Abstract
Anguina tritici is a seed-borne nematode and survives inside the infected seeds (seed galls). The cockles are the sole source of nematode inoculum, and the disease initiates when seed galls are sown along with the healthy seeds. In the present study, the effects of seed priming with Trichoderma viride, T. harzianum, T. atroviride, T. virens and T. asperellum and seed soaking with their culture filtrates were examined on the mortality and emergence of quiescent juveniles of A. tritici from the seed galls as well as on the germination of seeds, and plant growth of wheat cv. PBW-343 inoculated with 10,000 J2 of A. tritici per pot. The Trichoderma spp., especially T. harzianum and T. viride colonialized the seed-galls within 15 days, covering almost entire gall with the mycelium and spores. The fungus invaded the cockles and parasitized the larvae of A. tritici inside, causing 100% mortality to them. The culture filtrate treatment (soaking of galls for 24 h) also induced mortality to the nematode juveniles and prevented their out-migration from the galls, but the overall impact of culture filtrate treatment was significantly less than the seed priming. The seed priming treatments also promoted the germination of healthy wheat seeds and prevented nematode attack leading to the healthy growth of wheat seedlings. The study has demonstrated the potential scope of seed priming with T. harzianum or T. viride for the management of ear cockle (seed gall) disease of wheat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant nematodes constitute a major group of pathogens which cause significant economic loss to all kinds of agricultural crops (Khan et al. 2021). However, the nematode damage to crops often remains unrecognized by growers, consequently negligence towards their management leads to significant yield loss to the crop (Khan 2023a). Plants nematodes are generally inhabited in the soil, but a few such as Anguina tritici is seed borne in nature, and invades leaves, flower, buds, and seeds (Khan 2008; Haque and Khan 2021).
Wheat (Triticum aestivum) is a most important staple and essential cereal crop. It is a major and strategic cereal and contributes to nearly one-third to the global food grain production (Kumari and Singh 2016). It is grown over 220 mha with 760.9 million tons production world over with an average productivity of 3474.4 kg/ha (FAOSTAT 2022). The leading wheat producer is China, followed by India, USA, and Russia (FAOSTAT 2022). Wheat is an important cereal crop in India, and the country has made a landmark in producing the wheat with 109.5 million tons with the national average productivity of 3521 kg/ha (INDIASTAT 2022). Plant nematodes are one of the important biotic factors responsible for suppression of plant growth and yield of cereals including wheat (Khan 2023b). Wheat crop is attacked by a number of nematodes such as, Anguina tritici, Heterodera avenae, Pratylenchus thornei, Meloidogyne naasi, etc. (Owen et al. 2023).
The seed-gall is an important disease in wheat, which has almost been eliminated from the western hemisphere with the use of seed cleaning techniques (El-Saadony et al. 2021), but the disease still occurs in isolated areas in several African and Asian countries including India, where grains from older lots are used as seeds to grow the crop in the next season (Khan and Athar 1996, 1998). In India, the disease occurs in the U.P., Punjab, Western part of Bihar, etc., and is popularly known as “sehun” disease. The disease may cause up to 30% yield loss and may make the wheat flour unfit for making breads etc. (Nandal et al. 2010). The larvae of A. tritici, in association with a plant pathogenic bacterium, Rathayibacter (= Corynebacterium) tritici, incite yellow ear rot disease or bacterial spike blight of wheat (Gupta and Swarup 1968). This disease complex may cause up to 77% yield loss in wheat (Paruthi et al. 1987; Nandal et al. 2010). Further, the nematode also transmits a fungus, Dilophospora alopicurai causing ear “twist disease in wheat”.
In view of continuous disregard to chemical pesticides, and increasing use of biopesticides, effectiveness of seed priming with fungus biomass and seed soaking with the culture filtrates of Trichoderma viride, T. harzianum, T. virens, T. atroviride and T. asperellum was evaluated against seed-gall nematode by determining the impact in inducing mortality to the A. tritici juveniles inside and outside the cockles, colonizing the cockles and nematode larvae, seed germination and seedling growth of wheat cultivars PBW-343 under in-vivo and in-vitro conditions.
