Abstract
Maize is susceptible to colonization by Aspergillus flavus, which is the major aflatoxins producing fungus. Aflatoxins are fungal secondary metabolites that have been reported to adversely affect normal cell metabolism and seed germination. In this study, 106 maize seed samples were sampled across 13 states throughout India, and were screened for incidences of fungal infection. Among the seed samples collected across India nearly all maize seed samples were found to be infected with Aspergillus. All morphologically confirmed isolates belonging to Aspergillus section Flavi were subjected to various aflatoxin, biochemical, physiological and genotype assays, as well as phylogenetic analyses. Among 60 isolates tested, eight were non-aflatoxigenic in nature as determined by icELISA, and seven of those eight isolates showed absence of one or more aflatoxin cluster genes in PCR assay. The morphological, physiological and biochemical characterizations failed to show variation based on their toxigenicity. The phenetic and molecular genetic diversity could be observed based on RAPD and ISSR analyses and little isolate diversity could be determined based on geography or aflatoxigenicity. An inferred ITS sequence phylogeny segregated isolates into only two lineage groups: a large group I (including Ia and Ib) and small group II contained A. flavus isolates closely related to A. parasiticus. The current study highlights inherent diverse A. flavus infections, exhibiting a range of aflatoxin concentrations, that are present in maize seeds and they are important because of the potential threat to agricultural production and consumption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Maize (Zea mays Link) is one of the most important food staples, not only for humans, but also for animals, and is equally important as raw material for the manufacture of many industrial products (Nayaka et al. 2009; White 1999). India has 3.4% of its agricultural area covered in maize cultivation, and now ranks sixth in world production (Rasul 2016). Maize is grown in most parts of the India, ranging from extreme humid to sub-humid regions and most popularly in north-eastern and western regions (Joshi 2005). Even though hybrid seeds are used in most maize growing regions, traditional farmers still depend on their own saved seeds for subsequent plantings (Aiyaz et al. 2015a; Morris et al.1998; Mudili et al. 2014; Rauniyar and Goode 1992). Maize seeds are rich in carbohydrates, which may attract a wide range of fungal pathogens during pre- and post-harvest storage, and from a seed stock perspective Aspergillus-infected seeds often exhibit poor germination, of seed leading to poor yield and growth (Aiyaz et al. 2015b; Solorzano and Malvick 2011). It is possible that compounds produced by these fungi (including toxic metabolites) have the potential to adversely affect normal cell metabolism and seed germination (Aiyaz et al. 2015c; Nayaka et al. 2008; Prasad et al. 1997). Likewise, it is demonstrated that, among the fungal pathogens, moulds such as Aspergillus are able to thrive under adverse conditions of temperature (13–37 °C) and moisture (approximate minimum aw 0.82) (Lawley et al. 2008). Aflatoxin (AF) biosynthesis exists in several Aspergillus species, but the most widely known and researched of those species is A. flavus (Divakara et al. 2015; Godet and Munaut 2010). Aflatoxin B1 is considered has Class 1 human carcinogen by International Agency for Research (Anttila et al. 2002), hence, aflatoxin levels food products are carefully examined and regulated in most countries. In developed and developing countries most of them have established and proposed regulation for controlling aflatoxins in food products (Anttila et al. 2002). Production of AF involves at least 23 enzymatic reactions orchestrated by 25 genes, representing a well-defined gene cluster located in a 75 kb telomeric region of chromosome 3 (Carbone et al. 2007; Yu et al. 2004). Few A. flavus in nature lack the ability to synthesis aflatoxins due to either mutation in, or deletion of, any of the genes within the cluster (Criseo et al. 2008; Kiyota et al. 2011).
Conventional methods used for species identification of seed-borne fungal pathogens are mostly based on fungal morphology such as colony diameter, color and texture, as well as the size and texture of conidia and conidiophore structures (Chandra et al. 2013; Klich 2002). These fungi are also known to show variation in optimum pH, temperature, and required carbon and nitrogen sources (Chellapandi and Jani 2009). Differing or altering the carbon sources are known to alter secondary metabolite production which includes mycotoxins (Paranagama et al. 2007). Fungi produce various extracellular enzymes such as lipase, protease, cellulase, pectinase and amylase with roles in primary metabolism, plant pathogenicity and also in mycotoxin production (Alam et al. 2009; Fakhoury and Woloshuk, 1999; Ortega et al. 2013; Venkataramana et al. 2015). The phylogenetic nature of A. flavus is genetically complex with the presence of several cryptic species which at times makes species identification difficult (Geiser et al. 2000).
Fungal species identification has been made simple and fast, with the advancement in the field of molecular technique (Venkataramana et al. 2012; Vitale et al. 2011). Molecular technique like amplified fragment length polymorphism (AFLP), fragment length polymorphism (RFLP), random amplified polymorphic DNA (RAPD), amplification of the internal transcribed spacer region (ITS), restriction inter-simple sequence repeats (ISSR), and single-strand conformation polymorphism (SSCP), all of which are auxiliary tools in traditional methods (Nitschke et al. 2009; Dendis et al. 2003; Kumeda and Asoa 2001).
