Abstract
The ubiquitous occurrence, recalcitrance, bioaccumulation potential and toxicity of petroleum hydrocarbons have gained significant environmental concern. The petroleum hydrocarbon contaminations are frequent in the extreme environments where the temperature, pH, salt concentrations, and/or pressure vary from normal conditions. Bioremediation using naturally occurring microorganisms is a promising tool for removal of hydrocarbons from contaminated environments, since it is effective, inexpensive and eco-friendly. Degradation of hydrocarbons depends on various factors including structure and other properties of the target hydrocarbon, environmental conditions and, quantity and type of the microbes present at contaminated sites. Hydrocarbon-degrading microorganisms are usually present in diverse environments and the ability of these microbes to adapt under extreme environments are often exploited for bioremediation. In this review, the processes of extremophiles to degrade different constituents of petroleum hydrocarbons including polyaromatic and aliphatic hydrocarbons, have been explained for soil and marine ecosystems, in extreme environments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Interests of scientists in biodegradation of crude oil contamination in extreme environments have deepened in the last two decades. The group of microbes which resist the extreme environments are known as extremophiles. Extremophiles are broadly divided into five groups- thermophiles, psychrophiles, barophiles, alkaliphiles, acidophiles and halophiles, which are found in habitats such as hot springs, marine water, hydrothermal environment, high saline lakes, polar regions and glaciers or deep-sea sediments (Tango and Islam 2002; Arulazhagan et al. 2017b).
Crude oil and petroleum-based products are complex mixture of hydrocarbons which contain 60% of different types of alkanes, 30% aromatic compounds, while rest of it consists of heteroatoms such as sulfur (S), oxygen (O) and nitrogen (N) (Hazen et al. 2016). Petroleum-based products are the main resource of energy and serve as raw materials for some industries (Kvenvolden and Cooper 2003). It is estimated that 40% of the total energy requirement of the world is derived from crude oil (Brown and Skipsey 1986). However, leaks and accidental oil-spills that occur in the process of exploitation, transportation, refining, production and storage of petroleum and petroleum products causes serious degradation of land, air and water ecosystems (Holliger et al. 1997). The National Oil Spill Detection and Response Agency (NOSDRA) detected around 9343 incidents in the last 10 years (Ndimele et al. 2018). Though some countries have regulated the maximum amount of hydrocarbons that may be present in ballast water, yet it is assumed that the transportation of several tons of ballast water presents a serious threat to the marine ecosystems. It has been estimated that nearly, 7.5 million liters of ballast water gets discharged into the sea every hour (Nguyen and Dong 2018). There had been several oil spill accidents (Table 1) that have contaminated the oceans and shoreline environments.
Hydrocarbon contamination in the soil is known to cause extensive damage or mutations in animals and plants and therefore, had become a growing concern worldwide (Alvarez and Vogel 1991). The productivity of agricultural lands gets reduced (Dabbs 1996), and also, water bodies used for fishing become deceased due to hydrocarbon contaminations (Odokuma and Ibor 2002). It is well-known that contamination of hydrocarbons has damaged the coastal zones, seas, oceans and are hazardous to the Earth's health sustainability (McGenity et al. 2012). Previously, some areas of south and north poles, marine waters and saline lakes had been found to be highly contaminated with petroleum hydrocarbons (Li et al. 2019).
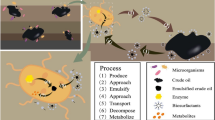
Bioremediation is the process where microorganisms acquire both, energy and carbon sources by metabolizing target organic pollutants (Thavasi et al. 2006). Microorganisms transform or detoxify organic pollutants into harmless products like CO2, H2O and other non-hazardous substances (Philp et al. 2005). Bioremediation of oil-contaminated soils by the natural population of microorganisms is efficient and, relatively cost-effective alternative to physiochemical approaches (Al-Mailem et al. 2010). However, occasionally, the absence of specialized microorganisms in contaminated soil or the poor quality of bacterial community is major limiting factor for the process of bioremediation (Ron and Rosenberg 2014).
The crude oil extraction and transportation sometimes involve, and therefore, contaminate extreme environments. In such environments, the traditional microorganisms used in bioremediation have lesser metabolic capacity and therefore, result in poor degradation, as they are either unable to perform optimally, or survive in extreme environments. Hence, extremophilic microbes should be considered for the bioremediation of crude oil contaminated extreme habitats, as extremophiles may adapt to adverse environmental conditions (Li et al. 2019).
There are several reviews on the degradation of petroleum hydrocarbons with several different perspectives, though this review is focused on their degradation in extreme environmental conditions. Pandey et al. (2016) reviewed different strategies for degradation of hydrocarbons by microorganisms and asserted on biodegradation by Pseudomonas, Mycobacterium, Rhodococcus, Arthrobacter, Aspergillus, Penicillium, Cyanobacteria, Chlorella and Candida, which are important for removal of hydrocarbon pollutants. There are several good reviews available which describe the role of microbes in the degradation of crude oil (Atlas 1981; Leahy and Colwell 1990). Nzila (2018a) reviewed the process of degradation of petroleum hydrocarbons by thermophiles and emphasized on their unique pathways for hydrocarbon catabolism. Si-Zhong et al. (2009) summarized the information available on bioremediation in cold environments, and also discussed the factors and fate of oil spilled in a cold environment. Another article on genomic characteristics of linear hydrocarbon degrading extremophiles under harsh environments has provided novel catabolic pathway for the bioremediation process (Park and Park 2018). Recently, Kotoky et al. (2018) explained the potential of omics approaches for better understanding of polycyclic aromatic hydrocarbons (PAHs) degradation in the rhizosphere, with relevant functional genes in soil microbiome that may maximize hydrocarbon degradation. Previously, Margesin and Schinner (2001) also summarized the bioremediation of hydrocarbon contaminants under extreme conditions. However, in this review, recent developments are described for the biodegradation potential of acidophilic, alkaliphilic or barophilic microorganisms, along with thermophiles, which has been elaborated. This has not been explained in detail in previous reviews.
Breakdown of petroleum hydrocarbons (PHs) in extreme environments
Microbial degradation of petroleum hydrocarbons is the main mechanism for the remediation of extreme environment polluted with petroleum products. An appropriate number of degraders, tolerant to environmental challenges are required for effective biodegradation (Thomassin-Lacroix et al. 2002). According to an estimate, microbial community of unpolluted environments comprises less than 1% of hydrocarbon degraders while crude-oil polluted soil and water may have 100% potential degraders (Atlas 1981). The extent and rate of degradation varies for different constituents of crude oil, for instance PAHs are difficult to degrade completely (Atlas and Bragg 2009). Still, extensive biodegradation of alkyl aromatic hydrocarbons by microorganisms have been reported in marine sediments (Jones et al. 1983). Bacterial strains had been reported to degrade petroleum hydrocarbons in the contaminated tropical stream in Lagos, Nigeria (Adebusoye et al. 2007). Several bacteria, namely Oleispira, Oleiphilus, Thalassolituus, Alcanivorax, and Cycloclasticus have been identified for removal of hydrocarbons from oil spills (Brooijmans et al. 2009). However, the petroleum hydrocarbons undergo a gradual weathering process which affects the metabolic activities and structure of microbial community (Atlas 1981).
The microbial hydrocarbon degradation may occur in either anaerobic or aerobic conditions; however, aerobic condition is considered superior for better degradation rate and degree of degradation (Bertrand et al. 1989). The hydrocarbon degradation in aerobic condition is facilitated by the activity of multicomponent dioxygenase, which catalyzes the incorporation of oxygen atoms into the aromatic nucleus to oxidize aromatic ring and transfer of two electrons from NADH to form cis-dihydrodiols which gets rearomatized to a diol intermediate. The subsequent dehydrogenation by an NAD+ dependent dehydrogenase yields dihydroxylated intermediates, these compounds are further oxidized through the ortho- or meta-cleavage pathways with the formation intermediates of the central metabolism such as acetyl-CoA, succinate, and pyruvate (Cerniglia 1992; Mueller et al. 1996; Mallick et al. 2011). Figure 1 illustrates the main principle of degradation of hydrocarbons.
Biodegradation of petroleum hydrocarbons involves a series of steps with the participation of different intra-and extracellular enzymes (Johnsen et al. 2005). These enzymes include peroxidases, dioxygenases, P450 monooxygenases, dehydrogenase, laccases, phosphatases, dehalogenases, nitrilases and nitroreductases (Singer et al. 2004; Chaudhry et al. 2005). Petroleum hydrocarbons can selectively be metabolized by an individual strain of microorganism, or microbial consortium of several strains belonging to either the same or different genera (Varjani and Upasani 2016). However, the microbial consortium has been reported to have more potential than individual strain for complete degradation of hydrocarbons (Varjani et al. 2015). The bioavailability of hydrophobic hydrocarbon to microbes is also important factor for their degradation. In fact, production of biosurfactant or bioemulsifier has been suggested to facilitate better attachment of bacterial cells to the substrate (Rahman et al. 2003).
Improved biodegradation of hydrocarbons by addition of biosurfactants producing extremophiles appears to be favorable for breakdown since they are stable at a wide range of pH, temperature, and salinity that enable it for use in adverse environments (Fig. 2). This enhances the bioavailability of pollutants and improve the solubility, hence, enhancing the rate of biodegradation (Sotivora et al. 2009). The cold-adapted microorganisms were reported to produce biosurfactants with low Krafft temperature and hence were recommended for the cold and harsh environments (Gu and Sjoblom 1992). It was observed that Acinetobacter and Rhodococcus were able to emulsify n-hexadecane (E24% > 50%) when n-hexadecane and diesel were utilized as carbon source at low temperature and high saline conditions. The Acinetobacter P1-1A was recorded with the highest emulsification ability (62.5%). The performance of biosurfactant production of Rhodococcus erythropolis P6-4P and Rhodococcus wratislaviensis P1-5P demonstrated that the surface tension was reduced to as low as 0.028 N m−1, which also stabilized the emulsion, and improved biosurfactant recovery through cell flocculation (Cai et al. 2014). Two efficient strains, Pseudomonas aeruginosa strain C450R and Halomonas sp. strain C2SS100 had been reported to remove 96.2% and 93.3% aliphatic hydrocarbons. P. aeruginosa was observed to degrade crude oil (2%, v/v), where the emulsifying activity and glycoside content was found to be recorded as 77 and 1.33 g l−1, respectively. This was due to the production of rhamnolipid biosurfactant which reduced the surface tension from 68 to 35.1 mN m−1. This was also thermally stable as supernatant retained the surface tension of 35.7 mN m−1 with E24 = 68% at a temperature range of 4–60 °C. However, above 60 °C, E24 was observed to change significantly and above 100 °C it was completely unstable. A halotolerant isolate of Bacillus licheniformis produced maximum amount of biosurfactant at 5% NaCl concentration, though it was able to produce the surfactant at 1–19% NaCl concentrations and 4.5–10.5 pH (Yakimov et al. 1995).
