Abstract
The pathogenic potential of three root-knot nematodes, Meloidogyne enterolobii, M. incognita, and M. javanica on the guava (Psidium guajava L.), cultivar Allahabad Safeda, was investigated in a greenhouse setting. The guava seedlings were inoculated with 0 (control), 100, 500, 1000, 1500, and 2000 s-stage juveniles (J2s) per plant. When compared to the control, nematode-inoculated plants showed significant differences in all three Meloidogyne spp., namely M. enterolobii, M. incognita, and M. javanica, in terms of growth parameters and nematode reproduction on the host plant. At 100 J2s and higher inoculum levels, such as 500, 1000, 1500, and 2000 J2s per plant, the reductions in growth were discernible compared to the control. As inoculum levels increased, root galling, egg mass production, and nematode populations all increased. The estimated soil and root populations of M. enterolobii and M. incognita gradually increased with increasing inoculum amounts. There was a noticeable increase in the population at all inoculum levels as compared to the lowest inoculum level (100 J2s). As the nematode inoculum increased, both the reproduction factor (RF) and multiplication factor (MF) decreased. The present study demonstrated that M. enterolobii is highly pathogenic, M. incognita is moderately pathogenic, and M. javanica is relatively less pathogenic to guava. At a minimum inoculum of 100 J2/plant, M. incognita can cause damage to guava by damaging the roots and inhibiting plant growth. At an initial population density of 100 J2s/plant of M. enterolobii, the guava crop suffers the most because of extensive multiple root galling and secondary infection by other soil-borne pathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Guava (Psidium guajava L.), a tropical fruit, is grown throughout many tropical and subtropical regions of the world and is a member of the Myrtaceae family (Carneiro et al. 2012). India is one of the main guava-producing nations on the globe. It is grown throughout the country, usually in backyard gardens and agroforestry production systems. The major guava-producing states in India are Uttar Pradesh, Bihar, Assam, Madhya Pradesh, Maharashtra, West Bengal, Orissa, and Tripura, among others. They have a combined area of 2,87,000 ha and produce roughly 43,04,000 MT of fruit annually (Anonymous 2020). Plant-parasitic nematodes are one of the main obstacles to the economic cultivation of guava, among other biotic and abiotic stresses, causing harm to the normal production of several essential horticultural crops like vegetables and fruits, including guava. Nematodes pose a severe threat to guava farmers, especially given their involvement in the guava decline problem (Singh 2020). With the increase of guava-growing areas in India, root-knot nematode infestation is becoming more prevalent among other plant-parasitic nematodes. Poornima et al. (2016) found a disease complex in guava in the presence of Fusarium solani and M. enterolobii. M. enterolobii alone is believed to affect the guava crop in Tamil Nadu to an estimated 80% of its area, resulting in a 10.47% avoidable yield loss (Anonymous 2018). Root-knot nematodes are becoming a serious threat to the cultivation of guava in various states, including UP, Tamil Nadu, Karnataka, Gujarat, and West Bengal (Khan et al. 2022). India experiences a 28% loss in guava production due to Meloidogyne spp., costing the country INR 2350.88 million ($32.65 million) annually (Kumar et al. 2020). Guava is infected by multiple species of Meloidogyne, and the extent of the damage is typically seen as a decline in the orchard and as slow-wilting symptoms in the plants in the presence of other soil-borne pathogens, mostly fungi. Apart from a poorly established root system that is damaged by small and big multiple galls, which causes the plant to die gradually, the afflicted plants show a severe drop in plant growth and yield in terms of quality and quantity. The guava root-knot nematode M. enterolobii (previously known as M. mayaguensis), is a new pest that is affecting numerous crops worldwide (Philbrick et al. 2020). It is comparatively more pathogenic and can, on its own, result in losses of up to 65%, which is greater than any other species known so far (Catagnone - Sereno, 2012; Castagnone-Sereno and Castillo 2014). Furthermore, Fusarium solani-immune trees were prone to significant root destruction by M. enterolobii (Gomes et al. 2012). The impact of Meloidogyne spp. on plant growth is determined by nematode species, physiological race, and initial nematode population density in the soil. For integrated pest management and sustainability, accurate identification, characterization, and population density assessment of Meloidogyne species in soil are crucial. This is important because there is no host-plant resistance among fruit crops, which could lower the initial nematode population density to tolerance threshold levels. The relative pathogenic potential, threshold values, and host-parasite interactions of many root-knot nematode species in guava are poorly understood. Therefore, it is crucial to carry out an inoculated experiment using different levels of inoculum density of nematode species against a susceptible host. Considering this, a study on the pathogenicity of M. incognita, M. javanica, and M. enterolobii on the guava cultivar Allahabad Safeda) was conducted to assess the relative pathogenic potential of common root-knot nematode species.
