Abstract
Interactive effects of ultraviolet radiation (UVR), photosynthetically active radiation (PAR) and exogenously supplied ammonium chloride (NH4Cl) was studied in the rice-field cyanobacterium Anabaena sp. HKAR-7. The cyanobacterium was cultured under varying NH4Cl concentrations i.e., 0, 50, 200, 500, 1000 and 5000 μM and 200 μM (concentration) was found to be optimum for the growth of the cyanobacterium. Detrimental effects of UV-B exposure were observed on photosynthetic pigments such as chlorophyll a (Chl a), carotenoids and phycocyanin (PC). However, damage to these pigments was less in the cyanobacterial samples supplemented with NH4Cl. Contents of Chl a and PC in cyanobacterial cells decreased upon UV-B exposure but decrement was less in the samples supplemented with NH4Cl. Upon UV-B exposure, carotenoids content enhanced initially (till 15 days) during the course of treatment (21 days) but significant decrease (in carotenoids content) was observed in later phase of the experiment. From the results of photosynthetic activity, maximum quantum efficiency of PSII (Fv/Fm) and maximum electron transport rate (ETRmax), it could be concluded that exogenous supplementation of NH4Cl (optimum concentration) helped in protecting the cyanobacterial cells from highly energetic UVR to certain extent. Another interesting observation was significantly higher levels of biosynthesis and accumulation of mycosporine-like amino acids (MAAs) in the cyanobacterial cells supplemented with NH4Cl in comparison to non-supplemented cells. The purified MAA was identified to be phorphyra-334 as evidenced by UV/VIS absorption spectra, high performance liquid chromatography (HPLC) and electrospray ionization-mass spectrometry (ESI–MS).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ultraviolet radiation (UVR), the comparatively low waveband radiation, is composed of highly energetic photons which reach the Earth’s surface along with solar radiation. In current scenario, UVR influx has increased on the Earth due to anthropogenically released ozone depleting compounds (Häder et al. 2015). Although, UV-B constitute less than 1% of the total incoming solar radiation (Vincent and Roy 1993), it severely affects crucial biomolecules such as DNA, RNA and proteins which are important for biochemical, physiological and genetic functioning of the cell (Sinha and Häder 2016; Rajneesh et al. 2019). Besides, in cyanobacteria, detrimental effects of UVR on pigmentation, phycobiliprotein composition, motility, N2 metabolism, DNA, protein profile and 14CO2 uptake have been well documented (Kannaujiya and Sinha 2015; Sinha and Häder 2016; Rajneesh et al. 2019). Enhanced production of reactive oxygen species (ROS) due to UVR leads to the destruction of D1 protein and photosystem (PS) II reaction centre and also disrupts photon absorption and electron transport (Xia et al. 2004). Decrease in photosynthetic quantum yields (Fv/Fm) has been observed in response to UVR in Fischerella sp. (Singh et al. 2017). Exposure to UVR results in breakage of the filaments (Qin et al. 2012) and inhibition of enzyme nitrogenase in N2-fixing cyanobacteria leading to decreased nitrogen uptake (Kumar et al. 2003; Pandey et al. 2020). However, with due course of evolution, these photoautotrophs have developed several protective strategies for overcoming the harmful effects of lethal UVR (Pathak et al. 2019a) which ranges from behavioral to molecular levels. Accumulation and biosynthesis of UV screening compounds such as mycosporine-like amino acids (MAAs) is one such protective mechanism adopted by cyanobacteria to survive and sustain under such abiotic stresses (Richa 2015). Different studies have correlated the accumulation and biosynthesis of MAAs in cyanobacteria and algae in response to UV exposure (Lesser et al. 1996; Karsten et al. 1998a; Hoyer et al. 2001; Rastogi and Incharoensakdi 2013; Richa 2015). MAAs are water-soluble, low molecular weight nitrogenous compounds having high molar extinction coefficients (ε = 28,100–50,000 M−1 cm−1) with absorption band ranging in between 310–362 nm (Richa 2015; Ahmed et al. 2019; Singh et al. 2020). Apart from synthesis of UV-screening compounds, other repair mechanisms against UVR exposure involve synthesis of several enzymes and protein cofactors (Roy 2000) and nitrogen limitation results in less efficient repair mechanisms, hence making the light driven process of photosynthesis more sensitive to UVR (Litchman et al. 2002). Korbee-Peinado et al. (2004) found that biosynthesis of MAAs was stimulated in response to external nitrogen supplementation in form of ammonium in red alga Porphyra columbina. However, there is wide controversy regarding the factors regulating the accumulation and induction of MAAs and information regarding the effect of nitrogen supplementation in form of ammonium chloride (NH4Cl) on biosynthesis and accumulation of MAAs in cyanobacteria are scarce (Banaszak and Neale 2001; Litchman et al. 2002). Therefore, the present investigation aims at studying the effects of UV-B and photosynthetically active radiation (PAR) on chlorophyll (Chl a), biliproteins, photosynthetic performance and biosynthesis of MAAs in Anabaena sp. HKAR-7, with and without the supplementation of optimum dose of exogenous nitrogen source in the form of NH4Cl. Such study would help in understanding the photoprotective mechanisms that allow phototrophic organisms such as cyanobacteria to sustain and reproduce in brightly lit and nutrient rich habitats.
Materials and methods
Experimental setup
The cyanobacterium, Anabaena sp. HKAR-7, isolated and purified from the rice-fields of Banaras Hindu University, Varanasi, India, was selected for the present study. Microscopic analysis was done using light (CX21i, Olympus Corporation, Tokyo, Japan) and scanning electron microscope (EVO18 research, Zeiss, UK) (Supplementary Fig. 1). Morphological identification was done through monographs and standard taxonomic keys (Desikachary 1959), and molecular characterization was done by 16S rRNA gene amplification and maximum likelihood method was utilized for phylogenetic tree mapping. Alignment of the sequence of 16S rRNA gene fragment against known sequences present in the GenBank database was done using the BLAST program of NCBI search (Altschul et al. 1990). CLUSTALW was used for producing multiple alignments. The 16S rRNA gene sequence of the cyanobacterium was classified into phylogenetic group as proposed by Desikachary (1959) to determine the genetic variability between and within the groups. A phylogenetic tree was constructed using the neighbor-joining algorithm (Saitou and Nei 1987) provided in MEGA 7 software (Kumar et al. 2016).