β-Ketoadipate metabolic pathway for the catabolism of aromatic compound by Halomonas organivorans (Zhuang et al. 2010)
Diversity of microorganisms involved in petroleum hydrocarbon degradation in extreme environments
The extremophiles are categorized into different categories, according to their adaptability to various extreme environments, and accordingly, the extremophilic hydrocarbon-degraders may be classified based on the environment, in which they degrade petroleum hydrocarbons. Few such examples are given in Table 2.
Thermophiles
High temperature significantly influences the bioavailability of PAH by decreasing the viscosity and increasing the diffusion coefficient (Arulazhagan et al. 2017a). Most of the extreme thermophilic and hyperthermophilic organisms are chemolithoautotrophs and heterotrophs. Microbial genera Pyrobaculum, Pyrodictium, Pyrococcus, Methanopyrus, Bacillus, Clostridium, and Thermus were found to grow up to 90–110 °C and metabolized naphthalene, phenanthrene, and anthracene (Muller et al. 1998). In another report, a Pseudomonas dominated consortium has been utilized for degradation of a wide range of aromatic hydrocarbons (Lugowski et al. 1997). Biodegradation pathways of phenanthrene in thermophilic bacterium Nocardia otitidiscaviarum are given in Fig. 3 (Nzila 2018b). The adaptability and stability of thermophilic bacteria at high temperature is associated with the presence of thermophilic proteins with stable structure, complex lipids in the membrane, and modified metal ions that increase the stability of the protein. Furthermore, thermophilic bacteria possess enzymes with special amino acids, hydrogen bond and ion pair (Li and Shao 2000; Shen and Shen 2010). In thermophiles, the branched chain C15 fatty acid content has been found to be iso-C15 fatty acid. The fatty acid composition (iso-fatty acids) in bacteria like Bacillus spp. have been observed to vary with increase in temperature. The iso-C15 content was found to be higher (30–50%) than that of ante-iso-C15 (lower than 10%) in Bacillus megaterium which can thrive between 45 and 70 °C (Sprott et al. 1997; Koga 2012). The thermophilic members of Bacillaceae family were observed to form endospore (varying in size), produced extracellular gelatinous materials, and formed well-structured cell walls while utilizing petroleum hydrocarbon (PAHs and NOS-asphaltene compounds) as sole source of carbon (Mohamed et al. 2006).
Phenanthrene biodegradation pathway in thermophilic bacterial strain Nocardia otitidiscaviarum (Nzila 2018a)
A thermophilic hydrocarbon degrader, Nocardia otitidiscaviarum TSH1 was able to metabolize a broad range of different hydrocarbons (phenol, n-alkanes and other PAHs) at 50 °C. Approximately 55, 10 and 40% of the initial amount of phenanthrene, anthracene, and pyrene, respectively, was observed to be metabolized by this strain (Zeinali et al. 2007). Thermophilic biosurfactant producing bacteria P. aeruginosa AP02-1 was able to degrade 99% of crude oil (v/v) and 2% (v/v) diesel oil at the optimum temperature of 45 °C within 7 days of incubation. The study was conducted with a combination of treatment as, biostimulation with NPK, application of rhamnolipid biosurfactant, and bioaugmentation with Geobacillus thermoleovorans T80, a thermophilic bacterium that led to the two folds faster removal of n-hexadecane at high temperature (60 °C). The degradation rate was found to be higher at 60 °C i.e. 91% and 48.5% at 18 °C. Expression of alkane monooxygenase gene (alkB) was induced in the presence of alkanes at 55 °C (Perfumo et al. 2006).
Chen and Taylor (1995, 1997) reported degradation of benzene, toluene, ethylbenzene, xylenes (BTEX) by co-metabolism of two different Thermus isolates. Bacillus and Geobacillus strains isolated from the volcanic island were found to degrade crude oil at high temperature, as they can degrade up to 46.64–76.6% and 56.53–87.68% respectively, of aliphatic compounds within 10 days of inoculation. Presence of alkane hydroxylase alkJ genes in ten isolates were confirmed by PCR analysis (Meintanis et al. 2006). Viamajala et al. (2007), isolated three Geobacilli sp. from compost that were able to solubilize phenanthrene at 60 °C. To understand the relationship between the biodegradation and temperature, phenanthrene solubilization at different temperature was experimented, and it was found that the zero-order phenanthrene solubilization rate was 1.31 ± 0.14 mg (l h)−1 at 60 °C, within 0–2 h. On the other hand, it was 0.019 ± 0.001 mg (l h)−1 at 20 °C. This result suggested that the biodegradation rate of phenanthrene was increased with elevated temperature. Geobacillus stearothermophilus A-2 isolated from a high temperature (73 °C) Dagang petroleum reservoir, has been reported to degrade long-chain n-alkanes (> C22-nC33) and aromatic hydrocarbons (naphthalene, phenanthrene, fluorene, benzo[b]fluorenes) and the highest degradation rate (89.83%) was observed (Zhou et al. 2016). Bacillus stearothermophilus which was isolated from an oil-polluted Kuwaiti desert was able to grow on pentadecane (C15), hexadecane (C16) and heptadecane (C17), while longer and shorter chains of n-alkanes were not readily utilized (Sorkhoh et al. 1993). A bacterial strain of Thermus brockii was found to degrade aliphatic and polyaromatic hydrocarbons at 60–70 °C optimum temperature. This strain showed high degradation rate of pyrene (40 mg h−1) and hexadecane (1000 mg h−1) mixture at high temperature (70 °C) and 6–7 pH range (Feitkenhauer et al. 2003).
Also, n-alkane degradation in thermophiles was reported to be initiated by the action of monooxygenase or dioxygenase leading to the formation of alcohol and fatty acids, which are further subjected to β-oxidation to generate energy and CO2. Genes involved in the degradation of n-alkane had been studied in mesophiles, especially in the genus Psuedomonas. The operon, alkBFGHJKL encode components of alkB system and the other operon alkT and alkS encode rubredoxin reductase that regulates the expression of the entire operon. The alkB gene had been reported to be present in thermophilic bacteria such as Geobacillus and Aeribacillus (Van Beilen and Funhoff 2007; Nie et al. 2014; Tourova et al. 2016). In G. thermoleovorans T70, the expression of alkB was observed to be induced by n-hexadecane which was able to degrade n-alkanes, at an optimum temperature of 55 °C (Marchant et al. 2006). The pathway for aerobic alkane degradation by Acinetobacter sp. M1 at high temperature (Park and Park 2018) is given (Fig. 4).
Aerobic alkane degradation pathway by Acinetobacter sp. M1 at high temperature (Park and Park 2018)
Psychrophiles
Cold-adapted microorganisms include bacteria, cyanobacteria, fungi and psychotropic archaea (Margesin and Schinner 2001). Some microorganisms have been reported to degrade petroleum hydrocarbons and alkanes at 4 °C and can degrade PAHs with high efficiency at low temperature (Siren et al. 1995; Michaud et al. 2004; Brakstad and Bonaunet 2006). At low temperature, the proportion of unsaturated and short-chain fatty acids had been reported to increase in these organisms, enriching the fluidity of the cell membrane. Colwellia psychrerythraea 34H was found to change the ratio between cis- and trans-esterified fatty acids in the phospholipids that enhanced membrane fluidity at low temperature (Methe et al. 2005). Psychrophilic Bacillus sp. has higher anteiso-C15 (around 50%) than iso-C15 (10–30%) (Koga 2012).
Generally, biodegradation rate of petroleum hydrocarbons decrease with the decrease in temperature, which may be attributed to reduced enzymatic activity rates. Also, the viscosity of the oil increases at low temperatures, which reduce the oil spreads in soil and aquatic matrices. Reduced solubility of oil in water at low temperature also retards the volatilization of short-chain alkanes (< C10) (Whyte et al. 1998; Niehaus et al. 1999; Bisht et al. 2015). Hazen et al. (2010) observed that at low temperatures (4–6 °C), half-lives of C13 to C26 alkanes was 1–2 days in deep water horizon plume samples, which was due to low microbial diversity. Similarly, Brakstad et al. (2015) observed half-life of alkanes was 1–2 weeks, while for PAHs it was up to 8 weeks at Norwegian coastal sea waters having low temperature (5 °C).
Psychrophilic microorganisms produce hydrocarbon-degradation enzymes encoded by genes which are capable to express and degrade hydrocarbons at low temperature. Such microorganisms produce cryoprotein, in low temperature, which affects the process of protein synthesis and protein folding (Xin and Zhou 1998; Phadtare 2004). Two psychrotrophs with ability to grow on hydrocarbon were identified as Pseudomonas spp. They were able to degrade n-alkanes (C5–C12), toluene and naphthalene at 5 °C and at 25 °C, through alk and nah catabolic pathways (Whyte et al. 1997). Presence of four alkane monooxygenase (albB1, alkB2, alkB3, and alkB4) in psychrotrophic Rhodococcus sp. strain Q15 has indicated that each monooxygenase was specific for a definite range of alkane (Whyte et al. 2002). C. psychrerythraea have been reported to harbor putative dioxygenases and monooxygenases for catabolism of complex petroleum hydrocarbon constituents. The enzymes responsible for the degradation of proteins and peptides were located external to the cytoplasm and also have σ-70 transcription factor for regulating extracellular polysaccharide biosynthesis in cold environments. The three-dimensional protein homology modeling and canonical discriminant analysis were utilized to induce the changes in 3D structure of proteins, which improved their catalytic activity for hydrocarbon degradation. When aspartate was replaced with glutamate, and polar moieties were reduced from the surface of the protein, there was increase in the flexibility and decrease in the thermostability of hydrocarbon degrading enzymes (Methe et al. 2005).
In an interesting report, several psychrotrophs were isolated from Antarctic soil, contaminated with crude oil. These isolates had the ability to degrade n-alkanes (from C6 to C20), which were the major constituents of pollutant (Bej et al. 2000). Different species of hydrocarbon-degrading bacteria at low temperatures include Halomonas, which was isolated from Antarctic sea (Milva et al. 2005), while Rhodococcus, or Sphingomonas dominated in Antarctic and Arctic soil (Lo Giudice et al. 2010; Yi-bin et al. 2014). The mixed communities of PAHs degraders were enriched from four different soil samples at 7 °C or 20 °C (Eriksson et al. 2003). It was reported that isolate Pseudomonas sp. DhA-91, n-alkane degrader and Sphingomonas sp. DhA-95 n-alkane and Jet fuel degrader were able to grow on respective substrate at 4–30 °C, while Pseudomonas sp. IpA-92, an aromatic hydrocarbon degrader grew at the range of 4–22 °C (Lo Giudice et al. 2010).