The objectives of the present study were to investigate the occurrence of aflatoxin production associated with fungal infection in maize seed used for planting, and molecular analysis of the genetic diversity of A. flavus isolated from infected maize seed.
Methods
Sample collection
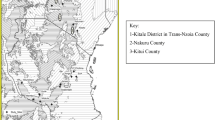
A survey was conducted across maize growing regions of India during the years of 2008–2011, a total of 106 seed samples (Table 1) were collected from agricultural Universities, seed corporations, research institutes and farm seed stock, irrespective of storage conditions. A minimum of 2.5 kg of maize seeds were collected per sample and labeled. The sampling points were documented using a Garmin handheld GPS 76CSx recorder and collection points were located at various agro-climatic regions across 13 states in India (Gopal et al. 2009). The GPS coordinates were converted to ‘shapefiles’ using Quantum GIS Lisboa where the attributes were layered to generate a study area (Fig. 1).
Assessment of fungal contamination
All maize seed samples were examined by standard blotter method (SBM) (ISTA 2005). To assess seed-borne fungi, seeds from each sample (Table 1) maize seeds were placed on wet filter paper in sterile pertiplates. Plated seeds were kept moist by frequently wetting filter paper with sterile distill water in two days interval. Fungal contamination was evaluated across all the seeds after seven days. Fungal identification was done based by using stereomicroscope under different magnifications and identified based on the morphological and colony characters (Singh et al. 1991). Isolates tentatively determined belonging to Aspergillus section Flavi were maintained as pure cultures on potato dextrose agar (PDA) medium at 4 °C and used for further studies.
Characterization of Aspergillus flavus isolates
A representative sample of 60 Aspergillus flavus isolates was characterized using various optimal media such as Potato Dextrose Agar (PDA), Aspergillus Flavus Parasiticus Agar (AFPA), Yeast Extract Sucrose (YES) agar and Czapek dox (CZ) agar, and Physiological characterization for all isolates involved growing them on PDA with modified pH levels of 4, 6, 8, 10 and 12 using tartaric acid and NaOH. Additionally, the ability of these Aspergillus isolates to grow in PDA at different temperatures (4, 15, 30 and 40 °C) was analyzed.
The ability of isolates to produce extracellular enzymes like amylase, lipase, protease, cellulase and pectinase was tested using culture plate assays (Sunitha et al. 2013). The isolates were point-inoculated in each medium type and their colony characteristics were observed after 7 days after incubation. High-Performance Thin Layer Chromatography (HPTLC) analysis was also performed for all isolates to determine their aflatoxin-producing potential (Criseo et al. 2001). Further aflatoxins were quantified indirect competitive (ic) ELISA (Reddy et al. 2009; Hariprasad et al. 2013).
Molecular identification of Aspergillus flavus isolates
All 60 Aspergillus isolates were subjected to a molecular confirmation of their preliminary species identification. DNA was extracted from the isolates using a Hi PurA™ Plant Genomic DNA Miniprep Purification Spin Kit (Hi media, India) according to manufacturer’s instructions. A genera-specific PCR assay was then performed for confirmation of Aspergillus species using Aspergillopepsin primers PEPO1 (CGACGTCTACAAGCCTTCTGGAAA) and PEPO2 (CAGCAGACCGTCATTGTTCTTGTC), PCR amplification was carried and genra specific identification of Aspergillus was done presence of specific band when visualized under UV transilluminator (Logotheti et al. 2009; Priyanka et al. 2013). Furthermore, these isolates were confirmed at the molecular species level according White et al. (1990). Multiplex PCR (mPCR) reactions were performed with primers for specific AF cluster genes selected: aflR, ordA, norA, omtA, and ver1 (Table 2) (Yin et al. 2009).
Phylogenetic and phenetic analyses
Sequences obtained from the ITS regions of all isolates, along with an outlier sequence of Aspergillus parasiticus (EU982020.1) from NCBI, were aligned using ClustalW version 1.7 (Thompson et al. 1994). The resulting phylogenetic tree was inferred by maximum likelihood method using the Mega 5.0 program (Tamura et al. 2011). Genetic distance was calculated using the Kimura 2-parameter model (Kimura et al. 1980).
RAPD analysis was performed by selecting 20 random decamer primers (Kini et al. 2002). These RAPD primers were first evaluated for their proven ability to amplify genomic DNA fragments for a few of our Aspergillus isolates. A total of 15 primers were used (Table 3). The PCR reaction was carried and amplified products were then separated in a 1.5% agarose gel stained with ethidium bromide and compared with Mass Ruler Express DNA ladder (Kini et al. 2002). ISSR analysis, was carried out by 21 different set of primers (Table 4) and band visualization was done according to Divakara et al. (2015).
Unambiguous ISSR and RAPD fragments were scored for the presence (1) or absence (0) of ISSR patterns in each of the 60 isolates. In some cases, isolates failed to amplify any product with certain primers. In these cases, missing data was scored as "?". Those isolates exhibiting similar banding patterns were referred to as monomorphic, whereas those that exhibited a different pattern were referred to as polymorphic (Rohlf et al. 1998). The data produced by the RAPD and ISSR methods were analysed (Felsenstein et al. 1993). The consistency of the phylogenetic trees drawn by the NJ method was explored by bootstrap resampling using the SEQBOOT program (PHYLIP 3.5) 100 replicates, of random seed 81 for RAPD, ISSR and combined data.