Materials and methods
Isolation of seed gall nematode, Anguina tritici
The seed galls collected from wheat fields in Aligarh in 2018 were used in the present study. These seed galls were stored in glass bottles at room temperature, away from exposure to direct sunlight. The direct isolation method was used to isolate the larvae of A. tritici Chitwood, from the cockles (Khan and Athar 1996). In this method, the collected cockles of wheat were soaked with water for 24 h and teared in a cavity block to release the mass of second stage juveniles (J2) of A. tritici. The solution was kept for a week to enable the nematode juveniles to resume the suspended metabolic activities and mobility. After seven days, the nematode suspension was analyzed under a stereomicroscope to observe the mobility in the larvae.
Culture of Trichoderma species
Pure cultures of Trichoderma viride Pers., T. atroviride Karsten, T. virens Arx, T. asperellum Lieckfeldt & Nirenberg, and T. harzianum Rifai, were procured from the ITCC, IARI, New Delhi. Trichoderma spp. were cultured in potato dextrose broth in conical flasks of 500ml capacity containing around 250 ml broths. The flasks were incubated at 25 ± 2ºC for 10 days. During the incubation, the flasks were visually examined for the growth of Trichoderma spp. The broths were filtered through Whatman filter paper to separate fungus biomass (mycelium/spores) and the filtrate for use in the experiments.
Relationship of size and weight of seed-galls with the population of Anguina tritici
In order to ascertain the relationship of the gall size and gall weight with the population density of A. tritici inside the galls, the wheat galls on the basis of size and weight were categorized into three groups: large, moderate, and small galls. The large galls weighed 9.2 mg (7.9–9.8 mg), moderate galls 5.6 mg (4.3–6.1 mg), and small galls weighed 3.5 mg (2.3–4.2 mg). The frequency of different size of galls was also determined by considering 100 galls randomly. The procedure was repeated ten times. Ten galls from each of the three categories (large, moderate, and small galls) were processed for determining the nematode population.
The cockles were soaked in water for about 24 h. Thereafter, the cockles are transferred to a cavity block and teared in 1–2 ml water. Upon tearing, the nematode mass emerged out from the cockles. The contents of the cavity block were transferred to 250 ml conical flask, and the volume of the nematode suspension was made to 100 ml. Identity of the nematode larvae present in the suspension was confirmed using the morphological characters. The nematode larvae present in the suspension were counted by taking 1 ml in a counting dish under the stereomicroscope. The process was repeated five times by taking another 5 ml of the suspension. The average number of nematodes was calculated and multiplied by the total volume of the nematode suspension to compute the nematode population in one cockle.
Effect of fungus biomass (seed priming) and culture filtrates (seed soaking) of Trichoderma species on nematode mortality inside the cockle
To examine the effect of seed priming with Trichoderma spp. on the mortality to the second-stage juveniles of A. tritici inside the cockles, the cockles and healthy seeds were primed with T. viride, T. atroviride, T. virens, T. asperellum, T. harzianum @ 10 g fungus/kg seed. The CFU load in the fungus biomass was determined by the dilution plate method which ranged 16–18 × 107 CFU/g fungus. The cockles were first soaked with water for 24 h, followed by soaking with tissue paper to remove excess moisture. Thereafter, 5% sucrose solution was sprinkled on the cockles to enhance adhesion of the biomass of Trichoderma spp. which was applied at the rate of 10 g/kg seeds. The cockles were left for 2–3 h. to allow excess moisture to evaporate. The cockles and healthy seeds were placed in the petri plates (5 cockles/ plate), containing two layers of blotter paper soaked in sterilized water and covered with the lid that also had the soaked papers. For culture filtrate treatments, cockles and healthy wheat grains were soaked in the culture filtrates of the five Trichoderma spp. for 24 h separately. Thereafter, the cockles and healthy grains were placed in petri plates lined with water-soaked blotter paper. The plates were sealed and incubated at 25ºC in an incubator for 15 days. The cockles at the 1,3,5,7,9,11,13,15 days of intervals were teared to liberate the larvae which were examined to determine the larval mortality according to the following formulae. The straight and immobile larvae were considered as dead. Further, approximately 100 larvae were treated with cotton blue and examined under compound microscope to determine infection with Trichoderma spp.