Materials and methods
Raising, maintenance of test plants, nematode inoculum preparation, and inoculation method
The guava seeds, cultivar Allahabad Safeda, were treated with 10% HCl and then soaked in water for the entire night, which helped to break down the tough seed coat and facilitate faster and better germination. In clay pots (15 cm diam.), each containing 1 kg of sterile pot mixture (7 parts clay: 3 parts sand: 1 part farmyard manure), treated seeds were planted. 20 days after sowing the seeds, at the four-leaf stage, the seedlings were transplanted into pots. Only one seedling per pot was maintained throughout the experiment. The single egg mass culture populations of Meloidogyne incognita, M. javanica, and M. enterolobii were separately maintained on eggplants. The nematode inoculum was obtained from heavily infested eggplant roots on which pure cultures of all three Meloidogyne spp. were maintained. The egg masses from these infected plants were handpicked with the help of sterilized forceps. The egg masses, after being washed with distilled water, were placed in 10 cm diameter sieves and lined with two layers of tissue paper. Furthermore, freshly hatched second-stage juveniles (J2s) were collected regularly every 24 h and transferred to a beaker. The water suspension of J2s was thoroughly stirred to homogenise the suspension just before counting, and the total number of nematodes per ml was counted in a counting dish under a stereoscopic microscope, and these juveniles were used as inoculum for conducting the experiments. The feeder roots of four-week-old guava seedlings were exposed without causing any damage to the roots. Using a sterile pipette, the required amount of J2s suspension was evenly dispensed around the exposed roots and instantly covered.
Experimental details
All three species, viz., M. incognita, M. javanica, and M. enterolobii were each studied separately for the three sets that made up the entire study for comparison of relative pathogenic potential under similar conditions. The plants were placed in a complete randomized block design with six replications and various inoculum amounts of 0, 100, 500, 1000, 1500, and 2000 J2 per pot. Regular watering was given as required. The plants were uprooted 60 days after inoculation, and measurements of the shoot and root length (cm), fresh shoot and root weight (g), number of galls per plant, number of egg masses, number of eggs per egg mass, the final population of nematodes from the soil, and root population (mature and immature females) were taken. The root galling severity was determined by root-knot index (RKI) on a 1–5 scale:1 = no gall/egg mass; 2, 1–10; 3, 11–30; 4, 31–100 and 5, > 100 galls/egg masses in a root system (Khan et al. 2014). Nematodes were extracted by Cobb’s decanting and sieving technique (Cobb 1918) followed by the modified Baermann’s funnel method (Schindler 1961) and from the root system by the NaOCl-Acid Fuchsin method (Byrd et al. 1983). The rate of reproduction factor (Rf = Pf/Pi) was estimated from the total nematode population (Pf) (root + soil) to the initial inoculum levels (Pi) (Oostenbrink 1966; Vovlas et al. 2008). The nematode multiplication factor [(the number of egg masses × the number of eggs per egg mass) ÷ nematode inoculum level] was determined according to Kumari et al. (2016).
Identification of Meloidogyne species
Before inoculation to the pot, the single egg mass populations of Meloidogyne incognita (Kofoid & White) Chitwood, 1949, Meloidogyne javanica (Treub, 1885) Chitwood, 1949, and Meloidogyne enterolobii Yang and Eisenback, 1983 were initially identified based on perineal pattern (Jepson 1987), and then confirmed by using sequence characterized amplified regions (SCAR) markers (Zijlstra et al. 2000; Tigano et al. 2010).