Two strains of Acinetobacter spp. T4 and 8mS were reported to degrade 20–34% of Arabian light crude oil, 14–27% of Dubai crude oil, 14–25% of Shanghai crude oil and 12–19% of Maya crude oil at 20 °C (Sugiura et al. 1997). Pham et al. (2014) isolated 11 oil-degrading bacterial strains that efficiently degraded 36–100% of oil and grew well at 10 °C. Other bacteria, including Pseudomonas simiae G1-100, Pseudomonas taiwanensis Y1-4, and P. koreensis Gwa2 showed 100% degradation while Rhodococcus frederiksbergensis G2-2, Pseudomonas arsenicoxydans Y2-1, Rhodanobacterumsongensis Gwa3, Pseudomonas migulae Gwa5, Rhodococcus jialingiae Y 1-l, and Rhodococcus qingshengii Y2-2 showed > 70% degradation of oil. Dias et al. (2015) analyzed the effect of fish meal, a complex organic nutrient source on biodegradation of hydrocarbon and composition of the bacterial community of Antarctic soil having mean temperature of 0.9 °C (max—7.7 °C and min—5.5 °C). They found that the Phylum Proteobacteria dominated the overall bacterial community, while the abundance of Actinobacteria was increased, and 71% of total hydrocarbon was removed after 30 days. More than 80% removal rate of petroleum hydrocarbon pollutants was achieved when oxygen content was increased through the soil tillage, in the Arctic diesel-contaminated soil (Paudyn et al. 2008). Similarly, bioaugmentation of psychrotolerant microbes resulted in removal of more than 80% of petroleum hydrocarbons from the soil of polar cold regions (Mohn et al. 2001). Equivalent to 62.3, 61.6 and 60.9% of crude oil (1000 mg l−1) was degraded by Chryseobacterium, Bacillus, and Pseudomonas respectively, at 10 °C, pH 7 and salinity of 10 g l−1. Also, application of these strains on artificially contaminated soil resulted in more than 70% removal of oil in 5 months duration at 10 °C (Wang et al. 2015).
Analysis by GC–MS and Raman spectrum of degradation products revealed that Planococcus sp. NJ41 and Shewanella sp. NJ49 can degrade diesel, n-hexadecane, PAHs and other petroleum hydrocarbons with high efficiency at low temperature (0–10 °C). GC–MS analysis showed the decomposition of a long straight chain of hydrocarbons into short-chain hydrocarbons and Raman spectrum showed the production of proteins and carbohydrates by these two bacteria during their growth and biodegradation, allowing it to adapt the extremely low temperature in Antarctica (Yi-bin et al. 2014). Biodegradation of diesel fuel in the microcosm of soil collected from defense establishment at Canada was observed to be slow, however it was significant at 0 °C, while at a temperature below 0 °C there was no evidence of the same process. Increase in the bioavailability of the substrates stimulated the hydrocarbon degradation by freeze–thaw cycles which lead to affect the composition of the microbial community (Eriksson et al. 2001). Rike et al. (2003) reported degradation of petroleum hydrocarbons at temperature below 0 °C, in situ condition in frozen soils at Svalbard.
Acidophiles
Acidophiles survive in highly acidic environments with pH < 5 (Brock et al. 1972). Hydrocarbon contaminated acidic environments such as mine drainage basins occasionally have elevated temperatures. Some studies on hydrocarbon degradation by acidophilic microorganisms have directed the existence of hydrocarbon degrading acidophiles (Margesin and Schinner 2001). Many acidophilic bacteria have been isolated and most of these isolates are autotrophic or mixotrophic, although heterotrophic acidophiles belonging to the genera Acidiphilium have been isolated from acidic mine effluent and were found to degrade a wide range of aliphatic hydrocarbons at pH 2–3 (Stapleton et al. 1998). Heterotrophic acidophiles and iron and sulfur-oxidizing chemolithotrophic acidophiles like Leptospirillum ferrooxidans and Acidithiobacillus ferrooxidans have been reported to exist by utilizing organic compounds (Johnson and Hallberg 2003).
High acidic environments can hinder the growth of microorganisms by the loss of catalytic enzyme activities and metabolisms. Only a few numbers of microorganisms are able to grow at high acidic conditions. In one of the report, 23 bacteria were isolated from acidic marine affluent which were acidophile and heterotrophs and these were able to utilize aliphatic hydrocarbons as carbon source at pH 3 (Alexander et al. 1987). Stapleton et al. (1998) reported two obligate acidophilic bacterial species Acidocella sp. strain LGS-3 and Acidiphilium facilities along with heterotrophic yeast Pichia sp. which have the ability to mineralize salicylic acid, naphthalene, phenanthrene and anthracene at pH 3. A mixed consortium of bacteria, fungus and yeast showed the degradation of more than 40% of total hydrocarbons and toluene into carbon dioxide and water. Recycle process of used oils results in the production of large amounts of acid resins. These resins contain mineral oil hydrocarbons, aromatic hydrocarbons, heavy metals and are extremely acidic in nature (0–1 pH). Acidophilic extremophiles were detected in the run-off zones of the resins (Kloos et al. 2006). A study on the bacterial communities in acidic soils with natural hydrocarbon seepage in Yellowstone national park having high sulfate concentration has revealed that heterotrophic acidophilic bacteria including Acidisphaera sp. and Acidiphilium sp. dominated the environment. Expression of alkB genes in the isolates (Acidithiobacillus ferrooxidans, Acidiphilium acidophilum, Acidocella sp., Acidiphilium acidophilum, Acidiphilium sp., Acidisphaera sp., Acidomonas methanolica, Gluconacetobacter sacchari, Acidisphaera rubrifaciens, Acidisphaera) suggested the hydrocarbon metabolism in sulfate contaminated acidic soils. It has been suggested that chemolithotrophic organism Acidithiobacillus ferrooxidans might supply Fe(III) to Acidiphilium sp. that could utilize hydrocarbons as the sole source of carbon and energy source and Fe(III) as an electron acceptor. The Acidisphaera related strain C197 possessed alkB gene and it has the ability to grow with 1% (v/v) n-dodecane or n-hexadecane as the sole source of carbon in minimal medium (pH 4.5) (Hamamura et al. 2005). Stenotrophomonas maltophilia strain AJH1 isolated from mineral mining site of Saudi Arabia was observed to degrade low molecularweight (LMW)-PAHs (anthracene, phenanthrene, naphthalene, fluorene) with a removal rate of 95% and high molecular weight (HMW)-PAHs (pyrene, benzo(e)pyrene and benzo(k) fluoranthene) with a removal rate of 80% at pH 2. The strain AJH1 was able to remove wastewater petroleum pollutants showing 89 ± 1.1% COD removal at acidic condition in a stirred reactor (Arulazhagan et al. 2017a).
Ivanova et al. (2014) isolated aerobic acidophilic bacteria Mycobacterium florentinum strain AGS10 from sulfur blocks of gas processing complex. The catabolic activity of hydroxylase families, alkB and cyp153 genes suggested the combined involvement in the degradation of hydrocarbons (iso-alkanes, toluene, naphthalene, phenanthrene, pristine, phytane as well as 2,2,4,4,6,8,8,-hepta-methylnonane, a branched hydrocarbon) by the strain at pH 2.5. An alkane with a longer chain, n-tricosane (C23H48) was found to be efficiently utilized (96% in 20 days) by this bacteria, butylcyclohexane was found to be an appropriate substrate for AGS10 degrading 93% in 20 days, however, degradation of n-hexacosane (C26H54) did not exceed 38%. Bacterial growth, hydrocarbon degradation, and surfactant production at different hydrocarbon mixtures showed a decrease in surface tension as low as 26.0 mN m−1, while interfacial tension against n-hexadecane was recorded as 1.3 mN m−1 (Ivanova et al. 2016). Microbial communities structure analysis suggested that Alphaproteobacteria belonging to Acidiphilium and Acidocella were predominantly found to be involved in natural petroleum degradation in the Dorset Coast, United Kingdom having pH 3.5 (Roling et al. 2006).
Alkaliphiles
Some microorganisms are able to grow in an alkaline environment by using sodium ions instead of proton for balancing inverted pH gradient and energy transformation (Arulazhagan et al. 2017b). Obligate alkaliphilic bacteria capable of producing enzymes with the potential of hydrocarbon degradation have been identified in mangrove ecosystems of Goa, West Coast of India. The alkaliphiles played a key role in the biogeochemical cycles and application in bioremediation of toxic aromatic compounds (Rasika et al. 2017). An alkaliphilic Mycobacterium sp. strain MHP-1 have been observed to degrade 50% pyrene during 7 days of incubation at high pH (9.0). Metabolite intermediates 4,5-phenanthrenedioic acid, 4-phenanthroic acid, and phthalic acid were detected in the culture medium. The strain possessed aromatic ring dioxygenase genes, which are homologs of nidAB genes from pyrene-degrading mycobacteria. The strain was also able to grow on fluoranthene, phenanthrene, and anthracene under alkaline condition (Habe et al. 2004). Pseudomonas toyotomiensis sp. HT-3T have been reported to decompose n-alkanes at pH level 6–10, isolated from a sample of soil immersed in water containing hydrocarbons in Toyotomi hot spring located in northern Hokkaido, Japan (Hirota et al. 2011). Facultative psychrophilic alkaliphilic Dietzia psychralcaliphila ILA-1T was identified to have the potential for in situ bioremediation of oil-contaminated soil and water. It was found to utilize d-glucose, d-fructose, propionate, valerate, 3-hydroxybutyrate, pyruvate, acetate, n-butyrate, isobutyrate, ethanol, n-tridecane, n-pentadecane, n-hexadecane, n-eicosane, n-tetracosane and pristine as a sole carbon source at optimum pH 10 (Yumoto et al. 2002).
Al-Awadhi et al. (2007) studied alkaliphilic hydrocarbon utilizing bacteria in Kuwaiti coast of the Arabian Gulf and hydrocarbon attenuation under alkaline and saline conditions. Alkaliphiles had been found to grow on different aromatic hydrocarbon compounds, crude oil, phenanthrene and n-alkane (C13–C40). The 16S rDNA analysis resulted in the identification of the degraders, which belonged to genera Marinobacter, Dietzia, Bacillus, Georgenia, Microbacterium, Stappia, Isoptericola, and Cellulomonas. The optimum pH for most of the genera involved in degradation process was 10, and NaCl concentrations ranged from 2.5 to 5%. Fahy et al. (2008) isolated benzene degrading strains from contaminated groundwater from SIReN aquifer, UK. Rhodococcus erythropolis, Arthrobacter, Actinobacteria, Hydrogenophaga, Pseudomonas strains were able to metabolize benzene, toluene, o-, m- or p-xylenes at a different pH range of 5.5–10. Alkaliphilic Bacillus licheniformis MTCC 5514 was observed to degrade the hydrocarbons, along with production of biosurfactant and enzymes at alkaline pH. The strain showed tolerance to pH 12 and degraded > 95% anthracene (300 ppm) within 22 days (Swaathy et al. 2014).