Results
Fungal contamination of seed samples
Of the 106 seed samples, the overall incidence of infection by Aspergillus section Flavi was 95%, although the occurrence of infection within the seeds of each 2.5 kg sample ranged from 1–88% (Table 1). Highest seed-borne incidence by Aspergillus section Flavi was observed in sample M81 which showed to have a contamination rate of 85%, and five seed samples (M37, M55, M73, M83 and M87) were free from any inherent Aspergillus infection (Table 1).
Characterization of Aspergillus flavus isolates
All 60 Aspergillus flavus isolates showed fast growth and early sporulation on PDA media, followed by CZ and YES, and diminished growth rate and sporulation were observed on AFPA medium. The isolates studied showed better growth at pH 8, and the least observed growth was at pH 4. The optimum temperature for the growth of the Aspergillus flavus isolates tested was 30οC, and least amount of growth was observed at 4οC.
All studied isolates were positive for extracellular amylase, cellulase and lipase enzyme production (Table 5). Among studied isolates for protease production, only two Aspergillus isolates (UOMMAF-11 and UOMMAF-25) were negative for production of the enzyme. Isolates UOMMAF-15, UOMMAF-37 and UOMMAF-54 were found to be negative for pectinase production. Based on the use of HPTLC, 27 of the 60 Aspergillus isolates exhibited production of both aflatoxins B1 and B2, 20 isolates produced only AF B1, five isolates produced only AF B2, and the remaining eight isolates (UOMMAF-2, UOMMAF-11, UOMMAF-26, UOMMAF-30, UOMMAF-33, UOMMAF-57, UOMMAF-64 and UOMMAF-71) were determined to be non-aflatoxigenic (Table 6). The icELISA confirmed the non-aflatoxigenic phenotype for seven of these eight isolates. Isolate UOMMAF-71, which appeared negative in HPTLC, exhibited the production of AF in the icELISA. All other isolates tested positive for AF production in icELISA, correlating positively with HPTLC (Table 6, Supplementary Table).
Molecular investigation of Aspergillus flavus isolates
PCR assays of genomic DNA with Aspergillopepsin primers were able to produce an expected amplicon fragment of about 200 bp in all isolates; therefore, the identities of the 60 morphologically identified isolates were confirmed to Aspergillus genus (Fig. 2). Further, PCR amplification with ITS primers produced an expected amplicon fragment of approximately 600 bp (Fig. 3), and their respective sequences were then BLAST-confirmed as Aspergillus flavus based on 94–100% identity and genBank accession numbers were obtained (Table 6).
Multiplex PCR results are shown in Fig. 4 and Table 6. Amplicon fragments of the norA, ver1, omtA, aflR, and ordA genes can be visualized at 399 bp, 472 bp, 594 bp, 873 bp and 1156 bp, respectively. Of the 60 isolates examined, 54 showed a pentaplex band pattern indicating the presence of the five target genes from the AF biosynthesis pathway. Non-aflatoxigenic isolates UOMMAF-2, UOMMAF-11 UOMMAF-26, UOMMAF-33, UOMMAF-57 and UOMMAF-64 showed banding patterns in which one or more bands were absent. Isolate UOMMAF-30; however, which was also found to be negative for AF production in both HPTLC and icELISA, exhibited the presence of all five bands in mPCR (Fig. 4).
Amplification products obtained by pentaplex PCR for aflatoxigenic and non-aflatoxigenic A. flavus isolates. Lane M represents marker DNA; lanes 2–14 correspond to UOMMAF isolates 1, 2, 11, 26, 30, 33, 64, 71, 57, 75, and 15 correspond to negative template control, respectively. Lanes 2–9 are non-aflatoxigenic isolates
Phylogenetic and phenetic analyses
A phylogenetic tree, inferred by the maximum likelihood method, and based on the ITS region, segregated the 60 isolates into two clades (Fig. 5). Clade A divided into two subclades supported by a bootstrap value of 62 which consists of the A. flavus lineage I isolates, whereas lineage II clade B consists of the out-group taxa A. parasiticus (EU902020.1) and three isolates of A. flavus lineage II (UOMMAF-2, UOMMAF-67 and UOMMAF-68), which were isolated from seed samples collected in Karnataka state and Andhra Pradesh. Both branches in the maximum likelihood method tree were strongly supported by high bootstrap values of > 90 that are labeled at nodal regions (Fig. 5).
The 15 selected decamer RAPD primers generated 123 unambiguous DNA bands; of which, 90 were polymorphic with an average of 6.0 polymorphic fragments per primer (Table 3). A phenetic UPGMA tree was inferred using dice similarity based on the coefficient dendrogram of RAPD data. The 60 Aspergillus isolates showed an ancestral bifurcation into two lineages, with isolate UOMMAF-28 being the only representative isolate for one of those lineages (noted as II in Fig. 6), and scoring a similarity coefficient of 0.44. Lineage I included the remaining isolates for which the similarity coefficient was 0.61 despite the high number of bifurcating branches observed (Fig. 6). In the phylogenetic NJ consensus tree (not shown) six clades were supported by bootstrap values higher than 50%. In one of them both isolates UOMMAF-32 and UOMMAF-38 were from Andhra Pradesh.