Statistical analyses
Both plant growth parameters and nematode pathogenicity data were first tested for normality and homogeneity of variance using Shapiro-Wilk and Bartlett’s tests, respectively. If normality was observed, analysis of variance (ANOVA) was performed to identify the significant difference in respective parameters in response to nematode population density (initial inoculum level). If significant variation (5% significance level) was observed, Tukey’s HSD test was conducted to identify significant differences among treatment groups. If normality was not followed, a non-parametric Kruskall-Wallis test was performed. If significant variation was observed, Pairwise Mann-Whitney U-test was conducted with ‘bonferroni’ correction to p-values. Significant variations among treatments were denoted by letter codes using the ‘multcompview’ package. All statistical analysis was performed in R (version R-4.2.1, R Core Team, 2021; https://www.r-project.org/) statistical software.
Results
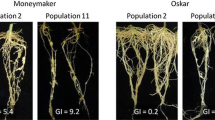
Compared to the control, nematode-inoculated plants in the guava cultivar Allahabad Safeda displayed significant differences in all three Meloidogyne spp. egg masses, eggs per egg mass, final population, plant growth parameters (shoot length, fresh shoot weight, root length, fresh root weight), root-knot index, and other growth-related metrics (Table 1). As the inoculum levels of M. enterolobii, M. incognita, and M. javanica increased from 100 to 2,000 J2s per pot containing 1 kg of sterilized soil after 60 days of inoculation, all plant growth parameters of guava gradually decreased. The affected plants’ growth seemed stunted (Fig. 1) and chlorotic at the highest inoculum level. It was observed that the galls formed at the highest inoculum level were more extensive and primarily formed in the roots, and the nematode species were found in large numbers in the root systems.
Pathogenic potential of Meloidogyne enterolobii on guava.
Figure 1 (A-D) shows the effects of M. enterolobii on the plant growth of guava cv. Allahabad Safeda. The treatment with no nematodes per pot (control) showed no significant change in shoot length, root length, shoot weight, or shoot and root weight (fresh), while the treatments with 100, 500, 1000, and 2,000 J2 per plant showed significant differences from one another. Similar to this, as the inoculum level grew from 100 to 2000 J2 per plant, the number of galls per root, egg masses per root, and the ultimate nematode population in the soil gradually increased (Fig. 3A-D). The inoculum level of 2000 J2s per plant resulted in the highest number of galls per root (Fig. 2), egg masses per root, and final nematode population in soil; the lowest number of galls per root, egg masses per root, and final nematode population in soil were observed in the treatment with 100 J2 per plant. The final nematode population in the soil did not substantially differ between the treatments with 1500 and 2,000 J2/plant, but these treatments did differ from the treatments with 100 and 1000 J2/plant (Fig. 3C), respectively, for galls per root, egg masses per gram root. With an increase in inoculum levels, the rate of reproduction (RF) of the root-knot nematode was significantly (Kruskall-Wallis χ2 = 24.27, p < 0.01) reduced; the rate of reproduction was highest (RF = 37.62) at the inoculum level of 100 J2s per plant, followed by 12.62 at 500 J2s per plant, and the lowest (RF = 4.74) at higher inoculum level at 2000 J2s per plant. A similar trend was observed in the case of multiplication factor (MF), where with increasing inoculum density, a significant (Kruskall-Wallis χ2 = 26.4, p < 0.01) reduction in MF was observed (Fig. 3D). The highest MF of 6.93 was observed with the initial inoculum density was 100, while the lowest MF (1.17) was documented at an inoculum density of 2000 J2s/plant. The lowest population level of M. enterolobii required to cause damage to guava plants and make them pathogens for the crop was found to be 100 J2/1000 cm3 of soil. The relative pathogenic potentials of all three species of Meloidogyne on guava are presented in Fig. 4 (A-D).