Autoclaved BG-11 (without nitrogen sources) medium was used for routine growth of the cyanobacterial cultures (Rippka et al. 1979) under axenic conditions at a temperature of 28 ± 2 °C, under continuous fluorescent white light (12 Wm–2). Cyanobacterial cultures were shaken manually four times a day in order to avoid clumping and shelf shading. Different concentrations of exogenous NH4Cl (0:Control, 50, 200, 500, 1000 and 5000 μM) were used in nutrient medium for screening purpose and 200 μM concentration of NH4Cl was found to be optimum for the growth of the cyanobacterium and hence was selected as optimum dose for further experiments. The homogeneous cultures of cyanobacteria (250 mL of culture with OD750 nm = 0.68 ± 0.2; Path length 1 cm) were taken in sterile glass Petri dishes (120 mm in diameter) and were treated with artificial UV-B radiation and PAR in a UV chamber with and without exogenous supplementation of 200 μM NH4Cl (HI Media, RM 717). The experiments were conducted under a 14:10 light/dark cycles with light intensity of 40 μmol photons m−2 s−1 at 25 °C. Cyanobacterial samples containing Petri dishes were placed under UV-B TL 40 W/12 fluorescence tubes (TL20 W/01RS, Philips, Germany) and UV-B intensity of ~ 1 W m–2 for 4 h/day (d) from 11:00 to 15:00 was maintained by adjusting the distance of Petri dishes from the UV-B tube in the chamber. Each experiment was performed in triplicates. Cut-off filter foils of 295 nm (Ultraphan; Digefra, Munich, Germany) were placed over each Petri dish for avoiding any exposure of UV-C radiation.
Measurement of photosynthetic pigments
Extraction of Chl a pigment was done by incubating the harvested cyanobacterial samples in 100% methanol for overnight at 4 °C in dark and quantification was done as per method given by Porra (2002), utilizing the absorbance values at 665.2 and 652 nm. Carotenoids were determined by the protocol described by Jensen (1978) with slight modification. Briefly, homogenized culture suspension was centrifuged at 10,000g for 10 min and supernatant was discarded. Pellet was dissolved in 85% acetone and calculation of carotenoids was done by recording the absorbance at 450 nm. For measurement of PC, cyanobacterial sample (10 mL) was centrifuged at 8000g for 15 min and dissolved the pellet in lysis buffer (pH = 8; 3 mL) followed by addition of 1 mg lysozyme in it. Samples were sonicated for 3–5 min and kept for overnight incubation at 4 °C followed by re-centrifugation at 10,000g for 5 min. Absorption spectra were recorded in the absorbance range of 200–700 nm against lysis buffer as a blank by using UV/VIS spectrophotometer (Hitachi 2900, Japan). The cellular PC content was calculated using equations described by Bryant et al. (1979).
Maximum quantum efficiency of PSII (Fv/Fm) and maximum electron transport rate (ETRmax)
Pulse-amplitude-modulation (PAM) fluorometer (PAM-2500, Heinz Walz GmbH, 2008, Effeltrich, Germany) was used for determination of Fv/Fm values. Treated cyanobacterial samples were dark-adapted for 30 min in order to complete the process of oxidation of PSII reaction centres and the maximum (Fm) and minimum (F0) fluorescent yields of PSII was observed in the dark-adapted state. The yields of Fm and F0 were used for calculating the Fv/Fm values as per the formula given by Schreiber (2004). The photosynthetic electron transport rate (ETR) was calculated as per the formula:
Fm′ = maximum fluorescence in light, Ft = steady state fluorescence in light, PPFD = photosynthetic photon flux densities.
Estimation of ETR was done from the operational PSII photochemical yield measured at different PPFD.
Extraction, partial purification and characterization of MAAs
For extraction of MAAs, cyanobacterial cells were harvested by centrifugation (Mikro 220R, Hettich, Germany) and pellets were resuspended in 100% methanol (HPLC grade), incubated overnight under dark conditions at 4 °C followed by homogenization. The aliquots were then centrifuged (5000g, 5 min) and supernatants were transferred to new microtubes and subjected to spectroscopic analysis between 250–700 nm using a UV–VIS spectrophotometer (U-2910, 2J1-0012, Hitachi, Tokyo, Japan). Analysis of the raw spectra (peaks) was done using UV Probe version software (Shimadzu Corp., Kyoto, Japan). The obtained supernatant (methanolic extracts) was evaporated at 40 °C in a vacuum evaporator (SPD111V, Thermo Electron Corp.) after spectroscopic analysis. The remaining residue was re-dissolved in 600 µL ultra-pure water. Chloroform (75 µL) was added to this solution followed by gentle vortexing and centrifugation (5000g, 5 min). After centrifugation, the water phase (uppermost) was transferred into fresh Eppendorf tubes to remove contamination by photosynthetic pigments (lipophilic) from the MAA (water-soluble). Finally, the samples were filtered by sterilized 0.2 μm pore size syringe filters (Axiva Sichem Biotech., New Delhi) and subjected to the high performance liquid chromatography (HPLC) analysis (Rastogi et al. 2012; Richa 2015).
HPLC analysis of UV-absorbing compound
Partially purified MAA was analyzed using HPLC (Waters, Elstree, UK), using a reverse phase semi-preparative column (symmetry prep C18, 7 µm particle size, 7.8 mm × 300 mm long) connected to an asymmetry guard column equipped with a Waters Photodiode array (PDA) detector. Samples (50 μL) were injected into the HPLC column and run at a flow rate of 1.0 mL min−1 using a mobile phase of 0.02% (v/v) acetic acid in ultra-pure water (Rastogi et al. 2012). The detection wavelength was 330 nm and the PDA scan wavelength was from 250–450 nm. The sharp peak, with a retention time (RT) of approximately 3.12 min was eluted and collected with the help of a fraction collector attached to the HPLC unit and quantification of MAA was performed by using the peak area (Richa 2015). Identification of the MAA was done by comparing the RT and absorption spectra.
Electrospray ionization-mass spectrometry (ESI–MS)
The HPLC purified fraction of MAA from Anabaena sp. HKAR-7 was subjected to ESI–MS to produce protonated molecules. Mass spectrum was recorded on an Amazon SL mass spectrometer (Bruker Daltonics Inc., Billerica, MA, USA). Cone voltage of 30 V was found to induce the formation of (M + H)1+ with a mass range of 100–1000 m/z. Data was analyzed using the software Data Analysis 4.0 (Bruker Daltonics Inc., Billerica, MA, USA).