Effect of fungus biomass (seed priming) and culture filtrates (seed soaking) of Trichoderma species on the seed germination and seedling growth of wheat
Plant culture
Paper cups of 7 × 10 cm dimension were filled two-third with autoclaved soil (sandy loam) and farmyard manure in a ratio of 3:1. The seeds primed with the biomass of Trichoderma spp. (10 g/kg seeds) or soaked with their culture filtrate (24 h) were sown in the cups (10 seeds/cup). The soil of the cups was inoculated with 10,000 J2 of A. tritici just before the sowing. One set of cups for each inoculated and uninoculated treatment was maintained as a control. Plants were grown for one month. After sowing, the pots were arranged under CRBD. One week after sowing the number of seedlings emerged in the cups were counted and thereafter thinned to one seedling per cup. Five pots (cups) for each treatment were maintained. At harvest, 30 days after sowing, the foliar symptoms such as leaf twisting and crinkling, and plant growth (shoot/root length, vigour index) and soil population of A. tritici using cobb’s decanting and sieving methods (Southey 1986) were determined.
Statistical analysis
Means were calculated from the data recorded from five replicates. The seed germination recorded on ten seeds sown in a cup was averaged and considered as one replicate. The data (5 replicates) were subjected to ANOVA. Three-factor ANOVA (nematode x BCAs x application methods) was conducted on seed germination, vigor index and plant growth, whereas for the rest of parameters, single-factor ANOVA was employed. The coefficient of variance (CV), standard errors for differences of the means (SED), and least significance difference (LSD) were computed at P ≤ 0.05 with the help of R software. The regression analysis was also performed on the data on juvenile mortality versus days of treatment.
Results
Relationship of size and weight of the cockles with the population density of Anguina tritici
On average a single cockle contained 10,116 (3834–16,533) juveniles of A. tritici with 16,533 J2 (14,917–17,908) / large cockle, 9982J2 (8108–11,021)/ medium and 3834 J2 (3074–4415)/ small cockle (Table 1). In randomly collected samples, the relative frequency of occurrence of large, medium and small sized cockles was 20, 35, and 45%, respectively (Fig. 1; Table 2).
Effect of fungus biomass and culture filtrates of Trichoderma species on the colonization of cockles and nematode mortality
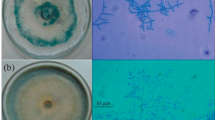
The seed priming with the biomass of Trichoderma spp. resulted in colonization of cockles and healthy wheat seeds with the fungus. The colonization became visible 5–7 days after the priming, and the mycelium of T. harzianum and T. viride fully covered the cockle within 13–15 days of the treatment (Fig. 2). The efficiency of Trichoderma spp. in colonizing the cockles and healthy grains of wheat was in the order: T. harzianum > T. viride > T. atroviride > T. virens > T. asperellum. The priming treatments also induced mortality to the A. tritici juveniles recovered from the cockles. The 100% and 91.9% mortality to the nematode juveniles was observed at 15th day of treatment with T. harzianum and T. viride, respectively, followed by 79.9% mortality with T. atroviride, 69.1% with T. virens, and 58.0% with T. asperellum (Table 3). In the control treatment, the average mortality to the second-stage juveniles was only 4–6%. A significant increase in the mortality was recorded from 7th day onwards with highest mortality on 15th day of treatment (Table 3). The regression on mortality rate versus days of treatment shows linear relationship with highest regression coefficient for T. harzianum and T. viride (Fig. 3). Generally, the dead nematode larvae were partially or fully parasitized by the fungus (Fig. 2C).
Soaking of wheat seeds in the culture filtrates of Trichoderma spp. led to mortality to A. tritici juveniles. However, a significant increase in the nematode mortality over control was recorded from 3rd day onward with a maximum impact during 11–15 days of the treatment with all Trichoderma spp. (Table 4). The culture filtrates of T. harzianum (80.4%) and T. viride (74.4%) induced highest mortality to A. tritici juveniles followed by T. atroviride (66.8%), T. virens (60.2%), and T. asperellum (52.1%). With all Trichoderma spp. the mortality percentage was significantly increased at 11th, 13th, and 15th day of treatment (Table 4). The dead nematode juveniles recovered from the filtrate treated cockles did not show infection with the Trichoderma spp., but the body was shriveled. The culture filtrate treatment was found relatively more effective than seed priming in inducing mortality up to 7th day whereas from 11th day onwards it was found significantly less effective than the seed-priming (Tables 3&4).