Effects of different inoculum levels of Meloidogyne enterolobii, M. incognita and M. javanica on the growth parameters of guava cultivar cv. Allahabad Safeda. The bars in the graph represent the mean ± SE of the data from six replications. Different letters above the error bars indicate statistically significant (p < 0.05) differences in respective plant growth parameters across inoculum levels based on Tukey HSD post hoc analysis
Effects of different inoculum levels of Meloidogyne enterolobii, M. incognita and M. javanica on the root-knot index (RKI) of guava cultivar cv. Allahabad Safeda. The bars in the graph represent the mean ± SE of the data from six replications. Different letters above the error bars indicate statistically significant (p < 0.05) differences in the RKI index across inoculum density levels based on Tukey HSD post hoc analysis
Effects of different inoculum levels of Meloidogyne enterolobii, M. incognita, and M. javanica on different aspects of reproductive potential (eggs / egg-mass, egg-mass / g of root, reproduction factor, and multiplication factor) on guava cultivar cv. Allahabad Safeda. The bars in the graph represent the mean ± SE of the data from six replications. Different letters above the error bars indicate statistically significant (p < 0.05) differences in respective parameters across inoculum density level based on Pairwise Mann-Whitney U-test or Tukey HSD post hoc analysis
Pathogenic potential of Meloidogyne incognita on guava.
Figure 1 (E-G) shows the impact of M. incognita on the plant growth of guava cv. Allahabad Safeda. As inoculum levels increased, the length of the shoot and the roots also decreased. In comparison to the control, shoot (F1,34 = 327, p < 0.05) and root length (F1,34 = 63.9, p < 0.05) significantly decreased with higher inoculum levels, such as 100, 500, 1000, and 2000 J2. The lowest inoculum level (100 J2) had the least impact on shoot weight (F1,34 = 44.8, p < 0.05) and root weight (F1,34 = 23.1, p < 0.05). When the inoculum levels were higher, between 500 and 2000 J2, the decreases in plant growth matrices were substantial and high. While the treatment with 100 J2 per plant recorded the fewest eggs, eggmasses per root, and ultimate low nematode population in soil (Fig. 3A-D). However, galling was noted in all the nematode-inoculated plants. With more inoculum present, there were more galls per root system (Fig. 2). The maximal inoculum level (2000 J2/1000 cm3 soil) had the lowest reproduction factor (RF = 2.13) and the 100 J2 level had the highest (RF = 15.44). The reproduction factor varied significantly (Kruskall-Wallis χ2 = 24.74, p < 0.01) across inoculum levels. Multiplication factor (MF) also showed significant (Kruskall-Wallis χ2 = 24.67, p < 0.01) variations across inoculum density, where the highest (2.57) MF was observed at 100 J2/plant, while the lowest (0.38) MF was documented in the case of 2000 J2/plant.
Pathogenic potential of Meloidogyne javanica on guava.
The effects of M. javanica on the growth of Guava cv. Allahabad are similarly shown in Fig. 1 (H-J). The treatment with no nematodes (control) per pot showed no significant change in shoot length, root length, and shoot and root weight (fresh), but the treatments with 100, 500, 1000, and 2,000 J2 per plant showed significant differences from one another. Similar to this, as the inoculum level grew from 100 to 2000 J2/per plant, the number of galls per root (Fig. 2), egg masses per root, and the final nematode population in the soil gradually increased (Fig. 3A-D). The inoculum level of 2000 J2/per plant resulted in the highest number of galls per root (root-knot index 3.0), egg masses per root, and final nematode population in the soil. The lowest number of galls per root, egg masses per root, and final nematode population in soil were observed in the treatment with 100 J2 per plant (Fig. 3A-D). Furthermore, it was found that although there was no significant difference between the treatments with 1000, 1500, and 2000 J2 per plant in respect of plant growth matrices, However, there was a significant difference between these treatments for egg masses per root, eggs per egg mass, and the final nematode population in soil compared to the treatments with 100 and 500 J2 per plant. Increases in inoculum levels resulted in a considerable reduction in the rate of root-knot nematode reproduction (RF) with significant variation across inoculum levels (Kruskall-Wallis χ2 = 17.37, p < 0.01); the highest RF (4.76) was obtained at the lowest inoculum level (100 J2/1000 cm3 soil), and the lowest RF (1.6) at the highest inoculum level of 2000 J2/plant (Fig. 3C). Thus, guava crops may suffer damage (galling severity greater than 3.0) due to the infection of M. javanica when the soil population level is very high (2000 per 1000 cm3 of soil). Moreover, the multiplication factor (MF) also showed significant (Kruskall-Wallis χ2 = 17.37, p < 0.01) variation across initial inoculum density (Fig. 3D).