Statistics
All the experiments were conducted with three replicates to evaluate the means and standard deviation (mean ± SD). For evaluating the significance of the data, one-way analysis of variance was used. The significant data was used to determine post hoc multiple comparisons by using the Tukey test at the significance level of 0.05.
Results
On the basis of microscopic analysis, morphological identification, molecular characterization and phylogenetic tree mapping, the cyanobacterium was confirmed to be Anabaena sp. It is a member of order Nostocales, family Nostocaceae and is a filamentous and heterocystous cyanobacterium. The 16S rRNA gene sequence of the cyanobacterium was submitted in NCBI database with an accession number KF857228. The phylogenetic tree revealed that the nearest homologues of Anabaena sp. HKAR-7 are Anabaena constricta MACC-177 (accession number MH702209) and Nostoc muscorum CCAP1453/8 (accession number HF678508) (Supplementary Fig. 2).
Interactive effects of UV-B radiation and ammonium (NH4Cl) on Anabaena sp. HKAR-7
Changes in Chl a content were utilized for estimating the growth of Anabaena sp. HKAR-7 for six concentrations of NH4Cl (0: Control, 50, 200, 500, 1000 and 5000 μM). We observed that 50, 200 and 500 μM concentrations of NH4Cl positively influenced Chl a content. However, higher doses of NH4Cl concentrations (1000 and 5000 μM) became toxic to the cyanobacterium. Chl a content increased gradually from initial value (0.20 μg mL−1) to a maximum value of 2.80 μg mL−1 in 200 μM NH4Cl followed by 50 μM NH4Cl (2.60 μg mL−1) treated samples at 21 days of experiment (Table 1).
Photosynthetic pigments and phycocyanin
Exogenous supplementation of NH4Cl aided in maintaining higher levels of Chl a in the cyanobacterial cells exposed to PAR and PAR + UV-B as compared to non-supplemented samples till 15 days of treatment (Table 2). However, in cyanobacterial samples exposed to PAR + UV-B along with externally supplied NH4Cl, Chl a content decreased after 21 days of treatment. Maximum Chl a content was observed in samples exposed to PAR + NH4Cl (1.7 folds) for 15 days and least in PAR + UV-B treated samples (without NH4Cl) (Table 2). It was observed that carotenoids content enhanced significantly in all the treated samples from initial value (25 μg mL−1) after 6 days of exposure, followed by a decrease in later phase of treatment. This increment in the content of carotenoids was high in PAR + UV-B + NH4Cl treated cyanobacterial samples (1.8 folds) as compared to PAR + UV-B (1.7 folds) treatment at 6 days. However, carotenoids content declined to about 1.9 and 1.3 folds in PAR + UV-B and PAR + UV-B + NH4Cl treated samples respectively, after 21 days of exposure (Table 3). Initial PC content in the cells of Anabaena sp. HKAR-7 was recorded to be 0.364 mg mL−1. Exposure of UV-B caused detrimental effects on PC content. However, this effect was quite less in the samples exposed to UV-B with exogenous NH4Cl supplementation. Maximum decrease in the PC content was recorded in PAR + UV-B exposed samples (without NH4Cl) at 21 days of treatment (Table 4).
Maximum quantum efficiency of PSII (Fv/Fm) and maximum electron transport rate (ETRmax)
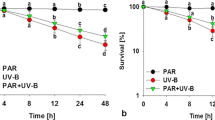
In order to assess the effects of given stress on tested cyanobacterium in terms of quantum efficiency of PSII (Fv/Fm) and ETRmax, we used PAM fluoremetre 2500. A strong correlation between values of Fv/Fm and healthiness of cyanobacterial samples was observed. For instance, control showed Fv/Fm value of 0.303. Anabaena sp. HKAR-7 maintained a relatively constant value of quantum yield, which increased slightly by 1.3 folds (0.3867 at 12 days) and onefold (0.3037 at 9 days) in PAR + NH4Cl and PAR treated samples respectively. The value of the Fv/Fm declined gradually in the samples exposed to PAR + UV-B (7.5 folds) and PAR + UV-B + NH4Cl (5 folds) and remained constant till 21 days of exposure (Fig. 1a). Values of ETRmax showed similar trend as observed in Fv/Fm (Fig. 1b) and were found to be comparatively high in samples exposed to PAR + NH4Cl as compared to PAR treatment. Exposure of UV-B without NH4Cl resulted in most pronounced detrimental effect on photosynthetic activity of the cyanobacterium.
Effect of PAR, UV-B radiation and NH4Cl on maximum quantum yield (Fv/Fm) (a) and maximum electron transport rate (ETRmax) (b) in Anabaena sp. HKAR-7. C control, P PAR, Salt: NH4Cl (200 μM). Results are expressed as means of three replicates. The error bars denote standard deviations of means (means ± S.D., n = 3)
Effect of UV-B radiation and NH4Cl on MAAs biosynthesis, partial purification and characterization
Absorption spectrum (UV/VIS) and HPLC analyses revealed significantly high induction of MAA phorphyra-334 (P-334) (RT = 3.12, λmax = 334 nm) in Anabaena sp. HKAR-7 when treated with combined stress of PAR + UV-B + NH4Cl for 21 days. Spectroscopic analysis of methanolic extracts of Anabaena sp. HKAR-7 showed absorption at 665 nm due to Chl a, at 470 nm due to carotenoids and absorption maxima for MAA at 334 ± 2 nm (Fig. 2a). Figure 2b depicts the absorption spectrum of partially purified MAA showing peak at 334 nm. HPLC chromatogram of purified MAA has been shown in Fig. 2c having the typical peak of MAA at RT of 3.12 min and Fig. 2d shows the absorption maximum for HPLC purified MAA P-334 at 334 nm. As mentioned earlier, HPLC purified MAA was utilized for production of protonated molecules by ESI–MS. Prominent ion peak of protonated molecules [M + H]+ at m/z 346.8 was observed in ESI–MS analysis (Fig. 3). Identification of the purified MAA and its quantification was done as per the method described earlier (Sinha et al. 1999). Interestingly, cyanobacterial samples exposed under different experimental conditions showed enhanced induction of MAA in the following increasing order i.e. PAR < PAR + NH4Cl < PAR + UV-B < PAR + UV-B + NH4Cl treatments. In Anabaena sp. HKAR-7 maximum induction of MAA P-334 was observed in the cyanobacterial samples exposed to combined stress of PAR + UV-B + NH4Cl (1.378 μmol/g dry wt) for 21 days (Fig. 4).