Barophiles
High pressure and low temperature are known to slow the rate of microbial activity and thus hydrocarbon degradation in deep oceans. Hydrocarbon degradation is dependent on ability of degrader microorganisms to adapt to high pressure (Marietou et al. 2018). Certain metabolic activities and enzymes responsible for hydrocarbon degradation were determined to be inhibited by high pressure. Rhodococcus qingshengii TUHH-12 had ability to degrade n-hexadecane at atmospheric pressure and high pressure conditions (0.1 MPa and 15 MPa). The growth rate of R. qingshengii TUHH-12 at 15 MPa was 0.16 h−1, while slightly higher rate (0.36 h−1) was observed at ambient pressure. The n-hexadecane degradation rate was found to be 0.035 mM h−1 at ambient pressure, and 0.019 mM h−1 at high pressure (Schedler et al. 2014). Similarly, significant differences in growth of Sphingobium yanoikuyae B1 with naphthalene was observed at different pressure. The pressure of up to 8.8 Mpa had shown little effect, however, growth was reduced with an increase in pressure and no growth was observed above 12 MPa. The effect of high pressure (20 Mpa), affected cellular components and processes like RNA transcription in bacteria. Membrane fluidity modifications and protein denaturation were observed at a pressure above 100 MPa and 400 MPa respectively. At 0.1 MPa, the naphthalene was observed to be completely degraded, with a degradation rate of 0.064 mM h−1 within 7–19 h. Substrate concentration was also observed to be decreased at a conversion rate of 0.054 mM h−1 (7–25 h), while 96.6% of naphthalene was found to be converted after 75 h of incubation (Aertsen et al. 2009; Schedler et al. 2014).
Hydrocarbon utilizing bacteria isolated from sediments at a depth of 4, 940 m consisted of Pseudomonas, Aeromonas, and Vibrio sp., which were observed to metabolize the hydrocarbons slowly under deep-sea conditions at 1 bar pressure (0.103 Mpa). In an old report, it was found that 93% of the hexadecane supplied in the medium was utilized within 4 weeks at 1 bar (0.103 MPa) and 4 °C, whereas it took 36 weeks to degrade similar amount at 500 bar (51.5 MPa) and 4 °C (Schwarz et al. 1975). Halotolerant bacterium Flavobacterium sp. isolated from a depth of 1945 m, was observed to tolerate 5% benzene, 10% toluene and 10% xylene and also degraded n-alkanes effectively. Another isolate identified as Bacillus sp. was observed to tolerate 10% benzene, 20% cyclohexane and 20% n-hexane and degraded PAHs (naphthalene, fluorene, anthracene, pyrene, chrysene, and 1,2-benzopyrene) (Aono and Inoue 1998). The microbial communities of the deep sea (2900 m) at Hatton–Rockall Basin were studied for the management of oil contamination, which had different temperature, and pressure conditions. The γ-proteobacteria, Pseudomonas and Colwellia, and several Bacteroidetes dominated at 30 MPa and 5 °C also, it was found that oil contamination did not exert a strong change in the bacterial community. The relative abundance was observed to be decreased for hydrocarbon degraders like Halomonas, Alcanivorax, and Marinobacter (Calderon et al. 2018). Piezosensitive hydrocarbonoclastic bacteria Alcanivorax jadensis KS_339 and Alcanivorax dieselolei KS_293 were found to grow rapidly after oil spills utilizing n-dodecane as the sole source of carbon at 10 MPa pressure corresponding to a depth of 500 m and 1000 m respectively (Scoma et al. 2016).
The survival of hydrocarbon degrader at the deep ocean and lithospheric sites require special adaptation for enzymatic actions, as high pressure can affect the binding capacity of enzymes to its substrates. Two outer membrane porin protein-encoding genes omgH and ompL were found to be abundantly expressed by Photobacterium sp. strain SS9 in high-pressure conditions up to 28 megapascals (MPa) (Arulazhagan et al. 2017b). Marietou et al. (2018) reported degradation of hydrocarbons under high-pressure conditions [0.1, 15 and 30 megapascals (MPa)] in the deep-water horizon at the Gulf of Mexico and observed Oleispira antarctica RB-8 related strains dominated in gamma-proteobacterial community. At 0.1 MPa, in crude oil degrading bacterial community, alphaproteobacterial members of the Sulfitobacter were found to be abundant, but at 30 MPa, the bacterial community shifted towards piezophiles, and the genus Photobacterium, was found to be abundant. Polaribacter sp. was abundant at 22 MPa. Bacteriodetes, Lutibacter sp. and Marinifilum sp. related with polycyclic hydrocarbon degradation, recovered from deep-sea horizon were found up to 15 MPa. Study also revealed the relative abundance of functional genes correlated with change in pressure. Also, they found that expression of alkB genes did not change in both 0.1 MPa and 15 MPa while upregulation of xylE genes was found to be six times higher at 0.1 MPa as compared to 15 MPa.
Saline environments
Halophiles are able to survive in extreme salt concentrations as they accumulate K+ and Cl− in its cells in order to maintain osmotic balance, which is attained by the action of the membrane-bound proton-pump bacteriorhodopsin. In hypersaline environment, the high water potential inside the cell protects the cellular proteins from denaturation and therefore, stabilizes the cellular architecture. The protein produced by halophiles is adapted to a high salt condition which also promotes folding of the riparophilic peptide chain and forms a functional protein which ensures the activity of the enzyme (Kong and Wang 2017; Mao and Guo 2018). Halophiles can be divided into three categories—slight halophiles (2–5% NaCl), moderate halophiles (5–20% NaCl) and extreme halophiles (20–35% NaCl) (Arulazhagan et al. 2017b). Numerous hypersaline environments such as saline industrial effluents, oil fields, natural saline lakes, salt marshes, and salt flats are found to be contaminated with petroleum hydrocarbons (Fathepure 2014). Seas, oceans and coastal zones are highly polluted by crude oil and it remains a major threat to the sustainability of planet Earth. It has been estimated that approximately 600,000 tons of petroleum reach the marine environment each year due to run-off from the land, off-shore oil drilling, and ballast water discharged from tankers (Kingston 2002; Ward et al. 2017). The higher molecular weight hydrocarbon degradation (C28–C36 of n-alkanes and 4–6 rings of PAHs) was found to be enhanced at the rate of 484% in extreme salinity and high internal resistance of the saline-alkali soil. This was believed to be due to enzyme activities and biological electron transfer leading to the enrichment of the species belonging to Flavobacteriia (Bacteroidetes), δ-Proteobacteria or Clostridium (Firmicutes). The study also revealed that activities of naphthalene dioxygenase (nah) and xylene monooxygenase (tol) were stimulated in bio-electrochemical remediation system (Li et al. 2016). The pathway of aromatic hydrocarbon degradation by halophiles has been outlined in Fig. 2.
Martins and Peixoto, (2012) estimated the rate of hydrocarbon degradation at a different range of salinities (0, 35, 50, 80, 120 and 160 g l−1). They observed 100% degradation of phenanthrene and dibenzothiophene at 35 g l−1, 75% of pristane and around 80% of noctadecane at salinities of 35 and 80 g l−1 respectively. Ahmadi et al. (2017) isolated three salt tolerant bacteria (Kocuria turfanesis strain M7, Halomonas alkaliphila strain R4 and Pseudomonas balearica strain Z8) from petrochemical wastewater of southwest of Iran containing 3% salinity. The bacterial consortium was efficient in the removal of COD by about 78.7–61.5% in saline wastewater, but the degradation rate was found to decrease with increased organic pollutant i.e. 0.33–1.21 kg COD m−3 day−1. Archaeal species grown on hypersaline environment showed the degradation of different types of polyaromatic hydrocarbons. Ten halophilic archaeal strains isolated from five different hypersaline environments were able to degrade mixture of benzoic acid, p-hydroxybenzoic acid, salicylic acid, naphthalene, anthracene, phenanthrene, pyrene, and benzo[a]anthracene in presence of 20% NaCl. 16S rDNA sequence analysis revealed that all the isolated species were found to belong to the genus Haloferax, and were identified as H. alexandrinus, H. alexandrines, H. prahovense, H. sulfurifontis strains. All the isolates were observed to degrade different polyaromatic hydrocarbons along with a reduction in COD in hypersaline crude oil reservoirs (Bonfa et al. 2011).
Recently, Xu et al. (2019) isolated two halotolerant phenanthrene degrading bacteria from Yellow River Delta, near Shengli oil field China, belonging to Delftia sp. and Achromobacter sp. which were able to degrade several polyaromatic (phenanthrene, pyrene, naphthalene, fluoranthene) as well as aliphatic (C12, C16, C20, C32) hydrocarbons with 7–8 optimum pH range at 28 °C. Maximum degradation was found to be 150–200 mg l−1, with k1 value 0.1974 and 0.1070 per day, respectively at a high salt concentration up to 4% (m/v). Degradation of phenanthrene was found to proceed through “phthalic acid” pathway by Delftia sp. and “naphthalene” metabolism pathway by Achromobacter sp. A NaCl-tolerant Enterobacter cloacae variant (MU-1) obtained by mutagenesis using atmospheric pressure glow discharge (APGD) plasmas, increased TPH degradation rate by 2.5 folds. Degradation of TPH was found to be higher (7.94%) in presence of 7.5% NaCl in case of mutant strain as compared to the wild type which was recorded as 3.17% (Hua et al. 2010). Neifar et al. (2018) reported the genomic characteristics of a halotolerant Halomonas desertis G11 that utilized crude oil as carbon source and had optimum growth at 10% NaCl. The strain had genes for alkane degradation that leads to β-oxidation and TCA cycle (alk pathway) and phenanthrene degradation through salicylate metabolism.
Petroleum-derived aromatic hydrocarbons (alkyl aromatics) and n-alkanes of crude oil have been reported to be degraded by Pseudomonas, Burkholderia, Mycobacterium, Anthrocebacter, Sphingomonas, and Rhodococcus in marine sediments (Jones et al. 1983). Nine bacterial strains namely, P. aeruginosa, P. fluorescents, Bacillus sp., B. subtilis, Acinetobacter lwoffi, Alcaligenes sp., Micrococcus roseus, Flavobacterium sp., and Corynebacterium sp. were observed to degrade petroleum hydrocarbons in a polluted tropical stream in Lagos, Nigeria (Adebusoye et al. 2007). Bacterial isolates from pristine and commercial sites of marine environment were found to be rapid aromatic degrader of hydrocarbons (Jones et al. 1983) and also, asphaltenes were degraded by co-metabolism using mix cultures of these isolates (Perry 1984; Rontani et al. 1985). A study on n-alkanes and polyaromatic hydrocarbons (PAHs) degradation in two communities of Niger Delta showed that microbial mineralization took place in both marine and freshwater environment. Naphthalene, acenaphthene, pyrene, and chrysene were degraded more in marine, while fluorene and anthracene are more degraded in freshwater. The presence of aerobic degraders in larger quantity in the marine environment, as compared to freshwater environment was suggested to be the reason for this observation (Onibiyo 2016). The 16S rDNA gene analysis attained six taxonomy classes of PAHs degrading bacteria in Indonesia marine areas viz. α-Proteobacteria (31%), γ-Proteobacteria (43%), Firmicutes Bacilli (12%), Actinobacteria; Micrococcales (9%), Actinobacteria; Propionibacteriales (2%), and Bacteroidetes; Flavobacteriia (3%). Bacteria showed the ability to degrade phenanthrene (20 isolates), dibenzothiophene (38 isolates), fluorene (25 isolates), naphthalene (25 isolates), phenothiazine (23 isolates), and pyrene (15 isolates) (Yetti et al. 2016).