Discussion
The accurate identification of Meloidogyne spp. and the efficient application of host plant resistance are essential for the management of the root-knot nematodes affecting guava plantations in guava-growing countries. Similarly to this, understanding how root-knot nematodes affect plant growth and yield are important for creating effective integrated management programmes and is required for farmers to comprehend the relevance of controlling the diseases caused by the nematode species. This study has demonstrated the significant effect on the growth of guava plants caused by the most commonly occurring M. enterolobii, M. incognita, and M. javanica. It also indicates the possible risk associated with the spread of these nematode species together with serious diseases. In India, root-knot nematode (M. enterolobii) causes a serious infestation in guava (Ghule et al. 2020). We attempted to understand the pathogenic potential of M. enterolobii under inoculated control conditions. The results revealed that as the inoculation level increased, the various growth characteristics decreased due to the increased level of nematode infection. Our studies also showed the high pathogenic potential of M. enterolobii on guava and at a higher inoculum level, the nematode impacted the growth substantially and displayed symptoms. Carnerio et al. (2012) also made a similar observation, noting that P. guajava ‘Paluma’ was extremely vulnerable to M. enterolobii, which had the greatest reproduction factor (182.4). M. enterolobii causes yellowing and stunting in guava plants as well as smaller fruits, dried branches, leaf loss, and decreased yields. The guava tree was doomed to decline and die as a result of the severe infestation of root-knot nematode, which caused significant root galling (Gomes et al. 2010, 2012; Jindapunnapat et al. 2013) found heavy root galling in addition to above-ground non-specific symptoms like yellowing, stunting, folded leaves, and blighted and wilted leaves caused by M. enterolobii infection in the orchards in central Thailand. Poornima et al. (2016) noted galls ranging in size from tiny to 5 cm in diameter on the collar area and lower stem regions of the guava trees.
The root-knot nematode, M. incognita, has been found associated with guava in Uttar Pradesh (Ansari and Khan 2012) and Haryana (Madhu et al. 2019) in India and is causing severe problems with its cultivation. Our findings on the Allahabad Safeda guava cultivar, which was used in the pathogenicity test with varying concentrations of M. incognita inoculum, demonstrated a substantial reduction in growth matrices and low to moderate root galling (RKI 2–4). Babatola and Oyedunmade (1992) established the host status of four guava cultivars inoculating M. incognita with more than 5,000 eggs per plant for 16 weeks and found all four cultivars as good hosts (RKI 1.8 -5.0), and noted severe infection at 40, 000 eggs per plant. The findings of Villota and Agudelo (1997) on M. incognita infecting all 23 guava cultivars provided support for our results. An increase in the inoculum level and a decline in the relative growth parameters were indicators of growth impairment. This result is consistent with the observations of Rodriguez et al. (1985) and Karim (1991), who found that with the increase in inoculum level, the number of galls, egg mass, and egg per eggmass all increased. Similar findings were made by Casassa et al. (1997) on the guava rootstock, P. gujava cultivar Criolla Roja; the reproduction factor was highest (3.47) at the lowest amount of inoculation and declined as the inoculation level increased. On the contrary, Carneiro et al. (2012) observed that the reproduction factor, when compared to M. enterolobii, was low (RF = 1.0). Therefore, the guava was not a good host for M. incognita. The present study also indicated a relatively low infection and reproduction rate (MF 1.57 vs. 4.61) of M. incognita as compared to M. enterolobii.
The growth parameters of the guava cultivar Allahabad Safeda exhibited no variation when M. javanica was inoculated even at higher levels, such as 1000, 1500, and 2000 J2 per pot. Furthermore, it was observed that M. javanica on guava in these trials displayed low root galling (RKI 2–3), a low reproduction factor (4.76 vs. 37.6 vs. 15.44 at an inoculum level of 100 J2/plant) in comparison to M. enterolobii and M. incognita. This was in agreement with the observation of Rossi et al. (2002), who reported a resistance response in three commercial guava cultivars inoculated with M. incognita race 2 and M. javanica for four months. Carneiro et al. (2012) assessed the host suitability of M. incognita race 1 (RF = 0.03), M. incognita race 2 (RF = 0.05), M. arenaria race 2 (RF = 0.0), M. javanica (RF = 0.0), and M. enterolobii (RF = 182.4) inoculating the guava cultivar Paluma with 10,000 eggs/plant for 12 months and conclusively shown, based on reproduction factor, that M. enterolobii is highly pathogenic to the guava.