Effect of fungus biomass (seed priming) and culture filtrates (seed soaking) with Trichoderma species on the seed germination and growth of nematode inoculated wheat seedlings
The seed priming or seed soaking treatments improved the germination of wheat seeds. The seed priming with T. harzianum showed the highest seedling emergence (93.30%), followed by T. viride (87.95%). T. atroviride (81.0%), T. virens (78.0%), and T. asperellum (73.5%) and 70.90% in the control (Table 5). Any significant impact of nematode inoculation on the seed germination was not recorded (Table 5). The culture filtrate treatment also promoted the seed germination, but the effect was 7–15% less than seed priming (Table 5).
The seeds priming with T. harzianum and T. viride resulted in 10% and 7% increase in the shoot length of wheat seedlings in the cups not inoculated with the A. tritici over control. The nematode inoculation significantly reduced the shoot length, but root growth was not affected (P ≤ 0.05). The seed priming with T. harzianum and T. viride improved the root length of nematode inoculated plants compared to the uninoculated control (Table 5).
The effect of seed soaking in the culture filtrates had no impact on the plant growth or vigour index of uninoculated plants, but in nematode inoculated pots, the wheat plants showed significantly better growth especially with T. harzianum treatments over inoculated control. The seeds primed with T. harzianum showed the highest vigor index (1427), followed by T. viride (1311), T. atroviride (1197), T. virens (1140), and T. asperellum (1058) compared to the uninoculated control (990), whereas, the seeds treated with culture filtrate of T. harzianum showed the highest vigor index (1363), followed by T. viride (1276), T. atroviride (1151), T. virens (1095), and T. asperellum (1046) compared to the inoculated control (664) (Table 5).
Discussion
The ear cockle caused by A. tritici is a commonly occurring problem in wheat, barley and rye, especially in economically backward and tribal areas where seeds from the old lots are used to grow above crops in the next season (Nandal et al. 2010). Due to easy availability of certified seeds, ear cockle occurrence in India, and other Asian and African countries has restricted to isolated areas, but on getting favorable conditions and spread to new areas, the nematode may cause damage to wheat plants to a greater extent (Khan and Athar 1998; Dababat and Fourie 2018). The nematode invasion may cause stunted growth with distorted and shortened stems and leaves (Khan and Athar 1998; Bridge and Starr 2007). The ears become broader and shorter; and sometimes, may not be formed (Bridge and Starr 2007). In infected wheat plant, generally, under sized earheads are formed, which contain few or several deformed and brown to black cockles (seed-galls) of varying size in place of normal grains (Nandal et al. 2010). The cockles contain a creamy mass of nematode larvae in anhydrobiotic quiescent state (Bridge and Starr 2007), The quiescent larvae can serve for over 30 years under dry conditions (Tulek et al. 2015). The nematode populations in the small, medium, and large cockles were recorded to be 3834–16,533 J2/ gall, with an overall average of around 10,000 larvae/gall. Nandal et al. (2010) have reported that the large galls may contain 27,160–32,870 J2, whereas a small gall may have as low as 3121–4884 J2.