Effect of PAR, UV-B radiation and NH4Cl on induction of P-334 concentration in Anabaena sp. HKAR-7. Results are expressed as means of three replicates. Vertical bars indicate standard deviation of the means. Similar letters over bar represent homogeneous mean group (P > 0.05). C control, P PAR; Salt: NH4Cl (200 μM)
Discussion
The physiological and biochemical response of any organism including cyanobacteria is greatly influenced by variation in their environment. For carrying out the process of photosynthesis and nitrogen fixation, cyanobacteria get exposed to high doses of damaging UVR (Balskus and Walsh 2010) which might result in photo-transformations in the genetic material (DNA) because of production of cyclobutane pyrimidine dimers, thymine-thymine pyrimidine-pyrimidone6–4) photoproducts and DNA–protein cross-links (Batista et al. 2009; Rajneesh et al. 2018; Pathak et al. 2019b). Activation of different lines of defense strategies including screening of UVR through UV-absorbing compounds such as MAAs increases resistance of cyanobacteria to high irradiances (Korbee-Peinado et al. 2004, 2005; Huovinen et al. 2006; Richa 2015; Rastogi et al. 2016). Photoprotective compounds, MAAs, not only play an important role in UVR screening but also act as antioxidant molecules, compatible solutes, intracellular nitrogen reservoir and aid in defense against thermal, desiccation and other stress conditions (Bandaranayake 1998; Oren and Gunde-Cimerman 2007; Rastogi et al. 2016; Richa et al. 2018). It has been found that low nitrogen nutrition results in decrement in the contents of Chl a and soluble proteins including RuBisCO in different cyanobacteria and algae (Beardall et al. 1991; Wulff et al. 2000). Supplementation of exogenous antioxidants and nitrogen source helps the organisms to overcome several abiotic stresses.
In the present study, certain doses of NH4Cl (50, 200 and 500 μM), positively influenced the growth of cyanobacterium Anabaena sp. HKAR-7 as indicated by changes in Chl a content. Here, 200 µM concentration of NH4Cl was found to be optimum as higher concentration of ammonium causes uncoupling of photophosphorylation in photoautotrophs and results in cellular toxicity, which becomes more pronounced under high light conditions (Britto and Kronzucker 2002; Zhu et al. 2000; Drath et al. 2008). Also, prolonged exposure to such combined stress (PAR + UV-B + NH4Cl) generates ROS (O2•¯, H2O2, OH•, 1O2) which results in significant reduction in the growth of cyanobacterium with increasing duration of UV-B exposure and concentration of NH4Cl. UV-B radiation exhibits detrimental effects on photosynthetic pigments which might be correlated to photoreduction of protochlorophyllide to chlorophyllide (Marwood and Greenberg 1996). Chlorophylls form complexes with proteins and lipids and thereby exist in a highly organized state. Hence, the decrement in Chl a content due to UVR exposure may be the result of degradation of lipids, proteins and their complexes associated with the thylakoid membrane (Prasad and Zeeshan 2005).
Carotenoids serve as important pigments which help in photoprotection against damaging effects of UVR. Increasing concentration of carotenoids in response to PAR + UV-B + NH4Cl stress is in accordance with its role as ROS scavenger in photoautotrophs, hence, providing crucial defense mechanism against photooxidation (Vincent and Quesada 1994; Pattanaik et al. 2008). Enhanced biosynthesis of carotenoids aids in increased utilization of light in the low and middle regions of the PAR spectrum and help in quenching the active oxygen species and free radicals (Paerl et al. 1983; Götz et al.1999). In this study, carotenoids content increased initially to prevent cyanobacterial cells from photooxidation. Under prolonged UV-B exposure, cells generated more ROS that might be a reason for decreased biosynthesis of carotenoids via photosynthesis, leading to marked decrease in its content. Besides, this decrease in carotenoids synthesis was least in nitrogen supplemented samples, which explains that its synthesis was more favourable under surplus nitrogen availability. Decrement in carotenoids content in turn affects PC, Chl a and thylakoid membrane adversely, resulting in the reduced photosynthetic efficiency of cyanobacteria. Phycobiliprotein in phycobilisomes are nitrogen storage compounds which funnel light energy to the underlying PSII reaction centres (Kannaujiya and Sinha 2015). Cyanobacterial phycobiliproteins are sensitive to degradation upon UV-B exposure as these are localized on the thylakoid’s outer surface membrane (Donkor and Häder 1991; Kannaujiya and Sinha 2015), however, this damage was quite less in cyanobacteria which were exposed to UV-B radiation along with supplementation of NH4Cl.
Damage to the photosynthetic apparatus on exposure to UV-B has been observed in several algae (Wulff et al. 2007; Bhandari and Sharma 2011). However, samples which were supplied with NH4Cl along with UV-B treatment seem to generate a quick repair mechanism after removal of UV-B stress. In cyanobacteria, D1 and D2 proteins of reaction centres (PSII) are sensitive to UVR and were found to be replaced immediately after UVR exposure and such rapid turnover of proteins of PSII reaction centre helps the organisms in acclimatizing to the stressed environment (Sicora et al. 2006). Repair of the damaged PSII occurs via energetically costly process of protein synthesis (Lesser et al. 1996) which might be one of the reasons for the increased repair capacity of the cells supplemented with external nitrogen source and this explains the least depressed Fv/Fm and ETRmax values in the cyanobacterial samples exposed to UV-B + NH4Cl. Phytoplankton show more sensitivity to UV-B radiation under nutrient-deficiency in comparison to nutrient-replete conditions and UV-B exposure under nutrient-deficiency damages the enzymes responsible for regulating the process of nitrate and ammonium uptake, hence, adversely affects the nitrogen metabolism (Döhler 1992; Lesser et al. 1994; Lohman et al. 1998). In this study also responses of cyanobacteria, mainly the recovery processes were modified during the UVR treatment on exogenous supplementation of NH4Cl in a dose dependent manner.