Using 20 ISSR primers to screen for their ability to elucidate polymorphic DNA bands, 131 unambiguous and reproducible amplification products were scored. Of the 131 amplified bands, 109 were polymorphic with an average of 5.45 polymorphic fragments per primer (Table 4). A phenetic UPGMA tree was inferred using dice similarity based on the coefficient dendrogram of ISSR data (Fig. 7). The 60 A. flavus isolates show an ancestral bifurcation into two lineages, with isolate UOMMAF-53 being the only representative in one of those lineages (noted as II in Fig. 7), and scoring a similarity coefficient of 0.49. Lineage I included the remaining isolates for which the similarity coefficient was 0.96 despite the high number of bifurcating branches observed (Fig. 7). In the phylogenetic NJ consensus tree (Fig. 8) four clades were supported by a bootsrap value higher than 50%. Three (UOMMAF-54, UOMMAF-55 and UOMMAF-71) of the four isolates of one clade were from Rajasthan, while three (UOMMAF-33, UOMMAF-37 and UOMMAF-49) of the nine isolates of the second clade were from Andhra Pradesh and two (UOMMAF-72 and UOMMAF-74) from Uttarakhand.
In the combined ISSR-RAPD NJ consensus tree nine clades were supported by bootstrap values higher than 50% (Fig. 9). Three (UOMMAF-32, UOMMAF-33 and UOMMAF-37) of the four isolates of one clade were from Andhra Pradesh, while two (UOMMAF-72 and UOMMAF-74) of the four isolates of the second clade were from Uttarakhand. In three clades both isolates (UOMMAF-2 and UOMMAF13; UOMMAF-11 and UOMMAF-18; UOMMAF-43 and UOMMAF-54) were from Karnataka.
Discussion
Aflatoxin producing fungi are native to warm arid, semi-arid and tropical regions, but changes in climate may result in greater prevalence of AF contamination outbreaks (Shearer et al. 1992; Bock et al. 2004). Types of aflatoxins and species of AF-producing fungi may differ based on geography and climatic conditions (Lisker et al. 1993; Horn and Dorner 1998). In the present work, maize seeds were collected from various agro-climatic regions in India. Aspergillus section Flavi consists of thirteen species of Aspergillus which have few morphological identification features which makes them difficult to identify (Divakara et al. 2014). Among the seed samples collected from Rajasthan and Andhra Pradesh state showed higher Aspergillus and aflatoxin contamination compared to other regions. According to previous reports, in maize A. flavus infection and AF contamination is prevalent and potentially severe during times of drought (Cotty and Jaime-Garcia 2007).
Aspergillus flavus is widely studied because of AF production, and its mode of action is well-established (Williams et al. 2004). This fungus may also contribute to a wide variety of diseases in plant systems (Amaike and Keller 2011). Unfortunately, no originated efforts have been made to address this problem in India. Scant reports are available in plant systems linked with Aspergillus infections. Interaction studies between A. flavus and maize seeds revealed that this fungus preferentially colonizes the embryo tissue upon infection, because of its rich nutrient content, and the endosperm is affected late in the destruction of the kernel (Watson 1987; Brown et al. 1994; Keller et al. 1994; Woloshuk et al. 1996). Aflatoxins are reported to be involved in host pathogenesis by affecting amylase activity during embryonic development (Chatterjee 1988).
Toxin analyses revealed that most (52-53/60) of the A. flavus isolates in this study are aflatoxigenic, but their aflatoxin levels were not uniform. No aflatoxin G1 or G2 production was found in the isolates. Among the aflatoxigenic strains, production varied from 10 to 320 ppb (Supplementary Table). Similar findings were reported from earlier study that variation in AF content is linked with differential expression of the AF biosynthesis genes among A. flavus isolates (Gendloff et al. 1992). In nature, strains of Aspergillus spp. may lose their ability to produce aflatoxins because of mutations in, or deletions of, biosynthesis pathway genes. Among the 60 isolates tested, eight isolates were found to be non-aflatoxigenic when analyzed by HPTLC; and of those, seven isolates was confirmed as non-aflatoxigenic based on icELISA methods. Additional support was uncovered by different patterns of deletion for AF biosynthesis genes through mPCR analysis. We observed evidence of gene deletion for most of the non-aflatoxigenic isolates, but in the cases of UOMMAF-30 and UOMMAF-71 all the tested genes amplified. This observation suggests that their non-aflatoxigenic phenotype is likely due to either a lack of other AF cluster genes not examined in this study, or the presence of mutations that affect the productivity of one or more AF cluster genes. Similar observations were reported whereby they observed large deletions of the AF gene cluster in atoxigenic A. flavus isolates (Chang et al. 2005). Eight distinct deletion patterns were reported, including one in which entire AF gene cluster was absent. Recently, several efforts have been made to distinguish aflatoxigenic and non-aflatoxigenic strains by using mPCR (Giovannetti et al. 2013; Navya et al. 2013; Rashmi et al. 2013). The morphological, physiological and biochemical characterization of the Aspergillus isolates failed to group them based on the chemotypic or taxonomic profiles. Optimum aflatoxin production by A. flavus occurs at a temperature of 25–30 °C and 0.99 aw conditions, and optimum growth occurs at 30 °C and 0.95 aw in the culture medium (Sanchis and Magan 2004). The enzyme α-amylase has a role in the production of aflatoxins by A. flavus, so a deficient α-amylase gene will produce lower quantities of the aflatoxins.