Cometabolism
Cometabolism has been used in bioremediation to improve biodegradation of pollutants as several compounds can not be utilized as carbon source by the microbes directly. Therefore, cometabolism approach permits the ability of the microorganisms to degrade such contaminants without using it as a substrate for growth but assimilate another compound to support its growth (Nzila 2013). Cometabolising microorganisms have been reported to be highly effective in bioremediation processes. For instance, benzo(a)pyrene has been found to be biomineralized to CO2 by Pseudomonas saccharophila in presence of growth substrate phenanthrene or salicylate (Chen and Aitken 1999). This strategy has been developed to improve the degradation of pollutants in mesophilic conditions (Nzila 2013). Qin et al. (2017) showed the use of glucose by Mycobacterium sp. strain M. CSW3 as growth promoting substrate but inhibited the expression of enzymes involved in PAHs degradation in mesophilic condition. Though, in another report, cometabolism in thermophiles has been reported, where the strain B. subtilis BUM, which was otherwise unable to utilize benzo(a)pyrene as sole substrate, degraded it when a growth promoting substrate phenanthrene was added in medium (Zhao and Wong 2010). Similarly, a consortium of Geobacillus sp. and Thermus sp. degraded acenaphthene, fluoranthene, pyrene and benzo(a)pyrene when n-alkane was used as a growth-promoting substrate (Feitkenhauer and Markl 2003). Thus, the prospects of using cometabolism are high, for better degradation of hydrocarbons by extremophiles. Metabolomics features analyzed by GC–MS and genetic systems of a marine isolate, Shewanella oneidensis MR-1 revealed co-metabolic degradation pathways of toxic metals and organic compounds when exposed to several carbon sources (Tang et al. 2007). Co-metabolism study on degradation of benzo(a)pyrene suggested that rate of degradation was reduced by the addition of glucose and other organic acids like malic, succinic and tartaric. Also, proteomic analysis indicated that usage of glucose hinders enzymes expression. Therefore, in co-metabolism it is also important to assess the influence of growth-promoting substrates on other unrelated physiological activities of bacteria (Nzila 2018a).
Future perspectives
There are several reviews that have addressed some of the unique features of extremophiles (Saxena et al. 2017; Nzila 2018a; Li et al. 2019). It’s recommended that the remediation of organic xenobiotics should be studied at the metaorganism level (Bell et al. 2014). Application of omics tools like metagenomics, proteomics and transcriptomics, data of functional genes present in communities of different contaminated environments such as sediments and marine water (DeLong et al. 2006; Yooseph et al. 2007), acid mine drainage (Tyson et al. 2005) have provided novel insights into the adaptation of organisms, and the process of metabolic breakdown of environmental pollutants with novel mechanisms has been discovered. Mukherjee et al. (2017) explored taxonomic and functional variation from different oil-polluted sites. Emerging techniques like high-throughput DNA sequencing provided abilities for discovering novel microorganisms with the capability to degrade hydrocarbons, with new dioxygenases (Fathepure 2014; Guerra et al. 2018). Better understanding of the community dynamics related to hydrocarbon degradation and diversity of catabolic pathways will be useful to design a reliable technology for bioremediation processes for extreme environments. Metagenomic analysis of thermophilic Anhoni and Tattapani hot springs in central India revealed the abundance of Pseudomonas stutzeri and Acidovorax sp. (in Anhoni) and Pyrobaculumaerophilum (in Tattapani) and the genes responsible for degradation of benzene, benzoate, toluene, xylene were observed (Saxena et al. 2017). The community analysis of extreme environments may be utilized further for designing culturomics strategies (Lagier et al. 2018), which may provide novel unexplored gene pools for degradation of oil in extreme environments (Fig. 5).
Functional metagenomics permits the identification of genes involved in the degradation of various pollutants (Ufarte et al. 2015). Further research can be done to isolate and confirm novel genes, directly from the metagenome, encoding for highly active and stable enzymes to degrade petroleum hydrocarbons in extreme conditions. Metabolomics can play an important role in providing the degradation pathways of microbial communities and their functional features because of which microorganisms survive on exposure to pollutants. Metabolite profiles were studied by GC–MS analysis during the removal of phenanthrene and several intermediates of TCA cycles were observed in Sinorhizobium sp. C4 (Keum et al. 2009). Metatranscriptomics provides a link between structure and functions of microbial activity and can also determine the active enzymes expressed under certain conditions which are encoded by the transcript. Proteomics combined with metabolomics and transcriptomics have been used to provide information on how proteins of thermophiles adapt to rise in temperature (Lacerda and Reardon 2009). These approaches will allow to study the conformational stability of the protein with microorganisms’ response when exposed to pollutants and changes that occur during degradation process. Microbial remediation in extreme environments can be made efficient by coupling metagenomic and metatranscriptomic strategies along with bioinformatics analysis. A computational network analysis for biodegradation will provide sufficient information related to enzymes and compounds to be degraded. In a study, network analysis of 15 degraders from hydrocarbon contaminated permafrost revealed that Burkholderia was found to co-occur with members of Sphingomonas, Novosphingobium, Nocardioides and Phenylobacetrium. Another node of Bradyrhizobium was connected to members of Rhodococcus, Arthrobacter and Burkholderia. Phenylobacterium, which is an uncommon degrader, often discovered from contaminated sites, was found to connect with well-known degraders in the network analysis (Yang et al. 2016). Researchers have already started using various sequencing techniques for remediation of extreme environments however it is also necessary to upgrade reference database for the standardized protocol. Nevertheless, another major challenge will be the production of cell-biomass at large scale, and also, development of suitable formulations for application of extremophiles at contaminated sites.
Conclusion
Extremophiles have been utilized for microbial degradation of crude oil, as they have been reported from contaminated areas under extreme temperature, pH, pressure and salt concentrations. This emphasizes on the metabolic capabilities of extremophilic microorganisms for the purpose of biodegradation, however, deeper understanding of degradation-mechanisms and related enzymes are still limited. Extremophiles adapted to more than one extreme environment have the potential to decontaminate habitats where diverse extremities prevail simultaneously. The mechanisms of these microorganisms to adapt extreme environments and specific ways to remove pollutants need attention and required to be explored. There is no sufficient information on degradation of nitrated halogenated contaminants and HMW-PAHs in extreme environments. The petroleum hydrocarbon contaminated environments in extreme environments are usually ignored for ecological risk assessment. Therefore, it’s also recommended that the bioremediation strategies designed with extremophiles should also focus on sustainable measures, and reduce the ecological risk. Also, it’s significant to mention that the technology developed for the degradation of petroleum hydrocarbons using the strategies mentioned here, will be of enormous commercial significance, in addition to their use for the environmental clean-up. Considering this, there is an imminent need for strategic and systematic research on extremophiles with focus on their environmental application. Hence, it will be important to explore and apply extremophilic microbes for the efficient remediation of petroleum hydrocarbon contaminated sites, in extreme conditions, in an eco-friendly manner.
References
Adebusoye SA, Ilori MO, Amund OO, Teniola OD, Olatope SO (2007) Microbial degradation of petroleum hydrocarbons in a polluted tropical stream. World J Microbiol Biotechnol 23(8):1149–1159
Aertsen A, Meersman F, Hendrickx MEG, Vogel RF, Michiels CW (2009) Biotechnology under high pressure: applications and implications. Trends Biotechnol 27:434–441. https://doi.org/10.1016/j.tibtech.2009.04.001
Ahmadi M, Jorfi S, Kujlu R, Ghafari S, Soltani RDC, Haghighifard NJ (2017) A novel salt-tolerant bacterial consortium for biodegradation of saline and recalcitrant petrochemical wastewater. J Environ Manag 191:198–208
Al Jazeera (2007) Tanker oil spill off S Korea coast
Al-Awadhi H, Sulaiman RHD, Mahmoud HM, Radwan SS (2007) Alkaliphilic and halophilic hydrocarbon-utilizing bacteria from Kuwaiti coasts of the Arabian Gulf. Appl Microbiol Biotechnol 77(1):183–186
Alexander B, Leach S, Ingledew WJ (1987) The relationship between chemiosmotic parameters and sensitivity to anions and organic acids in the acidophile Thiobacillus ferrooxidans. J Gen Microbiol 133:1171–1179
Al-Mailem DM, Sorkhoh NA, Al-Awadhi H, Eliyas M, Radwan SS (2010) Biodegradation of crude oil and pure hydrocarbons by extreme halophilic archaea from hypersaline coasts of the Arabian Gulf. Extremophiles 14:321–328. https://doi.org/10.1007/s00792-010-0312-9
Alvarez PJJ, Vogel TM (1991) Substrate interactions of benzene, toluene, and para-xylene during microbial degradation by pure cultures and mixed culture aquifer slurries. Appl Environ Microbiol 57(10):2981–2985
Annweiler E, Richnow HH, Antranikian G, Hebenbrock S, Garms C, Franke S, Michaelis AW (2000) Naphthalene degradation and incorporation of naphthalene-derived carbon into biomass by the thermophile Bacillus thermoleovorans. Appl Environ Microbiol 66. http://aem.asm.org/