Induction of light-dependent MAA biosynthesis was higher in UV-B radiation as compared to PAR in cyanobacteria (Sinha et al. 2002; Richa 2015). Similarly, PAR and PAR + NH4Cl exposed cyanobacterial samples showed higher rate of Fv/Fm and ETRmax as compared to UVR exposed samples (PAR + UV-B and PAR + UV-B + NH4Cl). MAA performs its photoprotective function by absorbing highly energetic UVR and dissipating it to the surroundings as heat (Conde et al. 2004). Here, UV-B radiation induced P-334 in a dose dependent manner with increased duration of exposure. Addition of NH4Cl further enhanced MAAs biosynthesis and synergistically effected its induction along with UVR in Anabaena sp. HKAR-7 which was in accordance with the previous findings (Singh et al. 2008). Some studies have questioned the photoprotective role of MAA as its induction and accumulation was not observed in response to UVR or PAR exposure, also, it failed to provide complete protection to the organisms against UVR (Garcia-Pichel et al. 1993; Neale et al. 1998; Gröniger et al. 1999; Yakovleva and Titlyanov 2001). The accumulation and biosynthesis of MAA is not always attributed by solar radiation alone as several other abiotic stresses/factors such as salinity, temperature and availability of nutrients also induce its biosynthesis (Bandaranayake 1998; Dunlap and Shick 1998; Karsten and Wiencke 1999; Singh et al. 2020). Combined stress of PAR + UV-B + NH4Cl significantly induced biosynthesis of P-334 in comparison to exposure of PAR, PAR + NH4Cl, and PAR + UV-B indicating an MAA-specific induction which was triggered by exposure of PAR + UV-B + NH4Cl. Induction of MAAs biosynthesis is dependent on the quality (wavelength) as well as duration of incident radiation (Karsten et al. 1998a, b; Karsten and Wiencke 1999; Franklin et al. 2001). This explains the reduced damage to the cyanobacterial photosynthetic apparatus on exposure to UV-B radiation which helped the cyanobacteria in maintaining the photosynthetic yield in spite of decrement in the Chl a content. However, role of repair mechanisms such as de novo synthesis of D1 and D2 protein of PSII and photoreactivation cannot be ruled out in the absence of UVR exposure. Photoreactivation helps in repair of damaged DNA by the enzyme “DNA photolyase” which utilize blue wavelength of solar radiation for correcting the modified nitrogenous bases of DNA to their normal forms (Senger 1982; Britt 1996; Todo et al. 1996; Sinha and Häder 2002; Zhang et al. 2013).
Basic skeleton of MAAs are made up of cyclohexenone and cyclohexenimine cores, which mainly consist of nitrogen and carbon. The requirement of nitrogen was completed by NH4Cl. However, deprivation of carbon availability might limit the efficacy of the MAAs biosynthesis as it was found that photoheterotrophic growth condition was required in Anabaena sp. for synthesis of MAAs (Singh et al. 2014). There is possibility that cyanobacterium utilizes other cellular carbon compounds for MAAs biosynthesis. Nitrogen fixation is an energetically expensive physiological process, hence, cyanobacteria do not fix atmospheric nitrogen in the presence of available nitrogen (Pandey et al. 2018). In presence of exogenous nitrogen source cyanobacteria can allocate this energy in the biosynthesis of MAAs explaining higher MAA biosynthesis in presence of NH4Cl in the present study. Results from present investigation may help in controlling the growth of harmful cyanobacteria in water bodies by reducing the nutrient availability and increasing the levels of UV-B exposure. Sustainable cultivation of cyanobacteria at commercial scale is the major limiting factor in their optimum utilization for the production of biofertilizers, energy and numerous secondary metabolites of nutritional and medicinal values. Optimization of exogenous supplementation of key elements such as nitrogen may help in enhanced biomass production of cyanobacteria and can serve as sustainable agricultural practice for obtaining very high value cyanobacterial biomass.
Conclusion
Limited doses of external nitrogen in form of NH4Cl supported the growth of Anabaena sp. HKAR-7 and aided the organism in tolerating the adverse effects of UV-B as indicated by its photosynthetic activity (Fv/Fm and ETRmax) and pigment composition. Our results suggest that in addition to quantity and quality of the incident radiation, nutrient availability (optimum dose of NH4Cl) significantly enhanced the levels of MAAs in Anabaena sp. HKAR-7. Higher levels of photoprotective compound P-334 might play important role in protecting the cyanobacterium from lethal effects of prolonged UV-B exposure. Ammonium protected the cyanobacterium against lethal UVR not only by enhancing resistance by inducing MAA biosynthesis, but also by increased recovery of the crucial process of photosynthesis. These results can be used as one of the various ways for enhanced and sustainable production of value added compounds such as MAAs for their possible applications in cosmetics and pharmaceutical industries.
References
Ahmed H, Pathak J, Rajneesh PP, Singh PR, Sinha RP (2019) Genomics and proteomics of photoprotective compounds mycosporine-like amino acids in cyanobacteria. In: Sinha RP, Pandey S, Ghoshal N (eds) Innovations in life science research. Nova Science Publishers, Hauppauge, pp 97–128
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Balskus EP, Walsh CT (2010) The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 329:1653–1656. https://doi.org/10.1126/science.1193637
Banaszak AT, Neale PJ (2001) Ultraviolet radiation sensitivity of photosynthesis in phytoplankton from an estuarine environment. Limnol Oceanogr 46:592–603. https://doi.org/10.4319/lo.2001.46.3.0592
Bandaranayake WM (1998) Mycosporines: are they nature’s sunscreens? Nat Prod Rep 15:15972. https://doi.org/10.1039/A815159Y
