) hearing loss among the elderly. However, this relationship is not linear [47].
The antioxidant effect of MT may have other otoprotective effects that may allow more extensible use of this hormone. In fact, this effect is 350 times greater than other antioxidants to reduce free radicals [48]. An interesting report showed in an animal model that transtympanic administration of low doses of MT had a positive effect on cisplatin-induced ototoxicity, based on hearing thresholds and cell cultures. These results indicated that the group that received melatonin had lesser threshold shift in DPOAE and ABR at all frequencies, and less epithelial loss and more TNF staining on cell cultures [49]. Normal OAE reappeared within 5 days when gentamicin or tobramycin was administered in combination with MT, without interference with the antibiotic function of aminoglycosides [50]. In addition, one study showed that administration of MT after or even before ionizing radiation might prevent high frequency hearing loss [51]. Chen et al. (2018) [52] gave an explanation of its effectiveness. High doses of radiation caused an increase in the regulation of prestin, a protein present in the cochlea, mainly in the lateral membrane above the outer hair cells (OHC), and MT would control this abnormal expression. In the case of NIHL, it has been found a significant difference in the activity of malondialdehyde and erythrocytic glutathione peroxidase between guinea pigs treated with MT and those untreated, and hearing thresholds showed a parallel trend in noise-exposed animals [53]. The dose of MT was 20 mg/kg, administered intramuscularly 24 h before noise exposure, immediately before noise exposure, and once a day for 60 h, until noise exposure was complete. In another comparative study [54], rats were treated with MT before and while being exposed for 4 h/day to 110 dB noise over a 14-day period. After a 21-day follow-up, the rats experienced an improvement in their hearing function, especially in the third week. This was due to the ability to reduce the gene expression of c-fos and TNF-a, factors involved in OHC injury, taking as reference DPOAE and ABR. As we have described, doses used in animal models are comparatively higher than those used in human patients. Considering that MT has much broad-spectrum and safety, it would be advisable to carry out studies in humans at higher doses in order to validate its effect in subsequent studies.
Role of Other Hormones in Otoprotection
Oxytocin
In addition to its regulatory effect on childbirth, oxytocin (OT) has anti-inflammatory and antioxidant properties potentially useful on hearing protection. It has been theorized that these properties are explained by interfering with GABA signaling transduction, regulating oxygen consumption, glutathione, and NADPH, thus playing a neuroprotective role [55]. Such protection appears to be equally effective in intratympanic as intraperitoneal administration in rats exposed to cisplatin ototoxicity, decreasing hearing damage, although with greater reduction in the first case [56]. In humans, a pilot study assessed the effects in the short/medium term in patients with tinnitus, in a small group of 15 patients, treated with intranasal OT. The reduction on tinnitus perception according to the results of the THI and global condition improvement (GCI) questionnaires was strong [57]. Surprisingly, at the beginning of the study, one-third of treated patients experienced immediate improvement with the first dose, with tinnitus volume reduction to a half, although differences with placebo were modest when compared with long-term treatment. These effects can be particularly explained by the modulation that OT exerts on the cerebral amygdala, a structure that communicates with the auditory pathway and that is involved, among others, in the tinnitus perception. Although this is a promising result, it is only a pilot study that evaluates a subjective symptom and may therefore bias the results [57].
ACTH
The use of ACTH analogs in noise-exposed rats reports significantly better results on ABR thresholds than the control group due to their anti-inflammatory effects. This anti-inflammatory effect would be explained by direct inhibition through their MC1, MC3, and MC5 receptors on several inflammatory cells, while stimulating their down-mediators, aldosterone, and corticosteroids [58], but also because they induce the expression of mineralocorticoid receptors in the SV and other cochlear structures [59]. In fact, aldosterone has interesting effects on hearing. Hearing recovery has been shown in MRL/MpJ-Faslpr autoimmune mice. This recovery, as in a previous study of the same author with corticoids, seems to be related to the ability of aldosterone to restore cochlear ionic balances, regulating Na+ and K+ entries [60]. In humans, those subjects with elevated aldosterone levels have better hearing test in noise. Long-term treatment with aldosterone in middle-aged mice slows the progression of ARHL, through central and peripheral effects, in a group in which this hormone gradually decreases [61]. As counterproductive effects and according to animal models, aldosterone might induce endolymphatic hydrops suppressing the expression of the Af9 gene. This hormone can produce epigenetic changes through this gene, which affect the sodium epithelial channel (eNAC) [62]. However, this effect has not been observed in patients with MD [63].