In summary, the present study demonstrated that M. enterolobii is highly pathogenic, M. incognita is moderately pathogenic, and M. javanica is not very pathogenic to guava. At the lowest inoculum of 100 J2/plant, M. incognita can cause harm to the guava and plant growth impairment. At the initial density of 100 J2/plant of M. enterolobii, the guava crop suffers the most due to severe damage to the root system. However, the present findings are based on the guava cultivar Allahabad Safeda. There might be variations in the host responses of different cultivars to the different species of Meloidogyne. The field soils are often infested with more than one species of Meloidogyne species, thus the infection of M. incognita or M. javanica induces root galling and thereby making the root system vulnerable to the attack of other soil-borne pathogens such as fungi, leading to disease complexes in guava.
References
Anonymous (2018) Annual Report, All India Coordinated Research Project on Nematodes in Agriculture, Coimbatore center, India
Anonymous (2020) National Horticultural Board, 2019-20. Government of India, http://nhb.gov.in/StatisticsViewer.aspx?enc
Ansari RA, Khan TA (2012) Parasitic association of root-knot nematode, Meloidogyne incognita on guava. e-J Sci Tech 5:65–67
Babatola JO, Oyedunmade EEA (1992) Host-parasite relationships of Psidium guajava cultivars and Meloidogyne incognita. Nematol Medit 20:233–235
Byrd DW Jr, Kirkpatrick T, Barker KR (1983) An improved technique for clearing and staining plant tissue for detection of nematodes. J Nematol 14:142–143
Carneiro RMDG, de Freitas VM, Mattos JK, Castro JMC, Gomes CB, Carneiro RG (2012) Major guava nematodes and control prospects using resistance on Psidium spp. and non-host crops. III Int Symp Guava and other Myrtaceae 959:41–49
Casassa AM, Matheus J, Crozzoli R, Bravo V, Gonzalez C (1997) Response of some selections of guava to Meloidogyne incognita. Mara County, Zulia State, Venezuela. Fitopatol Venezolana, 10:5–8
Castagnone-Sereno P (2012) Meloidogyne enterolobii (= M. mayaguensis): profile of an emerging, highly pathogenic, root-knot nematode species. Nematology 14:133–138
Castagnone-Sereno P, Castillo P (2014) Meloidogyne enterolobii (Pacara earpod tree root-knot nematode). https://www.cabi.org/isc/datasheet/33238
Cobb NA (1918) Estimating the nematode population of the soil, United States Department of Agriculture. Agric Cir 1:1–48
Ghule T, Phani V, Somavanshi VS, Patil M, Bhattacharyya S, Khan MR (2020) Further observations on Meloidogyne enterolobii (Nematoda: Meloidogynidae) infecting guava (Psidium guajava) in India. J Nematol e2020-120 52. https://doi.org/10.21307/jofnem-2020-120
Gomes VM, Souza RM, Correa FMC, Dolinsk C (2010) Management of Meloidogyne mayaguensis in commercial guava orchards with chemical fertilization and organic amendments. Nematol Brasil 34:23–30
Gomes VM, Souza RM, Midorikawa G, Miller R, Almeida AM (2012) Guava decline: evidence of nationwide incidence in Brazil. Nematropica 42:153–162
Jepson SB (1987) Identification of root-knot nematodes (Meloidogyne species). CAB International, Wallingford, UK
Jindapunnapat K, Chinnasri B, Kwankuae S (2013) Biological control of root-knot nematodes (Meloidogyne enterolobii) in guava by the fungus Trichoderma harzianum. J Dev Sustainable Agric 8:110–118. https://doi.org/10.11178/jdsa.8.110
Karim SA (1991) Effects and control of root-knot nematode, Meloidogyne incognita, on guava plants, Psidium guajava L. MARDI Res J 19:71–75