Ecological factors and selection pressures in the distinct agro-climatic regions have the potential to influence genetic diversity (Giovannetti et al. 2013). These influences may lead to variation in the ITS regions among Aspergillus species (Henry et al. 2000). The ITS region is often considered a good delineator of species (Schoch et al. 2012). Our ITS analysis revealed little sequence diversity among the 57 isolates which were from lineage I (subgroups Ia and IB) in Clade A. These isolates grouped separately, and all belonged to lineage II the clade B contained UOMMAF-2 (a non-aflatoxigenic isolate), UOMMAF-67, UOMMAF-68 and an A. parasiticus isolate from Greece. The presence of the two lineages in A. flavus is well known which well reported (Moore et al. 2009). It may be that lineage B isolates actually belong to A. parasiticus, because their ITS sequences are very similar to the ITS sequence (EU982020.1) of the isolate, which was identified as A. parasiticus by Krimitzas et al. (2013). To show more intra-species genetic diversity among the 60 A. flavus isolates may require using additional molecular markers, such as the intergenic regions of the AF gene cluster (Cary et al. 2005).
The 15 RAPD and 20 ISSR primers used in this study provided results suggesting considerable genetic diversity for a majority of Aspergillus isolates from 13 different agro-climatic regions of India. The RAPD and ISSR dendrogram exhibited extensive pattern diversity among the majority of the isolates, despite their similarity coefficients of 1.0 and 0.94. Our findings revealed that these Aspergillus isolates, which were randomly distributed across India, cannot be grouped based on geography. Furthermore, RAPD and ISSR patterns obtained with these primers failed to discriminate between aflatoxigenic and non-aflatoxigenic phenotypes and between ITS clades A and B. In the present study some isolates from the same region were phylogenetically related to each other based on the phylogenetic trees of ISSR, RAPD and combined ISSR-RAPD data. Its reported that calmodulin gene and ISSR patterns for investigating intra-species diversity among 30 A. flavus isolates from peanut in China, failed to separate isolates based on their toxigenic profiles or their regions of the origin (Zhang et al. 2013).
Conclusions
The present study has provided relevant information on the current status of A. flavus infection across different maize-growing regions of India. We show that diverse populations of aflatoxigenic A. flavus capable of producing varied levels of aflatoxins, are potential threats for agricultural production. Likewise, from the results of RAPD and ISSR it was concluded that distribution of two lineages of A. flavus across India is random, and these strains cannot be grouped based on their agro-climatic regions or based on aflatoxigenicity. Future studies involving a more holistic approach should be conducted to better understand the distribution and diversity of A. flavus and aflatoxin contamination in agricultural crops. This will aid in developing suitable strategies for the management of A. flavus and aflatoxins in maize.
Abbreviations
- RAPD:
-
Random amplified polymorphic DNA
- ISSR:
-
Inter simple sequence repeats
- ITS:
-
Internal transcribed spacer regions
- UPGMA:
-
Unweighted pair group method with arithmetic mean
- NTSYS-pc:
-
Numerical taxonomy and multivariate analysis system
References
Aiyaz M, Divakara ST, Chandranayaka S, Niranjana SR (2015a) Efficacy of seed hydropriming with phytoextracts on plant growth promotion and antifungal activity in maize. Int J Pest Manag 61:153–160. https://doi.org/10.1080/09670874.2015.1025116
Aiyaz M, Divakara ST, Nayaka SC, Hariprasad P, Niranjana SR (2015b) Application of beneficial rhizospheric microbes for the mitigation of seed-borne mycotoxigenic fungal infection and mycotoxins in maize. Biocontrol Sci Technol 25:1105–1119. https://doi.org/10.1080/09583157.2015.1020760
Aiyaz M, Divakara ST, Parthasarathy S, Hariprasad P, Nayaka SC, Niranjana SR (2015c) Genetic diversity and biocontrol potential of rhizospheric microbes isolated from tomato and maize. J Pure Appl Microbiol 9:239–248
Alam S, Shah H, Magan N (2009) Water availability affects extracellular hydrolytic enzyme production by Aspergillus flavus and Aspergillus parasiticus. World Mycotoxin J 2:313–322. https://doi.org/10.3920/WMJ2008.1108
Amaike S, Keller NP (2011) Aspergillus flavus. Annu Rev Phytopathol 49:107–143
Anttila A, Bhat RV, Bond, JA, Borghoff SJ, Bosch FX, Carlson GP, Castegnaro M, Cruzan G., Gelderblom WCA, Hass U, Henry SH, Herbert RA et al (2002) IARC Monographs on the evaluation of carcinogenic risks to humans: some traditional herbal medicines, some mycotoxins, naphthalene and styrene. In: IARC Monogr. Eval. Carcinog. Risks to Humans. https://doi.org/10.1002/food.19940380335