Thyroid Hormones
Thyroid hormones play an important role in the development of the cochlea [6]. Two isoforms are differentiated from their receptor, TRβ1 and TRβ2. While the absence of both receptors generates early-onset hearing loss [64], the lack of TRβ1 is involved in a late-onset hearing loss with degeneration of HC [65]. Lack of thyroid transporters is also involved in the loss of HC [66]. In humans, the severity of hypothyroidism correlates with the degree of hearing loss. Hypothyroid patients treated with levothyroxine appear to improve hearing thresholds, although in less than half of cases and modestly, so a standardized use of this hormone in the general population is, a priori, unpromising [67]. Thyroid hormones are involved in the maturation of the middle ear ossicular chain, so metabolic alterations of these hormones would explain the predisposition to an increased incidence of otitis media in these subjects [68]. As noted above, one report showed that human treatment with levothyroxine discreetly improved hearing, and this included patients with conductive hearing loss [67].
Erythropoietin
Although previous reports place erythropoietin (EPO) as an ototoxic agent, by inducing vasoconstriction [69], various animal models showed that it protects the inner ear from ototoxic damage, observed through cell cultures and ABR. Thus, expression of Epo and Epo-R was detected by immunohistochemistry and dual immunofluorescence staining using polyclonal antibodies directed against Epo and Epo-R, followed by confocal laser scanning microscopy following Epo adenovirus vector infection [70]. In Epo-transgenic mice, lesser ABR threshold shifts after aminoglycoside-induced hearing loss were found, compared with the control group [71]. On a mouse model for DFNB12 (Cdh23(erl/erl)), results showed that EPO can significantly decrease the ABR thresholds in the mice as compared with those of the untreated mice, with significantly higher DPOAE amplitudes in the EPO-treated Cdh23(erl/erl) mouse group compared with the untreated groups. In fact, cell cultures showed that the loss of outer hair cell loss at middle through basal turns of cochleae was significantly lower [72]. Moreover, it seems that neuronal EPO overexpression would have a role in ARHL, observed in aged mice, preserving high frequencies [73]. The mechanism of action is not entirely clear, but it seems that reduces the loss of SG neurons and HC, inhibiting oxidative stress and apoptosis. Moreover, EPO is expressed in the SG, and its receptor, EPO-R, is diffusely expressed in different structures of the inner ear [74]. In humans, some reports showed hearing improvement in patients with renal failure [75] and neural maturation in preterm infants [76, 77]. Systemic administration of EPO exerts a very discrete effect, given that only 1% of EPO passes through to the cerebrospinal fluid and systemic EPO may increase cardiovascular and carcinogenic risk. Therefore, for its therapeutic potential, studies aiming at its direct application to the inner ear should be developed [71].
Neuroregeneration in Hormone Therapy: Growth Hormone and Associated Hormones
So far, we have talked about a protective rather than a regenerating role on hearing function. However, promising data may indicate that there is a potential hormone repairing function. Specifically, growth hormone (GH) plays a major role in the development of the inner ear. There is a high immunoreactivity GH and GHR in the otic vesicle of neural chickens on their third embryonic day (ED3) [5]. Smeti et al. (2012) [78] reported, through transcriptomic analysis of developing mice and adults, an 89× GH increase in cochlear sensory epithelia, activating signaling pathways through JAK2-STAT transcription pathways. In a controlled environment with aged mice and subsequent cochlea extraction for RNA microarray, analysis revealed that up to 116 genes were involved in upregulation or downregulation between younger and older animals. Of these, the most important were prolactin (up to 108.2 times regulated) and GH (up to 43.94 times regulated) [79]. This group of hormones has shown in vivo and in vitro results, a promising role in acoustic nerve and HC regeneration. In a recent study of SG sensory cells in rats at different concentrations of GH, it was shown that, although there were no changes in relation to cell survival, neurite growth and branching were stimulated [80]. In addition, cell morphology remained stable, so the presence of GH maintains and allows the different cell subtypes to grow. Previous studies in zebrafish reported that treatment with GH regenerated post-traumatic HC through ciliary protection or recovery after exposure to acoustic trauma [81]. Apart from the direct effect of this hormone, it is able to release various mediators with neurotrophic and protective effects that also play a role in the inner ear, such as IGF-I, BDNF, and NT-3 [82, 83] Reports of hearing improvement in humans treated with GH are very rare. However, in a recent case-report, we have shown a clear improvement in hearing in a child with cerebral palsy who had moderate SNHL [84], perhaps due to the early treatment with GH and the effects of this hormone on stem cells [85].