Aono R, Inoue A (1998) Organic solvent tolerance in microorganisms. In: Horikoshi K, Grant WD (eds) Extremophiles: microbial life in extreme environments. Wiley-Liss, New York, pp 287–310
Arulazhagan P, Al-Shekri K, Huda Q, Godon JJ, Basahi JM, Jeyakumar D (2017a) Biodegradation of polycyclic aromatic hydrocarbons by an acidophilic Stenotrophomonas maltophilia strain AJH1 isolated from a mineral mining site in Saudi Arabia. Extremophiles 21(1):163–174
Arulazhagan P, Mnif S, Rajesh Banu J, Huda Q, Jalal MAB (2017b) HC-0B-01: biodegradation of hydrocarbons by extremophiles. In: Biodegradation and bioconversion of hydrocarbons, pp 137–162
Atlas RM (1981) Microbial degradation of petroleum hydrocarbons: an environmental perspective. Microbiol Rev 45(1):180–209
Atlas R, Bragg J (2009) Bioremediation of marine oil spills: when and when not—the Exxon Valdez experience. Microb Biotechnol 2(2):213–221
Barakat KM, Hassan SWM, Darwesh OM (2017) Biosurfactant production by haloalkaliphilic Bacillus strains isolated from Red Sea, Egypt. Egypt J Aquat Res 43:205–211
Baraniecki CA, Aislabie J, Foght JM (2002) Characterization of Sphingomonas sp. Ant 17, an aromatic hydrocarbon degrading bacterium isolates. Microb Ecol 43:44–45
Bej AK, Saul D, Aislabie J (2000) Cold-tolerant alkane-degrading Rhodococcus species from Antarctica. Polar Biol 23:100–105
Bell TH, Joly S, Pitre FE, Yergeau E (2014) Increasing phytoremediation efficiency and reliability using novel omics approaches. Trends Biotechnol 32(5):271–280
Bertrand JC, Caumette P, Mille G, Gilewics M, Denis M (1989) Aerobic biodegradation of hydrocarbons. In: Science progress. Oxford, pp 333–350
Bisht S, Pandey P, Bhargava B, Sharma S, Kumar V, Sharma KD (2015) Bioremediation of polyaromatic hydrocarbons (PAHs) using rhizosphere technology. Braz J Microbiol 46(1):7–21
Bland B (2018) Rescuers battle toxic oil blaze off China coast. Financial Times
Bonfa MRL, Grossman MJ, Mellado E, Durrant LR (2011) Biodegradation of aromatic hydrocarbons by Haloarchaea and their use for the reduction of the chemical oxygen demand of hypersaline petroleum produced water. Chemosphere 84(11):1671–1676
Brakstad OG, Bonaunet K (2006) Biodegradation of petroleum hydrocarbons in seawater at low temperatures (0–5 °C) and bacterial communities associated with degradation. Biodegradation 17:71–82
Brakstad OG, Throne-Holst M, Netzer R, Stoeckel DM, Atlas RM (2015) Microbial communities related to biodegradation of dispersed Macondo oil at low seawater temperature with Norwegian coastal seawater. Microb Biotechnol 8(6):989–998
Brock TD, Brock KM, Belly RT, Weiss RL (1972) Sulfolobus: a new genus of sulfur oxidizing bacteria living at low pH and high temperature. Arch Microbiol 84:54–68
Brooijmans RJ, Pastink MI, Siezen RJ (2009) Hydrocarbon-degrading bacteria: the oil-spill clean-up crew. Microb Biotechnol 2(6):587–594
Brown GC, Skipsey E (1986) Energy resources, geology, supply and demand. Open University Press, Milton Keynes
Burgherr P (2007) In-depth analysis of accidental oil spills from tankers in the context of global spill trends from all sources. J Hazard Mater 140:245–256
Cai Q, Zhang B, Chen B, Zhu Z, Lin W, Cao T (2014) Screening of biosurfactant producers from petroleum hydrocarbon contaminated sources in cold marine environments. Mar Pollut Bull 86:402–410
Calderon LJP, Gontikaki E, Potts LD, Shaw S, Gallego A, Anderson JA, Witte U (2018) Pressure and temperature effects on deep-sea hydrocarbon degrading microbial communities in subarctic sediments. Microbiol Open. https://doi.org/10.1002/mbo3.768
Cerniglia CE (1992) Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3:351–368. https://doi.org/10.1007/BF00129093
Chaudhry Q, Blom-Zandstra M, Gupta S, Joner EJ (2005) Utilizing the synergy between plants and rhizosphere microorganisms to enhance breakdown of organic pollutants in the environment. Environ Sci Pollut Res 12:34–48
Chen SH, Aitken MD (1999) Salicylate stimulates the degradation of high molecular weight polycyclic aromatic hydrocarbons by Pseudomonas saccharophila P15. Environ Sci Technol 33:435–439
Chen CI, Taylor RT (1995) Thermophilic biodegradation of BTEX by two Thermus species. Biotechnol Bioeng 48:614–624
Chen CI, Taylor RT (1997) Batch and fed-batch bioreactor cultivations of a Thermus species with thermophilic BTEX-degrading activity. Appl Microbiol Biotechnol 47:726–733
Dabbs WC (1996) Oil production and environmental damage. http://www.american.edu.TED/hpl.htm. Accessed 20 May 2019
Delille D (2000) Response of Antarctic soil bacterial assemblages to contamination by diesel fuel and crude oil. Microb Ecol 40(2):159–168
DeLong EF, Preston CM, Mincer T, Rich V, Hallam SJ, Frigaard NU et al (2006) Community genomics among stratified microbial assemblages in the ocean’s interior. Science 311:496–503. https://doi.org/10.1126/science.1120250
Dias RL, Ruberto L, Calabro A, Lo Balbo A, Del Panno MT, Mac Cormack WP (2015) Hydrocarbon removal and bacterial community structure in on-site biostimulated biopile systems designed for bioremediation of diesel-contaminated Antarctic soil. Polar Biol 38(5):677–687
Eriksson M, Ka J, Mohn WW (2001) Effects of low temperature and freeze–thaw cycles on hydrocarbon biodegradation in Arctic Tundra soil. Appl Environ Microbiol 67(11):5107–5112
Eriksson M, Sodersten E, Yu Z, Dalhammar G, Mohn WW (2003) Degradation of polycyclic aromatic hydrocarbons at low temperature under aerobic and nitrate-reducing conditions in enrichment cultures from Northern soils. Appl Environ Microbiol 69:275–284
Fahy A, Ball AS, Lethbridge G, Timmis KN, McGenity TJ (2008) Isolation of alkali-tolerant benzene-degrading bacteria from a contaminated aquifer. Lett Appl Microbiol 47(1):60–66. https://doi.org/10.1111/j.1472-765X.2008.02386.x
Fathepure BZ (2014) Recent studies in microbial degradation of petroleum hydrocarbons in hypersaline environments. Front Microbiol. https://doi.org/10.3389/fmicb.2014.00173
Feitkenhauer H, Markl H (2003) Biodegradation of aliphatic and aromatic hydrocarbons at high temperatures. Water Sci Technol 47:123–130
Feitkenhauer H, Muller R, Markl H (2003) Degradation of polycyclic aromatic hydrocarbons and long chain alkanes at 60–70 °C by Thermus and Bacillus spp. Biodegradation 14(6):367–372
Grossi V, Yakimov MM, Al Ali B, Tapilatu Y, Cuny P, Goutx M et al (2010) Hydrostatic pressure affects membrane and storage lipid compositions of the piezotolerant hydrocarbon-degrading Marinobacter hydrocarbonoclasticus strain#5. Environ Microbiol 12:2020–2033
Gu T, Sjoblom J (1992) Surfactant structure and its relation to the krafft point, cloud point and micellization: some empirical relationships. Colloids Surf 64:39–46
Guerra AB, Oliveira JS, Silva-Portela RC, Araujo W, Carlos AC, Vasconcelos ATR et al (2018) Metagenome enrichment approach used for selection of oil-degrading bacteria consortia for drill cutting residue bioremediation. Environ Pollut 235:869–880. https://doi.org/10.1016/j.envpol.2018.01.014
Habe H, Kanemitsu M, Nomura M, Takemura T, Iwata K, Nojiri H, Omori T (2004) Isolation and characterization of an alkaliphilic bacterium utilizing pyrene as a carbon source. J Biosci Bioeng 98(4):306–308. https://doi.org/10.1263/jbb.98.306
Hamamura N, Olson SH, Ward DM, Inskeep WP (2005) Diversity and functional analysis of bacterial communities associated with natural hydrocarbon seeps in acidic soils at Rainbow Springs, Yellowstone National Park. Appl Environ Microbiol 71(10):5943–5950. https://doi.org/10.1128/AEM.71.10.5943-5950.2005
Hao R, Lu A (2009) A biodegradation of heavy oils by halophilic bacterium. Prog Nat Sci 19:997–1001
Hazen TC, Dubinsky EA, DeSantis TZ, Andersen GL, Piceno YM, Singh N, Jansson JK, Probst A, Borglin SE, Fortney JL et al (2010) Deep-sea oil plume enriches indigenous oil-degrading bacteria. Science 330:204–208
Hazen TC, Prince RC, Mahmoudi N (2016) Marine oil biodegradation. Environ Sci Technol 50(5):2121–2129
Hirota K, Yamahira K, Nakajima K, Nodasaka Y, Okuyama H, Yumoto I (2011) Pseudomonas toyotomiensis sp. nov., a psychrotolerant facultative alkaliphile that utilizes hydrocarbons. Int J Syst Evolut Microbiol 61(8):1842–1848. https://doi.org/10.1099/ijs.0.024612-0
Holliger C, Gaspard S, Glod G et al (1997) Contaminated environments in the subsurface and bioremediation: organic contaminants. FEMS Microbiol Rev 20(3–4):517–523
Hua X, Wang J, Wu Z, Zhang H, Li H, Xing X, Liu Z (2010) A salt tolerant Enterobacter cloacae mutant for bioaugmentation of petroleum- and salt-contaminated soil. Biochem Eng J 49(2):201–206
Hwang H, Hu X, Zhao X (2007) Enhanced bioremediation of polycyclic aromatic hydrocarbons by environmentally friendly techniques. J Environ Sci Health Part C 25:313–352
Issa N, Vempatti S (2018) Oil spills in the Arabian Gulf: a case study and environmental review. Environ Nat Resour Res 8(2)
Ivanova AE, Sukhacheva MV, Kanat’eva AY, Kravchenko IK, Kurganov AA (2014) Hydrocarbon-oxidizing potential and the genes for n-alkane biodegradation in a new acidophilic mycobacterial association from sulfur blocks. Microbiology 83(6):764–772. https://doi.org/10.1134/s0026261714060095
Ivanova AE, Sokolova DS, Kanat’eva AY (2016) Hydrocarbon biodegradation and surfactant production by acidophilic mycobacteria. Microbiology 85(3):317–324
Johnsen AR, Wick LY, Harms H (2005) Principle of microbial PAH-degradation in soil. Environ Pollut 133:71–84
Johnson DB, Hallberg KB (2003) The microbiology of acidic mine waters. Res Microbiol 154:466–473
Jones DM, Douglas AG, Parkes RJ, Taylor J, Giger W, Schaffner C (1983) The recognition of biodegraded petroleum-derived aromatic hydrocarbons in recent marine sediments. Mar Pollut Bull 14(3):103–108
Kate B (2017) Ten years later: the lessons of the Cosco Busan oil spill. The six fifty
Keum YS, Seo JS, Qing XL, Kim JH (2009) Comparative metabolomic analysis of Sinorhizobium sp. C4 during the degradation of phenanthrene. Appl Microbiol Biotechnol 80:863–872
Kingston PF (2002) Long-term environmental impact of oil spills. Spill Sci Technol Bull 7:53–61