Bock CH, Mackey B, Cotty PJ (2004) Population dynamics of Aspergillus flavus in the air of an intensively cultivated region of southwest Arizona. Plant Pathol 53:422–433
Brown RC, Lemmon BE, Olsen OA (1994) Endosperm development in barley: microtubule involvement in the morphogenetic pathway. Plant Cell 6:1241–1252
Carbone I, Ramirez-Prado JH, Jakobek JL, Horn BW (2007) Gene duplication, modularity and adaptation in the evolution of the aflatoxin gene cluster. BMC Evol Biol 7:111–132. https://doi.org/10.1186/1471-2148-7-111
Cary JW, Klich MA, Beltz SB (2005) Characterization of aflatoxin-producing fungi outside of Aspergillus section Flavi. Mycologia 97:425–432
Chandra NS, Udayashankar A, Niranjana S, Prakash H, Mortensen C (2013) Laboratory protocols in fungal biology: curent methods in fungal biology, in: fungal biology. Springer Science & Business Media, Berlin. https://doi.org/10.1007/978-1-4614-2356-0
Chang PK, Horn BW, Dorner JW (2005) Sequence breakpoints in the aflatoxin biosynthesis gene cluster and flanking regions in non-aflatoxigenic Aspergillus flavus isolates. Fungal Genet Biol 42:914–923
Chatterjee D (1988) Inhibitory effects of aflatoxin B1 on amylase in maize seed. Lett Appl Microbiol 7:9–11
Chellapandi P, Jani AA (2009) Enhanced endoglucanase production by soil isolates of Fusarium sp. and Aspergillus sp. through submerged fermentation process. Turk J Biochem 34:209–214
Cotty PJ, Jaime-Garcia R (2007) Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int J Food Microbiol 119:10–115
Criseo G, Bagnara A, Bisignano G (2001) Differentiation of aflatoxin producing and non producing strains of Aspergillus flavus group. Lett Appl Microbiol 133:291–295
Criseo G, Racco C, Romeo O (2008) High genetic variability in non-aflatoxigenic A. flavus strains by using Quadruplex PCR-based assay. Int J Food Microbiol 125:341–343. https://doi.org/10.1016/j.ijfoodmicro.2008.04.020
Dendis M, Horvath R, Michalek J, Ruzicka F, Grijalva M, Bartos M, Benedík J (2003) PCR-RFLP detection and species identification of fungal pathogens in patients with febrile neutropenia. Clin Microbiol Infect 9:1191–1202
Divakara ST, Aiyaz M, Hariprasad P, Nayaka SC, Niranjana SR (2014) Aspergillus flavus infection and aflatoxin contamination in sorghum seeds and their biological management. Arch Phytopathol 47:2141–2156
Divakara ST, Aiyaz M, Moore GG, Venkataramana M, Hariprasad P, Nayaka SC, Niranjana SR (2015) Analysis of genetic and aflatoxin diversity among Aspergillus flavus isolates collected from sorghum seeds. J Basic Microbiol 55:1255–1264. https://doi.org/10.1002/jobm.201400951
Fakhoury AM, Woloshuk CP (1999) Amy1, the alpha-amylase gene of Aspergillus flavus: Involvement in aflatoxin biosynthesis in maize kernels. Phytopathology 2:313–322. https://doi.org/10.1094/phyto.1999.89.10.908
Felsenstein J (1993) PHYLIP (phylogeny inference package), v. 3.5c. In: Department of Genetics, University of Washington, Seattle, WA
Geiser DM, Dorner JW, Horn BW, Taylor JW (2000) The phylogenetics of mycotoxin and sclerotium production in Aspergillus flavus and Aspergillus oryzae. Fungal Genet Biol 31:169–179. https://doi.org/10.1006/fgbi.2000.1215
Gendloff E, Chee F, Leonard T (1992) Variation in regulation of aflatoxin biosynthesis amongst isolates of A. flavus. Experimentia 48:84–87
Giovannetti M, Sbrana C, Strani P, Agnolucci M, Rinaudo V, Avio L (2013) Genetic diversity of isolates of Glomusmosseae from different geographic areas detected by vegetative compatibility testing and biochemical and molecular analysis. Appl Environ Microbiol 69:616–624
Godet M, Munaut F (2010) Godet, Marie, and Françoise Munaut. Molecular strategy for identification in Aspergillus section Flavi. FEMS Microbiol Lett 304:157–168. https://doi.org/10.1111/j.1574-6968.2009.01890.x
Gopal S, Sarkar R, Banda K, Govindarajan J, Harijan BB, Jeyakumar MB (2009) Study of water supply and sanitation practices in India using geographic information systems: some design and other considerations in a village setting. Indian J Med Res 129:233–241
Hariprasad P, Durivadivel P, Snigdha M, Venkateswaran G (2013) Natural occurrence of aflatoxin in green leafy vegetables. Food Chem 138:1908–1913
Henry T, Iwen PC, Hinrichs SH (2000) Identification of Aspergillus species using internal transcribed spacer regions 1 and 2. J Clin Microbiol 38:1510–1515
Horn BW, Dorner JW (1998) Soil populations of Aspergillus species from section Flavi along atransect through peanut-growing regions of the United States. Mycologia 90:767–776