Batista LFZ, Kaina B, Meneghini R, Menck CFM (2009) How DNA lesions are turned into powerful killing structures: Insights from UV-induced apoptosis. Mutat Res Rev 681:197–208. https://doi.org/10.1016/j.mrrev.2008.09.001
Beardall J, Roberts S, Millhouse J (1991) Effects of nitrogen limitation on uptake of inorganic carbon and specific activity of ribulose-1, 5 biphosphate carboxylase/oxygenase in green microalgae. Can J Bot 69:1146–1150. https://doi.org/10.1139/b91-147
Bhandari RR, Sharma PK (2011) Photosynthetic and biochemical characterization of pigments and UV-absorbing compounds in Phormidium tenue due to UV-B radiation. J Appl Phycol 23:283–292. https://doi.org/10.1007/s10811-010-9621-8
Britt AB (1996) DNA damage and repair in plants. Annu Rev Plant Biol 47:75–100. https://doi.org/10.1146/annurev.arplant.47.1.75
Britto DT, Kronzucker HJ (2002) NH4+ toxicity in higher plants: a critical review. J Plant Physiol 159:567–584. https://doi.org/10.1078/0176-1617-0774
Bryant DA, Guglielmi G, Tandeau de Marsac N, Castlets AM, Cohen-Bazire G (1979) The structure of cyanobacterial phycobilisomes: a model. Arch Microbiol 123:113–127. https://doi.org/10.1007/BF00446810
Conde FR, Churio MS, Previtali CM (2004) The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem Photobiol Sci 3:960–967. https://doi.org/10.1039/b405782a
Desikachary TV (1959) Cyanophyta. Indian Council of Agriculture Research, New Delhi. https://doi.org/10.4319/lo.2000.45.5.1144
Döhler G (1992) Impact of UV-B radiation on the uptake of 15N-ammonia and 15N-nitrate by phytoplankton of the Wadden Sea. Mar Biol 112:485–489. https://doi.org/10.1007/BF00356294
Donkor VA, Häder D-P (1991) Effects of solar and ultraviolet radiation on motility, photomovement and pigmentation in filamentous, gliding cyanobacteria. FEMS Microbiol Ecol 86:159–168. https://doi.org/10.1016/0378-1097(91)90661-S
Drath M, Kloft N, Batschauer A, Marin K, Novak J, Forchhammer K (2008) Ammonia triggers photodamage of photosystem II in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Physiol 147(1):206–215. https://doi.org/10.1104/pp.108.117218
Dunlap WC, Shick JM (1998) UV radiation absorbing mycosporine-like amino acids in coral reef organisms: a biochemical and environmental perspective. J Phycol 34:418–430. https://doi.org/10.1046/j.1529-8817.1998.340418.x
Franklin LA, Kräbs G, Kuhlenkamp R (2001) Blue light and UV-A radiation control the synthesis of mycosporine-like amino acids in Chondrus crispus (Florideophyceae). J Phycol 37:257–270. https://doi.org/10.1046/j.1529-8817.2001.037002257.x
Garcia-Pichel F, Wingard CE, Castenholz RW (1993) Evidence regarding the UV sunscreen role of a mycosporine like compound in the cyanobacterium Gloecapsa sp. Appl Environ Microbiol 59:170–176
Götz T, Whidhovel U, Boger P, Sandmann G (1999) Protection of photosynthesis against ultraviolet-B radiation by carotenes in transformants of the cyanobacterium Synechococcus PCC 7942. Plant Physiol 120:599–604. https://doi.org/10.1104/pp.120.2.599
Gröniger A, Hallier C, Häder D (1999) Influence of UV radiation and visible light on Porphyraumbilicalis: photoinhibition and MAA concentration. J Appl Phycol 11:437–445. https://doi.org/10.1023/A:1008179322198
Häder D-P, Williamson CE, Wängberg SÅ, Rautio M, Rose KC, Gao K, Helbling EW, Sinha RP, Worrest R (2015) Effects of UV radiation on aquatic ecosystems and interactions with other environmental factors. Photochem Photobiol Sci 14:108–126. https://doi.org/10.1039/c4pp90035a
Hoyer K, Karsten U, Sawall T, Wiencke C (2001) Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar Ecol Prog Ser 211:117–129. https://doi.org/10.3354/meps211117
Huovinen P, Matos J, Pinto IS, Figueroa FL (2006) The role of ammonium in photoprotection against high irradiance in the red alga Grateloupia lanceola. Aquat Bot 84:308–316. https://doi.org/10.1016/j.aquabot.2005.12.002
Jensen A (1978) Chlorophylls and carotenoids. In: Craige IS, Hellebust JA (eds) Handbook of phycological methods: physiological and biochemical methods. Cambridge University Press, Cambridge, pp 59–70
Kannaujiya VK, Sinha RP (2015) Impacts of varying light regimes on phycobiliproteins of Nostoc sp. HKAR-2 and Nostoc sp. HKAR-11 isolated from diverse habitats. Protoplasma 252:1551–1561. https://doi.org/10.1007/s00709-015-0786-5
Karsten U, Wiencke C (1999) Factors controlling the formation of UV-absorbing mycosporine-like amino acids in the marine red alga Palmaria palmata from Spitsbergen (Norway). J Plant Physiol 155:407–415. https://doi.org/10.1016/S0176-1617(99)80124-2
Karsten U, Franklin LA, Lüning K, Wiencke C (1998a) Natural ultraviolet and photosynthetic active radiation induce formation of mycosporine-like amino acids in the marine macroalga Chondrus crispus (Rhodophyta). Planta 205:257–262. https://doi.org/10.1007/s004250050319
Karsten U, Sawall T, Hanelt D, Bischof K, Figueroa FL, Flores-Moya A, Wiencke C (1998b) An inventory of UV absorbing mycosporine-like amino acids in macroalgae from polar to warm-temperate regions. Bot Mar 41:443–453. https://doi.org/10.1515/botm.1998.41.1-6.443
Korbee-Peinado N, Abdala-Díaz RT, Figueroa FL, Helbling EW (2004) Ammonium and UV radiation stimulate the accumulation of mycosporine-like amino acids (MAAs) in Porphyra columbina (Rhodophyta) from Patagonia, Argentina. J Phycol 40:248–259. https://doi.org/10.1046/j.1529-8817.2004.03013.x
Korbee-Peinado N, Huovinen P, Figueroa FL, Aguilera J, Karsten U (2005) Availability of ammonium influences photosynthesis and the accumulation of mycosporine-like amino acids in two Porphyra species (Bangiales: Rhodophyta). Mar Biol 146:645–654. https://doi.org/10.1007/s00227-004-1484-6
Kumar A, Tyagi MB, Jha PN, Srinivas G, Singh A (2003) Inactivation of cyanobacterial nitrogenase after exposure to ultraviolet-B radiation. Curr Microbiol 46:380–384. https://doi.org/10.1007/s00284-001-3894-3