The present investigation has demonstrated that priming of cockles with Trichoderma spp. can effectively suppress the nematode population, and subsequently the disease. The fungus colonized the entire seed within 13–15 days and entered inside the cockles, parasitizing and killing all the nematode larvae inside. Generally, quiescent larvae become fully activate and migrate out from the cockles in 10–15 days (Haque and Khan 2021). The present study has shown that during this period (13–15 days), Trichoderma spp. colonized and entered inside the cockle and parasitized A. tritici juveniles before they could migrate out of the cockle. Researches have shown that, in addition to direct parasitism, the secondary metabolites synthesized by Trichoderma spp. inhibit the multiplication of plant pathogenic fungi and nematodes (Khan and Mohiddin 2018), and also stimulate the plant growth (Khan et al. 2009, 2022; TariqJaveed et al. 2021). Trichoderma spp. regulate root architecture and increase root growth, resulting in the enhancement of root efficiency to absorb nutrients (Sani et al. 2020). Trichoderma spp. produce harzianic acid, alamethicin, tricholin, peptaibols, antibiotics and enzymes (Contreras-Cornejo et al. 2016), which may adversely impact the nematode feeding. A similar effect of treatments with cultural filtrates of Trichoderma spp. was recorded on A. tritici as well as on the seed germination and vigor index of wheat plants in the present study. The impact of seed priming with the fungus biomass was recorded greater than the culture filtrates. This was apparently due to continuous exposure of the juveniles to the nematoxic substances and the entire root system to the growth promoting chemicals because of colonization of wheat seeds by Trichoderma spp. Whereas the impact of cultural filtrate would have been for a shorter period which would had been gradually diluted and diminished with the progress of time. For this reason, the seed priming with Trichoderma spp. supported better seed germination, seedling emergence and improved growth and vigor index especially with T. harzianum. Trichoderma spp. differ in their effectiveness (Debnath et al. 2020). Researches have demonstrated that T. harzianum has relatively greater effectiveness in suppressing the pathogen and improving the plant growth compared to other species (Fazeli-Nasab et al. 2021; Mohammed and Khan 2021). Among the Trichoderma spp. tested in the present study, T. harzianum proved highly effective and is recommended for seed priming treatment on wheat seeds. Besides suppressing the nematode and soil borne fungi, T. harzianum treatment may also offer additional benefits of promoting seed germination and plant growth.
Conclusion
The present research done to assess the suppressive potential of Trichoderma spp., against seed-gall nematode has revealed effectiveness of T. harzianum in inducing mortality to A. tritici larvae when applied as seed priming (fungus biomass) or seed soaking (culture filtrate). The seed priming treatment caused 100% mortality to the nematode juveniles, and significantly improved the seed germination, vigor index and plant growth of wheat. The study has demonstrated the potential scope of using biopesticides as replacements for high cost and poisonous chemical nematicides in the management of ear cockle in wheat. The treatment also served as a plant growth promotor and may be recommended as a general agronomic practice in wheat cultivation.
References
Bridge J, Starr JL (2007) Plant nematodes of agricultural importance: a color handbook. Academic press, pp 1–155
Contreras-Cornejo HA, Macías-Rodríguez L, Del-Val EK, Larsen J (2016) Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: interactions with plants. FEMS Microbiol Ecol 92(4):fiw036. https://doi.org/10.1093/femsec/fiw036
Dababat AA, Fourie H (2018) Nematode parasites of cereals. In Plant parasitic nematodes in subtropical and tropical agriculture. Wallingford UK: CAB International. pp. 163–221
Debnath S, Chakraborty G, Dutta SS, Chaudhuri SR, Das P, Saha AK (2020) Potential of Trichoderma species as biofertilizer and biological control on Oryza sativa L. cultivation. Biotecnol Veg 20(1):1–6
El-Saadony MT, Abuljadayel DA, Shafi ME, Albaqami NM, Desoky ESM, El-Tahan AM, Mesiha PK, Elnahal AS, Almakas A, Taha AE, Abd El-Mageed TA (2021) Control of foliar phytoparasitic nematodes through sustainable natural materials: current progress and challenges. Saudi J Biol Sci 28(12):7314–7326