Kloos K, Schloter M, Meyer O (2006) Microbial activity in an acid resin deposit: biodegradation potential and ecotoxicology in an extremely acidic hydrocarbon contamination. Environ Pollut 144(1):136–144. https://doi.org/10.1016/j.envpol.2005.12.022
Koga Y (2012) Thermal adaptation of the archaeal and bacterial lipid membranes. Archaea 2012:789652
Kong FJ, Wang XJ (2017) Halophilic microrganisms in salt lakes on Tibet Plateau and their potential application. Sci Technol Rev 35(12):27–30
Kostka JE, Prakash O, Overholt WA, Green SJ, Freyer G, Canion A, Delgardio J, Norton N, Hazen TC, Huettel M (2011) Hydrocarbon-degrading bacteria and the bacterial community response in Gulf of Mexico beach sands impacted by the deepwater horizon oil spill. Appl Environ Microbiol 77(22):7962–7974
Kotoky R, Rajkumari J, Pandey P (2018) The rhizosphere microbiome: significance in rhizoremediation of polyaromatic hydrocarbon contaminated soil. J Environ Manag 217:858–870
Kvenvolden KA, Cooper CK (2003) Natural seepage of crude oil into the marine environment. Geo Mar Lett 23(3–4):140–146
Lacerda CMR, Reardon KF (2009) Environmental proteomics: applications of proteome profiling in environmental microbiology and biotechnology. Brief Funct Genom Proteomic 8:75–87. https://doi.org/10.1093/bfgp/elp005
Lagier JC, Dubourg G, Million M, Cadoret R, Bilen M, Fenollar F, Levasseur A, Rolain JM, Fournier PE, Raoult D (2018) Culturing the human microbiota and culturomics. Nat Rev Microbiol 16:540–550
Leahy JG, Colwell RR (1990) Microbial degradation of hydrocarbons in the environment. Microbiol Rev 54(3):305–315
Leewis MC, Uhlik O, Leigh MB (2016) Synergistic processing of biphenyl and benzoate: carbon flow through the bacterial community in polychlorinated-biphenyl-contaminated soil. Sci Rep 6:22145
Li HY, Shao JY (2000) The thermophilic mechanism of thermophilic baeteria. Chin Bull Life Sci 12(1):30–33
Li X, Wang X, Zhang Y, Zhao Q, Yu B, Li Y, Zhou Q (2016) Salinity and conductivity amendment of soil enhanced the bioelectrochemical degradation of petroleum hydrocarbons. Sci Rep 6:32861. https://doi.org/10.1038/srep32861
Li H, Li X, Yu T, Wang F, Qu C (2019) Study on extreme microbial degradation of petroleum hydrocarbons. Mater Sci Eng 484:012040
Lo Giudice A, Bruni V, De Domenico M, Michaud L (2010) Psychrophiles—cold-adapted hydrocarbon-degrading microorganisms. In: Handbook of hydrocarbon and lipid microbiology. https://doi.org/10.1007/978-3-540-77587-4.139
Lugowski AJ, Palamteer GA, Boose TR, Merriman JE (1997) Biodegradation process for detoxifying liquid streams. Patent US5656169
Mallick S, Chakraborty J, Dutta TK (2011) Role of oxygenases in guiding diverse metabolic pathways in the bacterial degradation of low-molecular-weight polycyclic aromatic hydrocarbons: a review. Crit Rev Microbiol 37:64–90
Mao X, Guo JF (2018) Functional protein research progression in extremophiles. Chin Bull Life Sci 30(01):107–112
Marchant R, Sharkey FH, Banat SI, Rahman TJ (2006) The degradation of n-hexadecane in soil by thermophilic geobacilli. FEMS Microbiol Ecol 56(1):44–54
Margesin R, Schinner F (2001) Biodegradation and bioremediation of hydrocarbons in extreme environments. Appl Microbiol Biotechnol 56(5–6):650–663
Marietou A, Chastain R, Beulig F, Scoma A, Hazen TC, Bartlett DH (2018) The effect of hydrostatic pressure on enrichments of hydrocarbon degrading microbes from the Gulf of Mexico following the deepwater Horizon oil spill. Front Microbiol. https://doi.org/10.3389/fmicb.2018.00808
Martin L, Eddie O (2019) We cannot swim, we cannot eat’: Solomon Islands struggle with nation’s worst oil spill. Guardian (ISSN 0261-3077)
Martins LF, Peixoto RS (2012) Biodegradation of petroleum hydrocarbons in hypersaline environments. Braz J Microbiol 43:865–872
Mayra C, Steve A (2017) Keystone pipeline leaks 210,000 gallons of oil in South Dakota
Mazoomdaar J (2017) Oil spill: we’re well prepared on paper but sluggish response made preparedness a joke. Indian Express
McGenity TJ, Folwell BD, McKew BA, Sanni GO (2012) Marine crude-oil biodegradation: a central role for interspecies interactions. Aquat Biosyst 8:10
Meintanis C, Chalkou KI, Kormas KA, Karagouni AD (2006) Biodegradation of crude oil by thermophilic bacteria isolated from a volcano island. Biodegradation 17(2):105–111. https://doi.org/10.1007/s10532-005-6495-6
Methe BA, Nelson KE, Deming JW, Momen B, Melamud E, Zhang XJ, Moult J, Madupu R, Nelson WC, Dodson RJ, Brinkac LM, Daugherty SC, Durkin AS, DeBoy RT, Kolonay JF, Sullivan SA, Zhou LW, Davidsen TM, Wu M, Huston AL, Lewis M, Weaver B, Weidman JF, Khouri H, Utterback TR, Feldblyum TV, Fraser CM (2005) The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc Natl Acad Sci USA 102:10913–10918
Michaud L, Lo Giudice A, Saitta M, De Domenico M, Bruni V (2004) The biodegradation efficiency on diesel oil by two psychrotrophic Antarctic marine bacteria during a two-month long experiment. Mar Res Bull 49:405–409
Mike S (2019) Bunker fuel spilled from Maersk ship at port of Hong Kong. gCaptain
Milva P, Attilio C, Gianfranco L, Franco B (2005) An Antarctic psychrotrophic bacteria Halomonas sp. ANT-3b, growing on n-hexadecane, produces a new emulsifying glycolipid. FEMS Microbiol Ecol 53:157–166
Mohamed ME, Al-Dousarya M, Hamzaha RY, Fuchs G (2006) Isolation and characterization of indigenous thermophilic bacteria active in natural attenuation of bio-hazardous petrochemical pollutants. Int Biodeterior Biodegrad 58:213–223
Mohn WW, Radziminski CZ, Fortin MC et al (2001) On site bioremediation of hydrocarbon-contaminated Arctic tundra soil in inoculated biopiles. Appl Microbiol Biotechnol 57:242–247
Mueller JG, Cerniglia CE, Pritchard PH (1996) Bioremediation of environments contaminated by polycyclic aromatic hydrocarbons. In: Crawford RL, Crawford LD (eds) Bioremediation: principles and applications. Cambridge University Press, Cambridge, pp 125–194
Mukherjee A, Chettri B, Langpoklakpam JS, Basak P, Prasad A, Mukherjee AK et al (2017) Bioinformatic approaches including predictive metagenomic profiling reveal characteristics of bacterial response to petroleum hydrocarbon contamination in diverse environments. Sci Rep 7:1–22. https://doi.org/10.1038/s41598-017-01126-3
Muller R, Antranikian G, Maloney S, Sharp R (1998) Thermophilic degradation of environmental pollutants. In: Antranikian G (ed) Biotechnology of extremophiles. Advances in biochemical engineering, bio-technology, vol 61. Springer, Berlin, pp 155–169
PTI Mumbai (2010) Mumbai oil spill continues, 300 containers tumbled into water so far. The Times of India
Nazarov M, Gorodyankin G (2014) Oil spills into Black Sea near Russian port after pipeline leak. Reuters
Ndimele PE, Saba AO, Ojo DO, Ndimele CC, Anetekhai MA, Erondu ES (2018) Remediation of crude oil spillage. In: The political ecology of oil and gas activities in the Nigerian aquatic ecosystem. https://doi.org/10.1016/b978-0-12-809399-3.00024-0
Neifar M, Chouchane H, Najjari A, Hidri DE, Mahjoubia M, Ghedira K, Naili F, Soufia L, Raddadi N, Sghaier H, Ouzari HI, Masmoudi AS, Cherif A (2018) Genome analysis provides insights into crude oil degradation and biosurfactant production by extremely halotolerant Halomonas desertis G11 isolated from Chott El-Djerid salt-lake in Tunisian desert. Genomics
Nguyen XP, Dong VH (2018) A Report of the Impacts of Pollutants on Maritime Operation in Vietnam. Eur J Eng Res Sci 3(10):120–125
Nie Y, Chi CQ, Fang H, Liang JL, Lu SL, Lai GL, Tang YQ, Wu XL (2014) Diverse alkane hydroxylase genes in microorganisms and environments. Sci Rep 4:4968
Niehaus F, Bertoldo C, Kähler M, Antranikian G (1999) Extremophiles as a source of novel enzymes for industrial application. Appl Microbiol Biotechnol 51:711–729
Nzila A (2013) Update on the cometabolism of organic pollutants by bacteria. Environ Pollut 178:474–482
Nzila A (2018a) Current status of the degradation of aliphatic and aromatic petroleum hydrocarbons by thermophilic microbes and future perspectives. Int J Environ Res Public Health 15(12):2782. https://doi.org/10.3390/ijerph15122782
Nzila A (2018b) Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons under anaerobic conditions: overview of studies, proposed pathways and future perspectives. Environ Pollut 239:788–802
Odokuma LO, Ibor MN (2002) Nitrogen fixing bacteria enhanced bioremediation of crude oil polluted soil. Glob J Pure Appl Sci 8(4):455–468
Onibiyo S (2016) Biodegradation of petroleum hydrocarbons in contaminated coastal environments, Nigeria. Thesis, Georgia State University. https://scholarworks.gsu.edu/geosciences_theses/100. Accessed 14 May 2019
Pandey P, Pathak H, Dave S (2016) Microbial ecology of hydrocarbon degradation in the soil: a review. Res J Environ Toxicol 10(1):1–15
Park C, Park W (2018) Survival and energy producing strategies of alkane degraders under extreme conditions and their biotechnological potential. Front Microbiol 9:1081
Pashaei R, Gholizadeh M, Iran KJ, Hanifi A (2015) The effects of oil spills on ecosystem at the Persian Gulf. Int J Rev Life Sci 5(3):82–89
Paudyn K, Rowe K et al (2008) Remediation of hydrocarbon contaminated soils in the Canadian Arctic by landfarming. Cold Reg Sci Technol 53:102–114
Perfumo A, Banat IM, Marchant R (2006) The use of thermophilic bacteria in accelerated hydrocarbon bioremediation. WIT Trans Ecol Environ 88:67–77
Perry JJ (1984) Microbial metabolism of cyclic alkanes. In: Atlas RM (ed) Petroleum microbiology. Macmillan, New York, pp 61–98
Phadtare S (2004) Recent developments in bacterial cold-shock response. Curr Issues Mol Biol 6(2):125–136
Pham VH, Kim J, Jeong SW (2014) Enhanced isolation and culture of highly efficient psychrophilic oil-degrading bacteria from oil-contaminated soils in South Korea. J Environ Biol 35(6):1145–1149
Philp J, Bamforth S, Singleton I et al (2005) Environmental pollution and restoration: a role for bioremediation. In: Bioremediation: applied microbial solutions for real-world environmental cleanup, 1st edn
Qin W, Fan F, Zhu Y, Wang Y, Liu X, Ding A, Dou J (2017) Comparative proteomic analysis and characterization of benzo(a)pyrene removal by Microbacterium sp. strain M.CSW3 under denitrifying conditions. Bioprocess Biosyst Eng 40:1825–1838