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Lesser MP, Cullen JJ, Neale PJ (1994) Carbon uptake in a marine diatom during acute exposure to ultraviolet radiation: relative importance of damage and repair. J Phycol 30:183–192. https://doi.org/10.1111/j.0022-3646.1994.00183.x
Lesser MP, Neale PJ, Cullen JJ (1996) Acclimation of Antarctic phytoplankton to ultraviolet radiation: ultraviolet-absorbing compounds and carbon fixation. Mol Mar Biol Biotech 5:314–325
Litchman E, Neale PJ, Banaszak AT (2002) Increased sensitivity to ultraviolet radiation in nitrogen-limited dinoflagellates: photoprotection and repair. Limnol Oceanogr 47:86–94. https://doi.org/10.4319/lo.2002.47.1.0086
Lohman M, Döhler G, Huckenbeck N, Veridni S (1998) Effects of UV radiation of different wavebands on pigmentation, 15N-ammonium uptake, amino acid pools and adenylate contents of marine diatoms. Mar Biol 130:501–507. https://doi.org/10.1007/s002270050270
Marwood CA, Greenberg BM (1996) Effect of supplementary UV-B radiation on chlorophyll synthesis and accumulation of photosystems during chloroplast development in Spirodela oligorrhiza. Photochem Photobiol 64:664–670. https://doi.org/10.1111/j.1751-1097.1996.tb03121.x
Neale PJ, Banaszak AT, Jarriel CR (1998) Ultraviolet sunscreens in Gymnodinium sanguineum (Dinophyceae): mycosporine-like amino acids protect against inhibition of photosynthesis. J Phycol 34:928–938. https://doi.org/10.1046/j.1529-8817.1998.340928.x
Oren A, Gunde-Cimerman N (2007) Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol Lett 269:1–10. https://doi.org/10.1111/j.1574-6968.2007.00650.x
Paerl HW, Tucker J, Bland PT (1983) Carotene enhancement and its role in maintaining blue-green algal (Microcystis aeruginosa) surface blooms. Limnol Oceanogr 28:847–857. https://doi.org/10.4319/lo.1983.28.5.0847
Pandey A, Ahmed H, Singh V, Singh DK, Rajneesh PJ, Sinha RP (2018) Impacts of UV-B radiation on the enzymes of nitrogen metabolism in cyanobacteria. In: Sinha RP, Srivastava UP (eds) Trends in life science. Nova Science Publishers, Hauppauge, pp 243–287
Pandey A, Pathak J, Singh DK, Ahmed H, Singh V, Kumar D, Sinha RP (2020) Photoprotective role of UV-screening pigment scytonemin against UV-B-induced damages in the heterocyst-forming cyanobacterium Nostoc sp. strain HKAR-2. Braz J Bot 43:67–80. https://doi.org/10.1007/s40415-020-00589-5
Pathak J, Ahmed H, Rajneesh SSP, Häder DP, Sinha RP (2019) Genetic regulation of scytonemin and mycosporine-like amino acids (MAAs) biosynthesis in cyanobacteria. Plant Gene 17:100172. https://doi.org/10.1016/j.plgene.2019.100172
Pathak J, Rajneesh SPR, Häder D-P, Sinha RP (2019) UV-induced DNA damage and repair: a cyanobacterial perspective. Plant Gene 22:100194. https://doi.org/10.1016/j.plgene.2019.100194
Pattanaik B, Roleda MY, Schumann R, Karsten U (2008) Isolate-specific effects of ultraviolet radiation on photosynthesis, growth and mycosporine-like amino acids in the microbial mat-forming cyanobacterium Microcoleus chthonoplastes. Planta 227:907–916. https://doi.org/10.1007/s00425-007-0666-0
Porra RJ (2002) The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth Res 73:149–156. https://doi.org/10.1023/A:1020470224740
Prasad SM, Zeeshan M (2005) UV-B radiation and cadmium induced changes in growth, photosynthesis, and antioxidant enzymes of cyanobacterium Plectonema boryanum. Biol Plant 49:229–236. https://doi.org/10.1007/s10535-005-0236-x
Qin HJ, Peng CG, Liu YD, Li DH (2012) Differential responses of Anabaena sp. PCC 7120 (Cyanophyceae) cultured in nitrogen deficient and nitrogen-enriched media to ultraviolet-B radiation. J Phycol 48:615–625. https://doi.org/10.1111/j.1529-8817.2012.01162.x
Rajneesh CA, Pathak J, Ahmed H, Singh V, Singh DK, Pandey A, Singh SP, Richa HDP, Sinha RP (2018) Ultraviolet radiation-induced DNA damage and mechanisms of repair in cyanobacteria: an overview. In: Sinha RP, Shrivastava UP (eds) Trends in life science research. Nova Publisher, Hauppauge, pp 169–218
Rajneesh PJ, Richa Häder D-P, Sinha RP (2019) Impacts of ultraviolet radiation on certain physiological and biochemical processes in cyanobacteria inhabiting diverse habitats. Environ Exp Bot 161:375–387. https://doi.org/10.1016/j.envexpbot.2018.10.037
Rastogi RP, Incharoensakdi A (2013) UV radiation-induced accumulation of photoprotective compounds in the green alga Tetraspora sp. CU2551. Plant Physiol Biochem 70:7–13. https://doi.org/10.1016/j.plaphy.2013.04.021
Rastogi RP, Kumari S, Richa HT, Sinha RP (2012) Molecular characterization of hot spring cyanobacteria and evaluation of their photoprotective compounds. Can J Microbiol 58:719–727. https://doi.org/10.1139/w2012-044
Rastogi RP, Sonani RR, Madamwar D, Incharoensakdi A (2016) Characterization and antioxidant functions of mycosporine-like amino acids in the cyanobacterium Nostoc sp. R76DM. Algal Res 16:110–118. https://doi.org/10.1016/j.algal.2016.03.009
Richa SRP (2015) Biochemical characterization of sunscreening mycosporine-like amino acids from two Nostoc species inhabiting diverse habitats. Protoplasma 252:199–208. https://doi.org/10.1007/s00709-014-0674-4
Richa PP, Sonker AS, Singh V, Sinha RP (2018) Potential applications of natural bioactive cyanobacterial UV protective compounds. In: La Barre S, Bates S (eds) Blue technologies: production and uses of marine molecules. Wiley VCH, Weinheim, pp 693–717
Rippka R, Denuelles J, Waterbury JB, Herdman M, Stanier RY (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Roy S (2000) Strategies for the minimization of UV-induced damage. In: de Mora SJ, Demers S, Vernet M (eds) The effects of UV radiation in the marine environment. Cambridge University Press, Cambridge, pp 177–205
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Schreiber U (2004) Pulse-amplitude-modulation (PAM) fluorometry and saturation pulse method: an overview. In: Godvindjee GC (ed) Chlorophyll-a fluorescence: a signature of photosynthesis. Kluwer Academic Publishers, Dordrecht, pp 279–319