Khan MR, Jain RK, Ghule TM, Pal S (2014) Root-knot nematodes in India-a comprehensive monograph. All India Coordinated Research Project on Plant parasitic nematodes with Integrated approach for their control. Indian Agricultural Research Institute, New Delhi, India, p 78
Khan MR, Poornima K, Somvanshi VS, Walia RK (2022) Meloidogyne enterolobii: a threat to guava (Psidium guajava) cultivation. Arch Phytopathol Pl Protec 55:1961–1997 https://. https://doi.org/10.1080/03235408.2022.2132623
Kumar V, Khan MR, Walia RK (2020) Crop loss estimations due to plant-parasitic nematodes in major crops in India. Nat Acad Sci Lett 43:409–412. https://doi.org/10.1007/s40009-020-00895-2
Kumari C, Dutta TK, Banakar P, Rao U (2016) Comparing the defence-related gene expression changes upon root-knot nematode attack in susceptible versus resistant cultivars of rice. Sci Rep 6:22846. https://doi.org/10.1038/srep22846
Madhu MR, Verma KK, Vinod K (2019) Distribution, prevalence and intensity of guava decline in western Haryana. J Entomol Zool Stud 7:521–524
Oostenbrink M (1966) Major characteristics of the relation between nematodes and plants. Mededbogesch Wageningen 66:3–46
Philbrick AN, Adhikari TB, Louws FJ, Gorny AM (2020) Meloidogyne enterolobii, a major threat to tomato production: current status and future prospects for its management. Front Pl Sci 11:606395. https://doi.org/10.3389/fpls.2020.606395
Poornima K, Suresh P, Kalaiarasan P, Subramanian K (2016) Root-knot nematode, Meloidogyne enterolobii in guava (Psidium guajava L.) a new record from India. Madras Agric J 103:359–365
Rodriguez H, Fernandez E, Shesteperov AA (1985) Adverse effect of Meloidogyne infection on guava (Psidium guajava L.) and factors influencing its development. Byulleten’Vsesoyuznogo Instituta Gel’mintologii im. KI Skryabina 41:48–56
Rossi CE, Ferraz LCCB, Montaldi PT (2002) Resistance of fruit tree rootstocks to Meloidogyne incognita race 2 and M. javanica. Arquivos do Instituto Biol 69:43 – 9
Schindler AF (1961) A simple substitute for a Baermann funnel. Plant Dis Reporter 45:747–748
Singh N (2020) Emerging problem of guava decline caused by Meloidogyne enterolobii and Fusarium oxysporum f.sp. Psidii. Indian Phytopathol 73:373–374. https://doi.org/10.1007/s42360-020-00198-y
Tigano M, Siqueira Kde Castagnone-Sereno P, Mulet K, Queiroz P, Santos M, dos Teixeira C, Almeida M, Silva J, Carneiro R (2010) Genetic diversity of the root-knot nematode Meloidogyne enterolobii and development of a SCAR marker for this guava-damaging species. Pl Pathol 59:1054–1061. https://doi.org/10.1111/j.1365-3059.2010.02350.x
Villota BJV, Agudelo FV (1997) Evaluation of guava material (Psidium guajava L.) for the -damage behaviour of Meloidogyne incognita. Fitopatol Colombiana 21:31–38
Vovlas N, Lucarelli G, Sasanelli N, Troccoli A, Papajova I, Palomares-Rius JE, Castillo P (2008) Pathogenicity and host‐parasite relationships of the root‐knot nematode Meloidogyne incognita on celery. Pl Pathol 57:981–987. https://doi.org/10.1111/j.1365-3059.2008.01843.x
Zijlstra C, Donkers-Venne DTHM, Fargette M (2000) Identification of Meloidogyne incognita, M. javanica and M. arenaria using sequence characterized amplified region (SCAR) based PCR assays. Nematology 2:847–853
Acknowledgements
The authors thank the Post Graduate School, ICAR- Indian Agricultural Research Institute, New Delhi, and the Division of Nematology for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patil, M., Khan, M.R. & Mondal, S. Pathogenic variation among three major root-knot nematodes (Meloidogyne spp.) affecting guava (Psidium guajava L.) cv. Allahabad Safeda. Indian Phytopathology 76, 551–558 (2023). https://doi.org/10.1007/s42360-023-00621-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-023-00621-0