IGF-1
Among all the mediators of the effects of GH, there is a hormone with important neurotrophic effects, such as the insulin-like growth factor 1 (IGF-1). IGF-1 in humans regulates the development, growth, and cell differentiation of the inner ear. In the cochlea, it plays a role in the proliferation and survival of the otic neuron. IGF-1 is expressed, as in many other tissues and organs, in the inner ear, specifically in the SV, organ of Corti, and in the greater epithelial ridge [86]. Only a small proportion of IGF-1 found in plasma circulates in free-form (the biologically active form). Most of it is bound to IGF-1-binding proteins (IGFBP) which regulate the amount of free IGF-1 and therefore its activity. IGFBPs 2–5 are expressed in the cochlea during embryonic and postnatal days, indicating that they play a role in cochlear development through cell pattern and differentiation [7]. Decreased IGF-1 levels are associated with different types of hearing loss, including ARHL. IGF-1 levels in mice decrease with age; in fact, there is a relationship between hearing loss and retinal degeneration [87]. Mutations in IGF-1 genes are rare; when they occur (usually in consanguineous male patients) hearing loss is not always present. On the other hand, mutations in the IGF-1 receptor (IGF1R) have not been associated with profound hearing loss in heterozygous people, so a small amount of IGF-1 interacting with its receptor IGF1R may be sufficient for the correct development of the inner ear. Igf1 knockout mice develop bilateral hearing loss, affecting all frequencies, as well as a delay in the response of the acoustic stimulus, so that (in addition to cochlear impairment) damage to the auditory pathway would also occur. This loss of IGF-1 produces in the prenatal mouse cochlea a decrease in activating Akt and ERK1/2, and an increase in the activation of the p38 kinase by the transcription factor FoxM1, and deficient expression of the transcription factors of the neural progenitors Six6 and Mash1. At the same time, a link was found between the absence of IGF-1 and the decrease in nuclear levels of MEF2, the accumulation of FoxM1, and the corresponding decrease in the inhibitor of cyclin-dependent nuclear kinase p27Kip1 [88]. Different experiments on ex vivo and in vivo animals have shown that IGF-1 protects cochlear HC in various situations, such as exposure to noise, ototoxic drugs, ischemia, and SNHL [14].
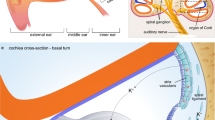
In humans, patients with Laron syndrome treated preventively with IGF-1 develop normal hearing [89]. It has been demonstrated that topical application of IGF-1 in the round window, prior tympanostomy, of patients with sudden hearing loss is a safe and effective therapy with minor side effects for those patients in whom initial treatment with systemic corticosteroids failed [90]. This effectiveness, however, was not superior to intratympanic dexamethasone salvage therapy. Physiological levels of IGF-1 are linearly associated with a lower risk of hearing impairment, so it would be interesting to develop future trials of IGF-1 supplementation [91]. Figure 1 schematizes the sites where different hormones and receptors are expressed in the inner ear.
Hormone and/or receptor expression sites in the inner ear. ER, estrogen receptor; EPO, erythropoietin; EPO-R, erythropoietin receptor; MCR, melanocortin receptor; VR, vasopressin receptor; SST, somatostatin receptor; CRF, corticotropin-releasing factor; CRFR2, corticotropin-releasing factor type 2; TRβ, thyroid receptor beta; IGF-1, insulin-like growth factor-1
Interestingly and paradoxically given its usually inhibitory effects, in vitro studies, upon exposure to an ototoxic agent, somatostatin (SS) is able to decrease HC loss [92, 93]; in fact, mammals present SST1 and SST2 receptors in HC [2]. Although the explanation is unclear, it appears to be related to its regulation in glutamatergic excitotoxicity, inhibiting the release of potassium-induced glutamate [93].
Bone Metabolism and Middle Ear Pathology
A known effect of two hormones, GH and calcitonin, is their therapeutic ability for bone remodeling, regulating metabolically bone resorption produced by osteoclasts and osteoblastic accumulation, thereby involving other hormones, neurotrophic factors, multiple cytokines (such as interleukins, TNF), and local prostaglandins [94]. This is why the use of both hormones can be useful in middle ear pathology. Thus, in patients with Turner syndrome, while GH supplementation is common to grow, the administration of the hormone appears to improve the incidence of middle ear otitis in those with a hypoplasia of the skull base and mastoid cavity phenotype [95]. Although reports showed that endogenous GH may, in cases of acromegaly, increase the risk of conductive hearing loss due to bone growth, the use of exogenous GH has been shown to be safe, with isolated cases of conductive hearing loss reported for this reason [96]. Regarding calcitonin, several articles have described auditory improvement in patients with Paget’s disease [97, 98]. More recently, the door has been opened to use this hormone in the treatment of otosclerosis. Lacosta et al. (2003) [99], demonstrated, first through a case-report and later in a prospective study, that intranasal doses of calcitonin improve hearing in this pathology, with a significant gain in acoustic transmission both in the airway and in the bone. However, hearing improvement and tinnitus disappearance happened in a small group of subjects (19.4% and 25% respectively).
In Fig. 2, the effect of different hormones, particularly GH, on the bone metabolism in the middle ear is shown.