FAOSTAT (2022) https://www.fao.org/faostat/en
Fazeli-Nasab B, Sayyed RZ, Piri R, Rahmani AF (2021) Biopriming and Nanopriming: green revolution wings to increase plant yield, growth, and development under stress condition and forward dimensions. In: Antioxidants in plant-microbe interaction, pp 623–655
Gupta P, Swarup G (1968) On the ear-cockle and yellow rot Disease of wheat. I. symptoms and histopathology. Indian Phytopathol 21(3):318–323
Haque Z, Khan MR (2021) Hand book of Invasive Plant-Parasitic nematodes. CABI, London, UK, p 544
INDIASTAT (2022) https://www.indiastat.com/data/agriculture/wheat-17195/data-year/2008-2023
Khan MR (2008) Plant nematodes methodology, morphology, Systematics, Biology and Ecology. Science Publishers, New Hampshire, USA, p 360
Khan MR (2023a) In: Khan, Quintanilla M (eds) Plant nematodes, hidden constraints in the global crop production. Nematode Diseases of crops and their sustainable management M. R. Elsevier Publishers
Khan MR (2023b) In: Khan MR (ed) Nematode pests of agricultural crops, a global overview. Novel Biological and Biotechnological Applications in Plant Nematode Management. Springer Publishers
Khan MR, Athar M (1996) Response of wheat cultivars to different inoculum levels of Anguina tritici. Nematol Mediterr 24:269–272
Khan MR, Athar M (1998) Effect of Anguina tritici on the growth and yield of wheat cultivars. Tests of Agrochemicals and Cultivars 132:52–53
Khan MR, Altaf S, Mohiddin FA, Khan U, Anwer A (2009) Biological control of plant nematodes with phosphate solubilizing microorganisms. In: Khan MS, Zaidi A (eds) Phosphate solubilizing microbes for crop improvement. Nova Science Publishers, Inc., New York, pp 395–426
Khan MR, Mohiddin FA, Tuteja Editors (2018) Trichoderma: its multifarious utility in crop improvement. In: Prasad R, Gill SS (eds) New and Future developments in Microbial Biotechnology and Bioengineering: crop improvement through microbial biotechnology. Elsevier publications, Amsterdam, Netherlands, pp 263–291
Khan MR, Ahamad I, Shah MH (2021) Emerging important nematode problems in field crops and their management. Emerging Trends in Plant Pathology.Pp.33–62
Khan A, Ansari MSA, Irsad, Khan AA (2022) Role of Beneficial microbes for Plant growth improvement. From Chemicals to Biologicals, Plant Protection, p 141
Kumari A, Singh SK (2016) Impact of different tillage practices on soil organic carbon and nitrogen pool in rice-wheat cropping system. J AgriSearch 3(2):82–86
Mohammed RKA, Khan MR (2021) Management of root-knot nematode in cucumber through seed treatment with multifarious beneficial microbes under protected cultivation. Indian Phytopathol 74(4):1035–1043. https://doi.org/10.1007/s42360-021-00422-3
Nandal SN, Paruthi IJ, Khan MR (2010) Seed gall nematode infestation in wheat. In: Nematode Infestations Part I: Food Crops. National Academy of Sciences, India 171–191
Owen K, Walia RK, Yan G, Khan MR (2023) In: Khan MR, Quintanilla M (eds) Nematode problems in wheat and barley and their sustainable management. Nematode diseases of crops and their sustainable management. Elsevier Publishers, Amsterdam
Paruthi IJ, Singh M, Gupta DC (1987) Quantitative and qualitiative losses in wheat (Triticum aestivum) grains due to earcockle and ‘tundu’. Seed Res 15:83–86
Sani MNH, Hasan M, Uddain J, Subramaniam S (2020) Impact of application of Trichoderma and biochar on growth, productivity and nutritional quality of tomato under reduced NPK fertilization. Annals of Agricultural Sciences 65(1):107–115
Southey JF (1986) Laboratory methods for work with plant and Soil Nematodes. Her Majesty Stationary Office, London, U.K., p 202
TariqJaveed M, Farooq T, Al-Hazmi AS, Hussain MD, Rehman AU (2021) Role of Trichoderma as a biocontrol agent (BCA) of phytoparasitic nematodes and plant growth inducer. J Invertebr Pathol 183:107626
Tulek A, Kepenekci I, Dababat AA, Çiftçigil TH (2015) History and current status of the wheat gall nematode [Anguina tritici (Steinbuch) Filipjev] on wheat in Turkey. Nematodes of small grain cereals, p 37
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts among the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khan, M.R., Manzoor, S. & Ansari, M.S.A. Effectiveness of Trichoderma species in controlling the seed-borne infestation of Anguina tritici in wheat seed-galls. Indian Phytopathology 76, 1083–1090 (2023). https://doi.org/10.1007/s42360-023-00681-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-023-00681-2