Senger H (1982) The effect of blue light on plants and microorganisms. Photochem Photobiol 35:911–920. https://doi.org/10.1111/j.1751-1097.1982.tb02668x
Sicora CI, Appleton SE, Brown CM, Chung J, Chandler J, Cockshutt AM, Vass I, Campbell DA (2006) Cyanobacterial psbA families in Anabaena and Synechocystis encode trace, constitutive and UV-B-induced D1 isoforms. Biochim Biophys Acta Bioenerg 1757:47–56. https://doi.org/10.1016/j.bbabio.2005.11.002
Singh SP, Klisch M, Häder D-P, Sinha RP (2008) Role of various growth media on shinorine (mycosporine-like amino acid) concentration and photosynthetic yield in Anabaena variabilis PCC 7937. World J Microbiol Biotechnol 24:3111–3115. https://doi.org/10.1007/s11274-008-9831-2
Singh SP, Ha SY, Sinha RP, Häder D-P (2014) Photoheterotrophic growth unprecedentedly increases the biosynthesis of mycosporine-like amino acid shinorine in the cyanobacterium Anabaena sp., isolated from hot springs of Rajgir (India). Acta Physiol Plant 36:389–397. https://doi.org/10.1007/s11738-013-1420-9
Singh DK, Richa KD, Chatterjee A, Rajneesh PJ, Sinha RP (2017) Response of the cyanobacterium Fischerella sp strain HKAR-5 against combined stress UV-B radiation, PAR and pyrogallic acid. JSM Environ Sci Ecol 5:1049
Singh DK, Pathak J, Pandey A, Ahmed H, Rajneesh KD, Sinha RP (2020) UV-screening compound mycosporine-like amino acids (MAAs) in cyanobacteria: biosynthesis, functions and applications. In: Singh PK, Kumar A, Singh VK, Shrivastava AK (eds) Advances in cyanobacterial biology. Elsevier Academic Press, Cambridge, pp 219–233. https://doi.org/10.1016/B978-0-12-819311-2.00015-2
Sinha RP, Häder D-P (2002) UV-induced DNA damage and repair: a review. Photochem Photobiol Sci 1:225–236. https://doi.org/10.1039/B201230H
Sinha RP, Häder D-P (2016) Effects of global change, including UV and UV screening compounds. In: Borowitzka MA, Beardall J, Raven JA (eds) The physiology of microalgae. Springer, Cham, pp 373–379
Sinha RP, Klisch M, Häder D-P (1999) Induction of a mycosporine-like amino acid (MAA) in the rice-field cyanobacterium Anabaena sp. by UV irradiation. J Photochem Photobiol B Biol 52:59–64. https://doi.org/10.1016/S1011-1344(99)00103-7
Sinha RP, Sinha JP, Gröniger A, Häder D-P (2002) Polychromatic action spectrum for the induction of a mycosporine-like amino acid in a rice-field cyanobacterium, Anabaena sp. J Photochem Photobiol B Biol 66:47–53. https://doi.org/10.1016/S1011-1344(01)00274-3
Todo T, Ryo H, Yamamoto K, Toh H, Inui T, Ayaki H, Nomura T, Ikenaga M (1996) Similarity among the Drosophila (6–4) photolyase, a human photolyase homology, and the DNA photolyase-blue-light photoreceptor family. Science 272:109–112. https://doi.org/10.1126/science.272.5258.109
Vincent WF, Quesada A (1994) Ultraviolet radiation effects on cyanobacteria: implications for Antarctic microbial ecosystems. In: Weiler CS, Penhale PA (eds) Ultraviolet radiation in antarctica: measurements and biological effects. Antarctic Research Series, American Geophysical Union, Washington, D.C., pp 111–124
Vincent WF, Roy S (1993) Solar ultraviolet-B radiation and aquatic primary production: damage, protection and recovery. Environ Rev 1:1–12. https://doi.org/10.1139/a93-001
Wulff A, Wängberg SA, Sundbäck K, Underwood GJC, Nilsson C (2000) Effects of UV-B radiation on a marine microphytobenthic community growing on a sand-substratum under different nutrient conditions. Limnol Oceanogr 45:1144–1152. https://doi.org/10.4319/lo.2000.45.5.1144
Wulff A, Mohlin M, Sundback K (2007) Intraspecific variation in the response of the cyanobacterium Nodularia spumigena to moderate UV-B radiation. Harmful Algae 6:388–399. https://doi.org/10.1016/j.hal.2006.11.003
Xia J, Li YJ, Zou D (2004) Effects of salinity stress on PSII in Ulvalactucaas probed by chlorophyll fluorescence measurements. Aquat Bot 80:129–137. https://doi.org/10.1016/j.aquabot.2004.07.006
Yakovleva IM, Titlyanov EA (2001) Effect of high visible and UV irradiance on subtidal Chondrus crispus: stress, photoinhibition and protective mechanisms. Aquat Bot 71:47–61. https://doi.org/10.1016/S0304-3770(01)00167-X
Zhang F, Scheerer P, Oberpichler I, Lamparter T, Krauss N (2013) Crystal structure of a prokaryotic (6–4) photolyase with an Fe–S cluster and a 6,7-dimethyl-8-ribityllumazine antenna chromophore. Proc Nat Acad Sci USA 110:7217–7222. https://doi.org/10.1073/pnas.1302377110
Zhu Z, Gerendas J, Bendixen R, Schinner K, Tabrizi H, Sattelmacher B, Hansen U-P (2000) Inhibitor-dependent stimulation of photosynthetic electron transport by far-red light in NO3− and NH4+ grown plants of Phaseolus vulgaris. L. Plant Biol 2:558–570. https://doi.org/10.1055/s-2000-7498
Acknowledgements
DKS (09/013(0612)/2015-EMR-I), JP ((09/013/0515/2013-EMR-I), AP (09/013(0619)/2016-EMR-I) and VS (09/013(0568)/2014-EMR-I) are thankful to Council of Scientific and Industrial Research (CSIR), New Delhi, India, for the financial support in the form of senior research fellowships. HA (UGC-JRF-21/12/2014(ii)EU-V) acknowledges University Grants Commission, New Delhi, India, for providing funds in the form of fellowship. Department of Biotechnology (DBT) and DST-INSPIRE, Govt. of India, are gratefully acknowledged for providing fellowships to Rajneesh and DK respectively. We are thankful to the Interdisciplinary School of Life Sciences (ISLS) and Laboratory of Scanning Electron Microscopy, Department of Geology, BHU, Varanasi, for providing access to the ESI-MS and SEM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Singh, D.K., Pathak, J., Pandey, A. et al. Response of a rice-field cyanobacterium Anabaena sp. HKAR-7 upon exposure to ultraviolet-B radiation and ammonium chloride. Environmental Sustainability 4, 95–105 (2021). https://doi.org/10.1007/s42398-020-00146-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42398-020-00146-6