Interaction of hormones in the bone metabolism. Calcitonin and estrogens exert and inhibitory effect on osteoclasts, inhibiting bone resorption. Estrogens, GH, and IGF-1 stimulate osteoblasts. Thyroid hormones stimulate osteoclasts. RANKL (receptor activator for nuclear Factor κB ligand) stimulates osteoclasts, increasing bone resorption, while OPG (osteoprotegerin) inhibits osteoclasts
Vasopressin and Its Relationship with Endolymphatic Hydrops
Vasopressin is an antidiuretic hormone with a potential role in the homeostasis of the inner ear fluid. Its receptor, V2R, is expressed in the inner ear. In mice, subcutaneous administration via mini-osmotic pups induces the endolymphatic hydrops that characterizes Meniere’s disease (MD). In humans, several authors have reported that in patients with MD, plasma levels and expression of V2R’s mRNA are significantly higher than in controls [100, 101]. Specifically, one of these authors in a subsequent clinical trial, based on the evidence from the results, recommended stress management techniques to reduce vasopressin levels and improve symptoms. However, other reports have ruled out the relevance of this hormone in definitive MD, since they did not observe this association between plasma levels of vasopressin and the severity of the disease [102]. Anyway, in a subsequent meta-analysis, there does appear to be a relationship between vasopressin and the acute phase of the disease, although this hormone is a poor marker for controlling the evolution of MD [103].
Conclusion
Despite the vast evidence, according to animal models, of the therapeutic potential of hormones in the treatment of hearing impairments, just few selected reports in patients and isolated clinical trials are available (see Table 1). The door is open to new applications for otoprotection, neuroregeneration and modulating actions that would improve multiple pathological conditions of the ear. Many questions remain unanswered. For example, it would be necessary to standardize drug dosages, a factor that already occurs in the classic corticosteroid treatment. It is surprising that in some cases, such as it happens with melatonin, studies with higher doses have not been performed, given its potential in the treatment of auditory pathology. Another stumbling block to resolve is to contribute more studies that value the use of alternative routes to the systemic one. The presence of several hormone receptors in the cochlea suggests that future studies have to be carried out on this pathway. In addition, and although only one case has been reported [84], it is likely that an early treatment with GH may recover hearing loss in children with cerebral palsy given the effects of this hormone on the proliferation of neural cochlear stem cells.
References
Thomas L, Purvis C, Drew J, Abramovich D, Williams L. Melatonin receptors in human fetal brain: 2-[125I] iodomelatonin binding and MT1 gene expression. J Pineal Res. 2002;33:218–24.
Radojevic V, Hanusek C, Setz C, Brand Y, Kapfhammer J, Bodmer D. The somatostatinergic system in the mammalian cochlea. BMC Neurosci. 2011;12:89.
Stenberg A, Wang H, Fish J, Schrott-Fischer A, Sahlin L, Hultcrantz M. Estrogen receptors in the normal adult and developing human inner ear and in Turner’s syndrome. Hear Res. 2001;157:87–92.
Bonnard Å, Sahlin L, Hultcrantz M, Simonoska R. No direct nuclear effect of progesterone in the inner ear: other possible pathways. Acta Otolaryngol. 2013;133:1250–7.
Harvey S, Johnson CD, Sanders EJ. Growth hormone in neural tissues of the chick embryo. J Endocrinol. 2001;169:487–98.
Song L, McGee J, Walsh E. The influence of thyroid hormone deficiency on the development of cochlear nonlinearities. J Assoc Res Otolaryngol. 2008;9:464–76.
Okano T, Kelley M. Expression of insulin-like growth factor binding proteins during mouse cochlear development. Dev Dyn. 2013;242:1210–21.
Xipeng L, Ruiyu L, Meng L, Yanzhuo Z, Kaosan G, Liping W. Effects of diabetes on hearing and cochlear structures. J Otol. 2013;8:82–7.
Fang Q, Longo-Guess C, Gagnon LH, Mortensen AH, Dolan DF, Camper SA, et al. A modifier gene alleviates hypothyroidism-induced hearing impairment in Pou1f1dw dwarf mice. Genetics. 2011;189:665–73.
Basappa J, Graham C, Turcan S, Vetter D. The cochlea as an independent neuroendocrine organ: expression and possible roles of a local hypothalamic–pituitary–adrenal axis-equivalent signaling system. Hear Res. 2012;288:3–18.
Graham C, Vetter D. The mouse cochlea expresses a local hypothalamic-pituitary-adrenal equivalent signaling system and requires corticotropin-releasing factor receptor 1 to establish normal hair cell innervation and cochlear sensitivity. J Neurosci. 2011;31:1267–78.
Trune D, Kempton J. Aldosterone and prednisolone control of cochlear function in MRL/MpJ-Faslpr autoimmune mice. Hear Res. 2001;155:9–20.
Atkinson P, Wise A, Flynn B, Nayagam B, Richardson R. Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS One. 2014;9:e102077.
Ito J. Regenerative medicine for the inner ear: summary. In: Ito J, editor. Regenerative medicine for the inner ear. Tokyo: Editorial Springer Japan. Minatu-KU; 2014. p. 313–8.