International Seed Testing Association (ISTA) (2005). International rules for seed testing. Seed Sci Tech, p 27
Joshi PK (2005) Maize in India: Production Systems, CIMMYT
Keller NP, Butchko RAE, Sarr B, Phillips TD (1994) A visual pattern of mycotoxin production in maize kernels by Aspergillus spp. Phytopathology 84:483–488
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotides sequences. J Mol Evol 16(2):111–120
Kini KR, Leth V, Mathur SB (2002) Genetic variation in Fusarium moniliforme Isolated from seeds of different host species from Burkina faso based on random amplified polymorphic DNA analysis. J Phytopathol 150:209–212
Kiyota T, Hamada R, Sakamoto K, Iwashita K, Yamada O, Mikami S (2011) Aflatoxin non-productivity of Aspergillus oryzae caused by loss of function in the aflJ gene product. J Biosci Bioeng 111:512–517. https://doi.org/10.1016/j.jbiosc.2010.12.022
Klich MA (2002) Identification of common Aspergillus species. Centraalbureau voor Schimmelcult. https://doi.org/10.1099/mic.0.2007/007641-0
Krimitzas A, Pyrri I, Kouvelis VN, Kapsanaki-Gotsi E, Typas MA (2013) A phylogenetic analysis of greek isolates of Aspergillus species based on morphology and nuclear and mitochondrial gene sequences. Biomed Res Int 2013:260395
Kumeda Y, Asao T (2001) Heteroduplex panel analysis a novel method for genetic identification of Aspergillus section Flavi strains. Appl Environ Microbio 67:4084–4090
Lawley R, Curtis L, Davis J (2008) The food safety hazard guidebook, the food safety hazard guidebook. R Soc Chem. https://doi.org/10.1039/9781847558398
Lisker N, Michaeli R, Frank ZR (1993) Mycotoxigenic potential of Aspergillus flavus strains isolated form groundnuts growing in Israel. Mycopathologia 122:177–183
Logotheti M, Kotsovili-Tseleni A, Arsenis G, Legakis NI (2009) Multiplex PCR for the discrimination of A. fumigatus, A. flavus, A. niger and A. terreus. J Microbiol Meth 76:209–211
Moore GG, Singh R, Horn BW, Carbone I (2009) Recombination and lineage-specific gene loss in the aflatoxin gene cluster of Aspergillus flavus. Mol Ecol 18(23):4870–4887
Morris ML, Singh RP, Pal S (1998) India’s maize seed industry in transition: changing roles for the public and private sectors. Food Policy 23:55–71. https://doi.org/10.1016/S0306-9192(98)00014-1
Mudili V, Siddaih CN, Nagesh M, Garapati P, Naveen Kumar K, Murali HS, Yli Mattila T, Batra HV (2014) Mould incidence and mycotoxin contamination in freshly harvested maize kernels originated from India. J Sci Food Agric 94:2674–2683. https://doi.org/10.1002/jsfa.6608
Navya HM, Hariprasad P, Naveen J, Chandranayak S, Niranjana SR (2013) Natural occurrence of aflatoxin, aflatoxigenic and non-aflatoxigenic Aspergillus flavus in groundnut seeds across India. Afr J Biotechnol 12(19):2587–2597
Nayaka SC, Chunche A, Udaya G (2008) Molecular detection and characterisation of Fusarium verticillioides in maize (Zea mays. L) grown in southern India. Ann Microbiol 58:359–367
Nayaka SC, Shankar CU, Reddy MS, Mortensen N, Nayaka SC, Siddapura RN, Harishchandra SP, Hunthrike S, Shetty A (2009) Control of Fusarium verticillioides, cause of ear rot of maize, by Pseudomonas fluorescens. Pest Manag Sci Former Pestic Sci 65:769–775. https://doi.org/10.1002/ps.1751
Nitschke E, Nihlgard M, Varrelmann M (2009) Differentiation of eleven Fusarium spp. isolated from sugar beet, using restriction fragment analysis of a polymerase chain reaction-amplified translation elongation factor 1α gene fragment. Phytopathology 99:921–929
Ortega LM, Kikot GE, Astoreca AL, Alconada Magliano TM (2013) Screening of Fusarium graminearum isolates for enzymes extracellular and deoxynivalenol production. J Mycol. https://doi.org/10.1155/2013/358140
Paranagama PA, Wijeratne EMK, Gunatilaka AAL (2007) Uncovering biosynthetic potential of plant-associated fungi: effect of culture conditions on metabolite production by Paraphaeosphaeria quadriseptata and Chaetomium chiversii. J Nat Prod 70:1939–1945. https://doi.org/10.1021/np070504b
Prasad G, Sinha KK, Ali MM (1997) Effect of aflatoxin B1 on chlorophyll, nucleic acid and protein contents in maize. Biol Plant 39:47–50. https://doi.org/10.1023/A:1000352821598
Priyanka SR, Venkataramana M, Kumar GP, Rao VK, Murali HS, Batra HV (2013) Occurrence and molecular detection of toxigenic Aspergillus species in food grain samples from India. J Sci Food Agric 94(3):537–543
Rashmi R, Ramana MV, Shylaja R, Uppalapati SR, Murali HS, Batra HV (2013) Evaluation of multiplex PCR assay for concurrent detection of four major mycotoxigenic fungi from foods. J Appl Microbiol 114:819–827
Rasul G (2016) Managing the food, water, and energy nexus for achieving the Sustainable Development Goals in South Asia. Environ Dev. https://doi.org/10.1016/j.envdev.2015.12.001