Rahman KSM, Rahman TJ, Kourkoutas Y, Petsas I, Marchant R, Banat IM (2003) Enhanced bioremediation of n-alkane in petroleum sludge using bacterial consortium amended with rhamnolipid and micronutrients. Bioresour Technol 90(2):159–168
Rasika DG, Sunita B, Saroj B (2017) Utilisation of toxic aromatic compounds by alkaliphilic bacteria isolated from mangrove ecosystems of Goa. Int J Environ Sci Toxicol Res 5(2):36–40
Rike AG, Haugen KB, Borresen M, Engene B, Kolstad P (2003) In situ biodegradation of petroleum hydrocarbons in frozen arctic soils. Cold Reg Sci Technol 37:97–120
Rojo F (2010) Enzymes for aerobic degradation of alkanes. In: Handbook of hydrocarbon and lipid microbiology. Springer, pp 781–797
Roling WFM, Ortega-Lucach S, Larter SR, Head IM (2006) Acidophilic microbial communities associated with a natural, biodegraded hydrocarbon seepage. J Appl Microbiol 101(2):290–299
Ron EZ, Rosenberg E (2014) Enhanced bioremediation of oil spills in the sea. Curr Opin Biotechnol 27:191–194
Rontani JF, Bosser-julak F, Rambeloarisoa E, Bertrand JC, Giusti G, Faure R (1985) Analytical study of Asthart crude oil asphaltenes biodegradation. Chemosphere 14:1413–1422
Saxena R, Dhakan DB, Mittal P, Waiker P, Chowdhury A, Ghatak A, Sharma VK (2017) Metagenomic analysis of hot springs in central india reveals hydrocarbon degrading thermophiles and pathways essential for survival in extreme environments. Front Microbiol 7:2123. https://doi.org/10.3389/fmicb.2016.02123
Schedler M, Hiessl R, Juarez AGV, Gust G, Muller R (2014) Effect of high pressure on hydrocarbon-degrading bacteria. AMB Express 4:77
Schwarz JR, Walker JD, Colwell RR (1975) Deep-sea bacteria: growth and utilization of n-hexadecane at in situ temperature and pressure. Can J Microbiol 21:682–687
Scoma A, Barbato M, Hernandez-Sanabria E, Mapelli F, Daffonchio D, Borin S, Boon N (2016) Microbial oil-degradation under mild hydrostatic pressure (10 MPa): which pathways are impacted in piezosensitive hydrocarbonoclastic bacteria? Sci Rep 6:23526. https://doi.org/10.1038/srep23526
Shen JS, Shen B (2010) Thermostability mechanism of thermophilic enzyme. J Microbiol 2(30):80–85
Singer AC, Thompson IP, Bailey MJ (2004) The tritrophic trinity: a source of pollutant degrading enzymes and its implication for phytoremediation. Curr Opin Microbiol 7:239–244
Siren R, Pelletier E, Brochu C (1995) Environmental factors influencing the biodegradation of petroleum hydrocarbons in cold seawater. Arch Environ Contam Toxicol 28:406–416
Si-Zhong Y, Hui-Jun J, Zhi W, Rui-Xia H, Yan-Jun J, Xiu-Mei L, Shao-Peng Y (2009) Bioremediation of oil spills in cold environments: a review. Pedosphere 19(3):371–381
Sorkhoh N, Ibrahim A, Ghannoum MA, Radwan S (1993) High-temperature hydrocarbon degradation by Bacillus stearothermophilus from oil-polluted Kuwaiti desert. Appl Microbiol Biotechnol. 39:123–126
Sotivora A, Spasova D, Vasileva-Tonkova E, Galabova D (2009) Effect of rhamnolipid biosurfactant on cell surface of Pseudomonas aeruginosa. Microbiol Res 164(3):297–303
Sprott GD, Agnew BJ, Patel GB (1997) Structural features of ether lipids in the archaeobacterial thermophiles Pyrococcus furiosus, Methanopyrus kandleri, Methanothermus fervidus, and Sulfolobus acidocaldarius. Can J Microbiol 43(5):467–476
Stapleton RD, Savage DC, Sayler GS, Stacey G (1998) Biodegradation of aromatic hydrocarbons in an extremely acidic environment. Appl Environ Microbiol 64(11):4180–4184
Sugiura K, Ishihara M, Shimauchi T, Harayama S (1997) Physicochemical properties and biodegradability of crude oil. Environ Sci Technol 31:45–51
Swaathy S, Kavitha V, Pravin AS, Mandal AB, Gnanamani A (2014) Microbial surfactant mediated degradation of anthracene in aqueous phase by marine Bacillus licheniformis MTCC 5514. Biotechnol Rep 4:161–170
Swannell RPJ, Lee K, McDonagh M (1996) Field evaluations of marine oil spill bioremediation. Microbiol Rev 60:342
Tang YJ, Hwang JS, Wemmer DE, Keasling JD (2007) Shewanella oneidensis MR-1 fluxome under various oxygen conditions. Appl Environ Microbiol 73:718–729. https://doi.org/10.1128/AEM.01532-06
Tango MSA, Islam MR (2002) Potential of extremophiles for biotechnological and petroleum applications. Energy Sources 24:543–559
Thavasi R, Jayalakshmi S, Balasubramanian T, Banat IM (2006) Biodegradation of crude oil by nitrogen fixing marine bacteria Azotobacter chroococcum. Res J Microbiol 1:401–408
Thomassin-Lacroix E, Eriksson M, Reimer K, Mohn W (2002) Biostimulation and bioaugmentation for on-site treatment of weathered diesel fuel in Arctic soil. Appl Microbiol Biotechnol 59(4–5):551–556
Tourova TP, Sokolova DS, Semenova EM, Shumkova ES, Korshunova AV, Babich TL, Poltaraus AB, Nazina TN (2016) Detection of n-alkane biodegradation genes alkB and ladA in thermophilic hydrocarbon-oxidizing bacteria of the genera Aeribacillus and Geobacillus. Microbiology 85:693–707
Tyson GW, Lo I, Baker BJ, Allen EE, Hugenholtz P, Banfield JF (2005) Genome-directed isolation of the key nitrogen fixer Leptospirillum ferrodiazotrophum sp. nov. from an acidophilic microbial community. Appl Environ Microbiol 71:6319–6324. https://doi.org/10.1128/AEM.71.10.6319-6324.2005
Ufarte L, Laville E, Duquesne S, Potocki-Veronese G (2015) Metagenomics for the discovery of pollutant degrading enzymes. Biotechnol Adv 33:1845–1854
Van Beilen JB, Funhoff EG (2007) Alkane hydroxylases involved in microbial alkane degradation. Appl Microbiol Biotechnol 74:13–21
Varjani SJ, Upasani VN (2016) Biodegradation of petroleum hydrocarbons by oleophilic strain of Pseudomonas aeruginosa NCIM 5514. Bioresour Technol 222:195–201
Varjani SJ, Rana DP, Jain AK, Bateja S, Upasani VN (2015) Synergistic ex situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int Biodeterior Biodegrad 10:116–124
Viamajala S, Peyton BM, Richards LA, Petersen JN (2007) Solubilization, solution equilibria, and biodegradation of PAH’s under thermophilic conditions. Chemosphere 66(6):1094–1106. https://doi.org/10.1016/j.chemosphere.2006.06.059
Vince G (2003) Prestige oil spill far worse than thought. NewScientist
Wang J, Wang J, Zhang Z, Li Y, Zhang B, Zhanga Z, Zhang G (2015) Cold-adapted bacteria for bioremediation of crude oil-contaminated soil. J Chem Technol Biotechnol 91(8):2286–2297
Ward EJ, Adkison M, Couture J, Dressel SC, Litzow MA, Moffitt S et al (2017) Evaluating signals of oil spill impacts, climate, and species interactions in Pacificherring and Pacificsal mon populations in Prince William Sound and Copper River, Alaska. PLoS One 12(3):e0172898
Whyte LG, Bourbonniere L, Greer C (1997) Biodegradation of petroleum hydrocarbons by psychrotrophic Pseudomonas strains possessing both alkane (alk) and naphthalene (nah) catabolic pathways. Appl Environ Microbiol 63:3719–3723
Whyte LG, Hawari J, Zhou E, Bourbonniere L, Inniss WE, Greer CW (1998) Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp. Appl Environ Microbiol 64(7):2578–2584
Whyte LG, Smits THM, Labbe D, Witholt B, Greer CW, van Beilen JB (2002) Gene cloning and characterization of multiple alkane hydroxylase systems in Rhodococcus strains Q15 and NRRL B-16531. Appl Environ Microbiol 68:5933–5942
Xin M, Zhou P (1998) Advance of research for microbial life in low temperature environments. Acta Microbiol Sin 38(5):400–403
Xu X, Liu W, Wang W, Tian S, Jiang P, Qi Q, Yu H (2019) Potential biodegradation of phenanthrene by isolated halotolerant bacterial strains from petroleum oil polluted soil in Yellow River Delta. Sci Total Environ 664:1030–1038. https://doi.org/10.1016/j.scitotenv.2019.02.080
Yakimov MM, Kenneth N, Wray V, Fredrickson HL (1995) Characterization of a new lipopeptide surfactant produced by thermotolerant and halotolerant subsurface Bacillus licheniformis BA550. Appl Environ Microbiol 61:1706–1713
Yang S, Wen X, Shi Y, Liebner S, Jin H, Perfumo A (2016) Hydrocarbon degraders establish at the costs of microbial richness, abundance and keystone taxa after crude oil contamination in permafrost environments. Sci Rep 6:37473. https://doi.org/10.1038/srep37473
Yetti E, Thontowi A, Yopi (2016) Polycyclic aromatic hydrocarbon degrading bacteria from the Indonesian Marine Environment. Biodiversitas 17(2):857–864
Yi-bin W, Fang-ming L, Qiang L, Bi-juan H, Jin-lai M (2014) Low-temperature degradation mechanism analysis of petroleum hydrocarbon-degrading antarctic psychrophilic strains. J Pure Appl Microbiol 8(1):47–53
Yooseph S, Sutton G, Rusch DB, Halpern AL, Williamson SJ, Remington K et al (2007) The sorcerer II global ocean sampling expedition: expanding the universe of protein families. PLoS Biol 5:e16. https://doi.org/10.1371/journal.pbio.0050016
Yumoto I, Nakamura A, Iwata H, Kojima K, Kusumoto K, Nodasaka Y, Matsuyama H (2002) Dietzia psychralcaliphila sp. nov., a novel, facultatively psychrophilic alkaliphile that grows on hydrocarbons. Int J Syst Evolut Microbiol 52:85–90
Zeinali M, Vossoughi M, Ardestani SK (2007) Characterization of a moderate thermophilic Nocardia species able to grow on polycyclic aromatic hydrocarbons. Lett Appl Microbiol 45(6):622–628
Zhao Z, Wong JWC (2010) Rapid biodegradation of benzo[a]pyrene by Bacillus subtilis BUM under thermophilic condition. Environ Eng Sci 27:939–945
Zhou J, Li G, Xie J, Cui X, Dai X, Tian H, Gao P, Wu M, Ma T (2016) A novel bioemulsifier from Geobacillus stearothermophilus A-2 and its potential application in microbial enhanced oil recovery. RSC Adv 98
Zhuang X, Han Z, Bai Z, Zhuang G, Shim H (2010) Progress in decontamination by halophilic microorganisms in saline wastewater and soil. Environ Pollut. https://doi.org/10.1016/j.envpol.2010.01.007
Acknowledgements
PP, BB, ND gratefully acknowledge Department of Biotechnology, Government of India, for financial support. JR is grateful to University Grant Commission for the fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajkumari, J., Bhuyan, B., Das, N. et al. Environmental applications of microbial extremophiles in the degradation of petroleum hydrocarbons in extreme environments. Environmental Sustainability 2, 311–328 (2019). https://doi.org/10.1007/s42398-019-00065-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42398-019-00065-1