Stenberg A, Wang H, Sahlin L, Hultcrantz M. Mapping of estrogen receptors α and β in the inner ear of mouse and rat. Hear Res. 1999;136:29–34.
Frisina RD. Hormones and hearing: too much or too little of a good thing can be ototoxic. Semin Hear. 2012;33:231–41.
Lee J, Marcus D. Estrogen acutely inhibits ion transport by isolated stria vascularis. Hear Res. 2001;158:123–30.
Laugel GR, Dengerink HA, Wright JW. Ovarian steroid and vasoconstrictor effects on cochlear blood flow. Hear Res. 1987;31:245–51.
Tremere LA, Burrows K, Jeong JK, Pinaud R. Organization of estrogen-associated circuits in the mouse primary auditory cortex. J Exp Neurosci. 2011;5:45–60.
Chen C, Chen C, Yang C, Lin C, Cheng Y. Testosterone modulates preattentive sensory processing and involuntary attention switches to emotional voices. J Neurophysiol. 2015;113:1842–9.
Simonoska R, Stenberg A, Duan M, Yakimchuk K, Fridberger A, Sahlin L, et al. Inner ear pathology and loss of hearing in estrogen receptor-β deficient mice. J Endocrinol. 2009;201:397–406.
McCullar E, Oesterle EC. Cellular targets of estrogen signaling in regeneration of inner ear sensory epithelia. Hear Res. 2009;252:61–70.
Charitidi K, Meltser I, Canlon B. Estradiol treatment and hormonal fluctuations during the estrous cycle modulate the expression of estrogen receptors in the auditory system and the prepulse inhibition of acoustic startle response. Endocrinology. 2012;153:4412–21.
Stenberg A, Simonoska R, Stygar D, Sahlin L, Hultcrantz M. Effect of estrogen and antiestrogens on the estrogen receptor content in the cochlea of ovariectomized rats. Hear Res. 2003;182:19–23.
Price K, Zhu X, Guimaraes P, Vasilyeva O, Frisina R. Hormone replacement therapy diminishes hearing in peri-menopausal mice. Hear Res. 2009;252:29–36.
Horner K, Cazals Y, Guieu R, Lenoir M, Sauze N. Experimental estrogen-induced hyperprolactinemia results in bone-related hearing loss in the guinea pig. Am J Physiol Endocrinol Metab. 2007;293:E1224–32.
Al-Mana D, Ceranic B, Djahanbakhch O, Luxon L. Hormones and the auditory system: a review of physiology and pathophysiology. Neuroscience. 2008;153:881–900.
Souza D, Luckwu B, Andrade W, Pessoa L, Nascimento J, Rosa M. Variation in the hearing threshold in women during the menstrual cycle. Int Arch Otorhinolaryngol. 2017;21:323–8.
Jönsson R, Rosenhall U, Gause-Nilsson I, Steen B. Auditory function in 70- and 75-year-olds of four age cohorts. Scand Audiol. 1998;27:81–93.
McFadden D, Martin G, Stagner B, Maloney M. Sex differences in distortion-product and transient-evoked otoacoustic emissions compared. J Acoust Soc Am. 2009;125:239–46.
Chen H, Chung C, Chen V, Wang Y, Chien W. Hormone replacement therapy decreases the risk of tinnitus in menopausal women: a nationwide study. Oncotarget. 2018;9:19807–16.
Hedenstierna C, Hultcrantz M, Collins A, Rosenhall U. Hearing in women at menopause. Prevalence of hearing loss, audiometric configuration and relation to hormone replacement therapy. Acta Otolaryngol. 2007;127:149–55.
Curhan S, Eliassen A, Eavey R, Wang M, Lin B, Curhan G. Menopause and postmenopausal hormone therapy and risk of hearing loss. Menopause. 2017;24:1049–56.
Hultcrantz M, Simonoska R, Stenberg AE. Estrogen and hearing: a summary of recent investigations. Acta Otolaryngol. 2016;126:10–4.
Weiderpass E, Adami HO, Baron JA, Magnusson C, Bergström R, Lindgren A, et al. Risk of endometrial cancer following estrogen replacement with and without progestins. J Natl Cancer Inst. 1999;91:1131–7.
He ZY, Ren DD. Sex hormones and inner ear. In: Drevensek G, editor. Sex hormones in neurodegenerative processes and diseases. London: Editorial Intechopen; 2018. p. 329–46.
Alves C, Oliveira C. Hearing loss among patients with Turner’s syndrome: literature review. Braz J Otorhinolaryngol. 2014;80:257–63.
Veldman J. Immune-mediated sensorineural hearing loss. Auris Nasus Larynx. 1998;25:309–17.