Rauniyar GP, Goode FM (1992) Technology adoption on small farms. World Dev 20:275–282. https://doi.org/10.1016/0305-750X(92)90105-5
Reddy KRN, Reddy CS, Muralidharan K (2009) Detection of Aspergillus spp. and aflatoxin B1 in rice in India. Food Micro 26:27–37
Rohlf FJ (1998) NTSYSpc: numerical taxonomy and multivariate system. Exeter Softwar, New York
Sanchis V, Magan N (2004) Environmental conditions affecting mycotoxins. In: Magan N, Olsen M (eds) Mycotoxins in food. CRC Press, Boca Raton, p 177
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Levesque CA (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci 109(16):6241–62466
Shearer JF, Sweets LE, Baker NK, Tiffany LH (1992) A study of Aspergillus flavus/parasiticus in Iowa crop fields. Plant Dis 76:19–22
Singh K, Frisvad JC, Thrane U, Mathur SB (1991) An illustrated manual on identification of some seed-borne Aspergilli, Fusaria, Penicillia and their mycotoxins. Danish Government Institute of Seed Pathology, Denmark, p 133
Solorzano CD, Malvick DK (2011) Effects of fungicide seed treatments on germination, population, and yield of maize grown from seed infected with fungal pathogens. F Crop Res 122:173–178. https://doi.org/10.1016/j.fcr.2011.02.011
Sunitha VH, Nirmala D, Srinivas C (2013) Extracellular enzymatic activity of endophytic fungal strains isolated from medicinal plants. World J Agric Sci 9(1):01–09
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Bio Evol 28:2731–2739
Thompson JD, Higgins DG, Gibson T, Clustal W (1994) Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 11(22):4673–4680
Venkataramana M, Chandra NS, Murali H, Batra HV (2012) A novel PCR-DNA probe for the detection of fumonisin producing Fusarium species from major food crops grown in Southern India. Mycol Int J Fungal Biol 3(3):167–174
Venkataramana M, Chandranayaka S, Prakash HS, Niranjana SR (2015) Mycotoxins relevant to biowarfare and their detection. Biol Toxins Bioterrorism 2015:295–319. https://doi.org/10.1007/978-94-007-5869-8_14
Vitale S, Santori A, Wajnberg E, Sereno-Castagnone P, Luongo L, Belisario A (2011) Morphological and molecular analysis of Fusarium lateritium, the cause of Gray Necrosis of Hazelnut Fruit in Italy. Mycology 101(6):679–686
Watson SA (1987) Structure and composition in corn: chemistry and technology. In: Watson SA, Ramstad PE (eds) St. Paul, MN: American Association of Cereal Chemists), pp 53–82
White DG (1999) Compendium of Corn Diseases, 4th edn. The American Phytopathological Society, Agaricus of North America
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics in PCR protocols-a guide to methods and applications ed. MA. Innis, DH. Gelfand, JJ. Sninsky, TJ White (San Diego: Calif Academic Press), pp 315–322
Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D (2004) Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr 80(5):1106–1122
Woloshuk CP, Cavaletto JR, Cleveland TE (1996) Inducers of aflatoxin biosynthesis from colonized maize kernels are generated by an amylase activity from Aspergillus flavus. Phytopathology 87:164–169
Yin Y, Lou T, Yan L, Michailides TJ, Ma Z (2009) Molecular characterization of toxigenic and atoxigenic Aspergillus flavus isolates, collected from peanut fields in China. J Appl Microbiol 107:1857–1865
Yu J, Chang PK, Ehrlich KC, Cary JW, Bhatnagar D, Cleveland TE, Payne GA, Linz JE, Woloshuk CP, Bennett JW (2004) Clustered pathway genes in aflatoxin biosynthesis. Appl Environ Microbiol 70:1253–1262. https://doi.org/10.1128/AEM.70.3.1253-1262.2004
Zhang CS, Xing FG, Selvaraj JN, Yang QL, Zhou L, Zhao YJ, Liu Y (2013) The effectiveness of ISSR profiling for studying genetic diversity of Aspergillus flavus from peanut-cropped soils in China. Biochem Syst Ecol 50:147–153
Acknowledgements
This research is funded by the Department of Biotechnology, and the Government of India. Project number (Project – BT/PR9864/AGR/05/391/2007 Dated: 6-6-2008). We are grateful for the Institute of Excellence, Ministry of Human Resource Development (MHRD) at University of Mysore, for providing the infrastructure to perform this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no competing interests.
Availability of data and supporting materials section
Please contact author for data requests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aiyaz, M., Divakara, S.T., Konappa, N.M. et al. Genetic and chemotypic diversity of two lineages of Aspergillus flavus isolated from maize seeds of different agroclimatic niches of India. Indian Phytopathology 73, 219–236 (2020). https://doi.org/10.1007/s42360-020-00224-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-020-00224-z