Chang K, Park S, Yeo S, Suh B. Effects of testosterone in the treatment of immune-mediated sensorineural hearing loss. Eur Arch Otorhinolaryngol. 2003;260:316–9.
Hurtuk A, Dome C, Holloman CH, Wolfe K, Welling DB, Dodson EE, et al. Melatonin: can it stop the ringing? Ann Otol Rhinol Laryngol. 2011;120:433–40.
Lopez-Gonzalez M, Santiago A, Esteban-Ortega F. Sulpiride and melatonin decrease tinnitus perception modulating the auditolimbic dopaminergic pathway. J Otolaryngol. 2007;36:213.
Neri G, Baffa C, De Stefano A, Poliandri A, Kulamarva G, Di Giovanni P, et al. Management of tinnitus: oral treatment with melatonin and sulodexide. J Biol Regul Homeost Agents. 2009;23:103–10.
Costello RB, Lentino CV, Boyd CC, O’Connell ML, Crawford CC, Sprengel ML, et al. The effectiveness of melatonin for promoting healthy sleep: a rapid evidence assessment of the literature. Nutr J. 2014;13:106.
Maldonado M, Murillo-Cabezas F, Terron M, Flores L, Tan D, Manchester L, et al. The potential of melatonin in reducing morbidity-mortality after craniocerebral trauma. J Pin Res. 2007;42:1–11.
Biesalski H, Welker H, Thalmann R, Vollrath L. Melatonin and other serotonin derivatives in the guinea pig membranous cochlea. Neurosci Lett. 1988;91:41–6.
Helliwell R, Williams L. The development of melatonin-binding sites in the ovine fetus. J Endocrinol. 1994;142:475–84.
Lasisi A, Fehintola F. Correlation between plasma levels of radical scavengers and hearing threshold among elderly subjects with age-related hearing loss. Acta Otolaryngol. 2011;131:1160–4.
Lopez-Gonzalez M, Guerrero J, Rojas F, Osuna C, Delgado F. Melatonin and other antioxidants prolong the postmortem activity of the outer hair cells of the organ of Corti: its relation to the type of death. J Pin Res. 1999;27:73–7.
Demir M, Altintoprak N, Aydin S, Kosemihal E, Basak K. Effect of transtympanic injection of melatonin on cisplatin-induced ototoxicity. J Int Adv Otol. 2016;11:202–6.
Lopez-Gonzalez M, Guerrero J, Torronteras R, Osuna C, Delgado F. Ototoxicity caused by aminoglycosides is ameliorated by melatonin without interfering with the antibiotic capacity of the drugs. J Pin Res. 2000;28:26–33.
Karaer I, Simsek G, Gul M, Bahar L, Gürocak S, Parlakpinar H, et al. Melatonin protects inner ear against radiation damage in rats. Laryngoscope. 2015;25:E345–9.
Chen T, Zhang W, Liang Y, Li Q, Yang C, Yuan YX, et al. Effect of melatonin on expression of prestin protein in the inner ear of mice following radiotherapy. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;53:118–23.
Karlidağ T, Yalçin Ş, Öztürk A, Üstündağ B, Gök Ü, Kaygusuz I, et al. The role of free oxygen radicals in noise induced hearing loss: effects of melatonin and methylprednisolone. Auris Nasus Larynx. 2002;29:147–52.
Bas E, Martinez-Soriano F, Láinez J, Marco J. An experimental comparative study of dexamethasone, melatonin and tacrolimus in noise-induced hearing loss. Acta Otolaryngol. 2009;129:385–9.
Kaneko Y, Pappas C, Tajiri N, Borlongan C. Oxytocin modulates GABAAR subunits to confer neuroprotection in stroke in vitro. Sci Rep. 2016;6:35659.
Bekmez Bilmez Z, Aydin S, Şanli A, Altintoprak N, Demir M, Atalay Erdoğan B, et al. Oxytocin as a protective agent in cisplatin-induced ototoxicity. Cancer Chemother Pharmacol. 2016;77:875–9.
Azevedo A, Figueiredo R, Elgoyhen A, Langguth B, Penido N, Schlee W. Tinnitus treatment with oxytocin: a pilot study. Front Neurol. 2017;8:494.
Mutlu A, Ocal F, Erbek S, Ozluoglu L. The protective effect of adrenocorticotropic hormone treatment against noise-induced hearing loss. Auris Nasus Larynx. 2018;45:929–35.
Yao X, Rarey KE. Localization of the mineralocorticoid receptor in rat cochlear tissue. Acta Otolaryngol. 1996;116:493–6.
Trune D, Canlon B. Corticosteroid therapy for hearing and balance disorders. Anat Rec Oboken. 2012;295:1928–43.
Halonen J, Hinton A, Frisina R, Ding B, Zhu X, Walton J. Long-term treatment with aldosterone slows the progression of age-related hearing loss. Hear Res. 2016;336:63–71.
Quin L, Zhang B, Wang Q, Li D, Luo X, Zhong S. Effect of aldosterone on cochlear Af9 expression and hearing in guinea pig. Acta Otolaryngol. 2017;137:903–9.
Maateijsen DJ, Kingma CM, De Jong PE, With HP, Albers FW. Aldosterone assessment in patients with Menière’s disease. ORL J Othorhinolaryngol Relat Spec. 2001;63:280–6.
Rüsch A, Ng L, Goodyear R, Oliver D, Lisoukov I, Vennström B, et al. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci. 2001;21:9792–800.
Ng L, Cordas E, Wu X, Vella K, Hollenberg A, Forrest D. Age-related hearing loss and degeneration of cochlear hair cells in mice lacking thyroid hormone receptor β1. Endocrinology. 2015;156:3853–65.
Sharlin D, Ng L, Verrey F, Visser T, Liu Y, Olszewski R, et al. Deafness and loss of cochlear hair cells in the absence of thyroid hormone transporters Slc16a2 (Mct8) and Slc16a10 (Mct10). Sci Rep. 2018;8:4403.
Hussein M, Asal S, Salem T, Mohammed A. The effect of L-thyroxine hormone therapy on hearing loss in hypothyroid patients. Egypt J Otolaryngol. 2017;33:637.
Cordas E, Ng L, Hernandez A, Kaneshige M, Cheng S, Forrest D. Thyroid hormone receptors control developmental maturation of the middle ear and the size of the ossicular bones. Endocrinology. 2012;153:1548–60.
Frederiksen BL, Cayé-Thomasen P, Lund SP, Wagner N, Asal K, Olsen NV, et al. Does erythropoietin augment noise induced hearing loss? Hear Res. 2007;223:129–37.
Zhong C, Jiang Z, Guo Q, Zhang X. Protective effect of adenovirus-mediated erythropoietin expression on the spiral ganglion neurons in the rat inner ear. Int J Mol Med. 2018;41:2669–77.
Bächinger D, Horvath L, Eckhard A, Goosmann M, Honegger T, Gassmann M, et al. Neuronal erythropoietin overexpression is protective against kanamycin-induced hearing loss in mice. Toxicol Lett. 2018;291:121–8.
Han F, Yu H, Zheng T, Ma X, Zhao X, Li P, et al. Otoprotective effects of erythropoietin on Cdh23erl/erl mice. Neuroscience. 2013;237:1–6.
Monge Naldi A, Belfrage C, Jain N, Wei E, Canto Martorell B, Gassmann M. Neuronal erythropoietin overexpression protects mice against age-related hearing loss (presbycusis). Neurobiol Aging. 2015;36:3278–87.
Cayé-Thomasen P, Wagner N, Lidegaard Fredriksen B, Asal K, Thomsen J. Erythropoietin and erythropoietin receptor expression in the guinea pig inner ear. Hear Res. 2003;203:21–7.
Markowski J, Gierek T, Wiecek A, Klimek D, Chudek J. Assessment of hearing organ ability in high- frequency auditory in patients suffering from chronic renal failure treated by haemodialysis and human recombinant erythropoietin (rhPEO). Otolaryngol Pol. 2002;56:589–96.
Song J, Sun H, Xu F, Kang W, Gao L, Guo J, et al. Recombinant human erythropoietin improves neurological outcomes in very preterm infants. Ann Neurol. 2016;80:24–34.
Natalucci G, Latal B, Koller B, Rüegger C, Sick B, Held L, et al. Effect of early prophylactic high- dose recombinant human erythropoietin in very preterm infants on neurodevelopmental outcome at 2 years. JAMA. 2016;315:2079–85.
Smeti I, Assou S, Savary E, Masmoudi S, Zine A. Transcriptomic analysis of the developing and adult mouse cochlear sensory epithelia. PLoS One. 2012;7:e42987.
Marano R, Tickner J, Redmond S. Prolactin expression in the cochlea of aged BALB/c mice is gender biased and correlates to loss of bone mineral density and hearing loss. PLoS One. 2013;8:e63952.
Gabrielpillai CB, Geissler T, Stock M, Stöver T, Diensthuber M. Growth hormone promotes neurite growth of spiral ganglion neurons. Neuroreport. 2018;29:637–42.
Sun H, Lin C, Smith M. Growth hormone promotes hair cell regeneration in the zebrafish (danio rerio) inner ear following acoustic trauma. PLoS One. 2011;6:e28372.
Chia DJ. Minireview: mechanisms of growth hormone-mediated gene regulation. Mol Endocrinol. 2014;28:1012–25.
Martinez-Moreno CG, Fleming T, Carranza M, Avila-Mendoza J, Luna M, Harvey S, et al. Growth hormone protects against kainate excitotoxicity and induces BDNF and NT3 expression in chicken neuroretinal cells. Exp Eye Res. 2018;166:1–12.
Guerra J, Devesa A, Llorente D, Mouro R, Alonso A, García-Cancela J, et al. Early treatment with growth hormone (GH) and rehabilitation recovers hearing in a child with cerebral palsy. Reports. 2019;2:4.
Devesa J, Almengló C, Devesa P. Multiple effects of growth hormone in the body: is it really the hormone for growth? Clin Med Insights Endocrinol Diabetes. 2016;9:47–71.
Sanchez-Calderon H, Rodriguez-de la Rosa L, Milo M, Pichel J, Holley M, Varela-Nieto I. RNA microarray analysis in prenatal mouse cochlea reveals novel IGF-I target genes: implication of MEF2 and FOXM1 transcription factors. PLoS One. 2010;5:e8699.
Rodríguez-de la Rosa L, Lassaletta L, Calvino M, Murillo-Cuesta S, Varela-Nieto I. The role of insulin-like growth factor 1 in the progression of age-related hearing loss. Front Aging Neurosci. 2017;9:411.
Varela-Nieto I, Murillo-Cuesta S, Rosa LR, Lassatetta L, Contreras J. IGF-I deficiency and hearing loss: molecular clues and clinical implications. Pediatr Endocrinol Rev. 2013;10:460–72.
Attias J, Zarchi O, Nageris BI, Laron Z. Cochlear hearing loss in patients with Laron syndrome. Eur Arch Otorhinolaryngol. 2011;269:461–6.
Nakagawa T, Kumakawa K, Usami S, Hato N, Tabuchi K, Takahashi M, et al. A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC Med. 2014;12:219.
Lassale C, Batty G, Steptoe A, Zaninotto P. Insulin-like growth factor 1 in relation to future hearing impairment: findings from the English longitudinal study of ageing. Sci Rep. 2017;7:4212.
Caelers A, Monge A, Brand Y, Bodmer D. Somatostatin and gentamicin-induced auditory hair cell loss. Laryngoscope. 2009;119:933–7.
Brand Y, Radojevic V, Sung M, Wei E, Setz C, Glutz A, et al. Role of somatostatin receptor-2 in gentamicin-induced auditory hair cell loss in the mammalian inner ear. PLoS One. 2014;9:e108146.
Kenkre J, Bassett J. The bone remodelling cycle. Ann Clin Biochem. 2018;55:308–27.
Quigley CA, Crowe BJ, Anglin DG, Chipman JJ. Growth hormone and low dose estrogen in turner syndrome: results of a United States multi-center trial to near-final height. J Clin Endocrinol Metab. 2002;87:2033–41.
Davenport M, Roush J, Liu C, Zagar A, Eugster E, Travers S, et al. Growth hormone treatment does not affect incidences of middle ear disease or hearing loss in infants and toddlers with Turner syndrome. Horm Res Paediatr. 2010;74:23–32.
Lando M, Hoover L, Finerman G. Stabilization of hearing loss in Paget’s disease with calcitonin and etidronate. Arch Otolaryngol Head Neck Surg. 1988;114:891–4.
Aoki M, Tanahashi S, Mizuta K, Kato H. Treatment for progressive hearing loss due to Paget’s disease of bone – a case report and literature review. J Int Adv Otol. 2016;11:267–70.
Lacosta Nicolás A, Sánchez del Hoyo J, García Cano J. Posible beneficio de la calcitonina en el tratamiento de la otosclerosis. Acta Otorrinolaringol Esp. 2003;54:169–72.
Kitahara T, Doi K, Maekawa C, Kizawa K, Horii A, Kubo T, et al. Meniere’s attacks occur in the inner ear with excessive vasopressin type-2 receptors. J Neuroendocrinol. 2008;20:1295–300.
Aoki M, Asai M, Nishihori T, Mizuta K, Ito Y, Ando K. The relevance of an elevation in the plasma vasopressin levels to the pathogenesis of Meniere’s attack. J Neuroendocrinol. 2007;19:901–6.
Hornibrook J, George P, Gourley J. Vasopressin in definite Meniere’s disease with positive electrocochleographic findings. Acta Otolaryngol. 2011;131:613–7.
Wu J, Zhou J, Dong L, Fan W, Zhang J, Wu C. A mysterious role of arginine vasopressin levels in Ménièreʼs disease—meta-analysis of clinical studies. Otol Neurotol. 2017;38:161–7.
Acknowledgments
We acknowledge the Foundation Foltra (Teo, Spain) for the support given at the time of writing this review.
Contributors
JG and JD participated equally in the conception and writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Informed Consent
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Medicine
Rights and permissions
About this article
Cite this article
Guerra, J., Devesa, J. Hormone Therapy: Challenges for Treating Hearing Impairments. SN Compr. Clin. Med. 1, 603–615 (2019). https://doi.org/10.1007/s42399-019-00089-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42399-019-00089-y