Abstract
Yellow vein mosaic virus (YVMV) and enation leaf curl virus (ELCV) are major viral diseases of okra (Abelmoschus esculentus [L.] Moench) in the tropics threatening its commercial cultivation. The study aims to screen okra genotypes against viral diseases and to study inter-relationships between YVMV disease severity and whitefly population with leaf morphological parameters and to assess the extent of marketable yield loss due to YVMV infection. Out of 565 genotypes screened, only BCO-1 was found to be resistant against YVMV disease, while two genotypes IC111551 and IC433616 were found moderately susceptible against ELCV disease up to 60 days after sowing. Negatively significant correlations were recorded between adaxial and abaxial pubescence density and the number of whitefly adults throughout the growth stages of plant. Similar trend of correlations were also observed between leaf morphological parameters and YVMV disease severity. The marketable yield loss of YVMV ranged from 17.09 to 96.49%. The resistance of BCO-1 to YVMV and ELCV is based on antixenotic property of the genotype, and could be utilized in future dual disease resistant breeding programme.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Okra [Abelmoschus esculentus (L.) Moench] is placed in Malvaceae family and originated in tropical Africa but can be grown in tropical and sub-tropical regions of the world (Ali et al. 2012). It is one of the most edible vegetable crops and popular for its green tender fruits grown throughout India during summer and rainy seasons (Solankey et al. 2013). India is the largest okra producing country accounting for 72.9% of total production in the world. In India, okra is grown at an area of 501 thousand hectares with an average production of 5783 MT green pods (Anonymous 2017). Okra production in tropical regions is, however, constrained by several abiotic and biotic factors and yield losses due to biotic factors are quite substantial (Jellis 2009). One of the major disputes over which the average productivity of okra has remained very low and almost stagnant for the last few decades is because of lack of improved quality seeds coupled with occurrence of biotic stresses. The major limiting factor towards production of optimum yield are considerable biotic stresses which include mainly Yellow vein mosaic virus (YVMV) and enation leaf curl virus (ELCV) caused by Begomovirus, resulting in serious yield losses in existing varieties and hybrids (Venkataravanappa et al. 2013). Presently, the disease is common in all okra growing states of India, some of which are endemic, and mostly in epidemic form, thereby, threatening the cultivation of okra. In the recent past, frequent breakdown of the viral disease resistance has been reported in popular cultivars of okra like Parbhani Kranti, P-7, Arka Anamika, Arka Abhay (Sanwal et al. 2014). The emergence of new viral strains is considerd to be one of the major factors responsible for the breakdown of tolerance, as the tolerance in a majority of the cases has been found to be location specific. Further, the emergence of polyphagous “B” biotype of Bemisia tabaci, mixed cropping system, along with the large host range of more than 600 plant species have also resulted in gemini viruses infecting previously unaffected crops (Singh et al. 2013). In the classical approaches of resistance breeding the availability of a suitable source of resistance is an essential pre-requisite. But no varieties or hybrids of okra has shown absolute resistance till now owing to the emergence of different strains of the virus in different okra growing parts of the world. Therefore all available germplasm should be evaluated under natural as well as artificial epiphytotic conditions to address the immediate problem of viral diseases. Development of virus resistant varieties has been considered to be the most effective, economical and reliable means for controlling viral diseases. The first step, however, in any virus resistance programme is to identify a genotypes possessing immunity or resistance to the viruses. The main objective of the investigation was to evaluate large number of okra genotypes against YVMV and ELCV diseases and to study the relationship between whitefly population and YVMV disease severity with leaf morphological parameters as well as assess the yield loss of okra as a result of YVMV disease infection in the Gangetic plains of West Bengal.
Materials and methods
Experimental material
Five hundred sixty genotypes along with five checks [Arka Anamika, VRO-6, Parbhani Kranti, Pusa Sawani and BCO-1 (local check)] of okra were collected from ICAR-NBPGR, New Delhi during 2016. The pre-soaked seeds of all genotypes were sown in pre-irrigated plots during the 1st week of August (Kharif season), 2016 at 60 cm × 30 cm spacing between rows and plants in raised plots that were 3.0 m long and 1.8 m width, accommodating 30 (thirty) plants per plot following Randomized Block Design including the standard checks in the research plots of All India Coordinated Research Project on Vegetable Crops, Bidhan Chandra Krishi Viswavidyalaya, West Bengal, India (23.5° N, 89° E, 9.75 m). No plant protection measures against sucking insect pests of okra were undertaken to allow for a appreciable increase in the white fly population in and around the experimental plots.
Data recording
Data on days to 50% flowering, node number at first flowering, fruit diameter (cm), fruit length (cm), fruit weight (g), number of fruits per plant, and fruit yield per plant (g) were recorded from 20 randomly selected plants from each plot in each replication in seven genotypes. Fifteen randomly selected fruits of marketable maturity (7 days after anthesis) were sampled from the selected plants per replication to record the observations on fruit characters. The average number of fruits per plant and total weight of fruits per plant recorded as fresh fruit yield per plant (g) were quantified by counting and weighing all the harvested fruits from each plant.
Observation on leaf morphological parameters
The number of hairs present on the abaxial and adaxial leaf surfaces of the selected genotypes was quantified under the microscope which was standardized and expressed as number of hairs per square centimeter (cm2). The leaf thickness was measured using digital vernier caliper.
Population of whitefly during kharif season
The incidence and severity of YVMV and ELCV diseases depend on the presence of a virus source and population density of the vector (Bemisia tabaci). The Whitefly populations were monitored from August to October and were recorded from five leaves, two each from lower, middle and one from upper canopy of the plants during 5.30 a.m. to 6 a.m. from 5 randomly selected tagged plants from each plot at 7 ± 2 days-intervals from the date of sowing.
Estimation of yellow vein mosaic virus disease severity in okra genotypes
Out of 565 genotypes, no YVMV severity was observed in 18 genotypes throughout growth period but severity of ELCV disease occurred. We have recorded YVMV severity data from seven genotypes (BCO-1, IC-029119-A, IC-045817, Arka Anamika, Parbhani Kranti, VRO-6 and Pusa Sawani) and rest of the genotypes which showed more than 90% YVMV severity at 20 DAS were discarded from the analysis. First appearance of YVMV disease and percent disease index (PDI) on seven genotypes was computed at four stages at an interval of 15 days starting from 20 days after sowing (DAS) up to 60 DAS. No further progress of YVMV and ELCV disease severity was observed beyond 60 DAS. Vein clearing of any form in the plant was treated as disease incidence. The PDI was expressed as a percentage from all 20 plants using a disease severity scale (0–4) through visual evaluation. The disease severity rating was assessed as follows: 0, no disease; 1, up to 15% of leaf area affected; 3, 30–45% of leaf area affected; 5, 45–60% of leaf area affected; and 7, greater than 60% of leaf area affected. YVMV disease was scored in a scale of PDI values (Das et al. 2013), where in a resistant (R) had a PDI ≤ 10%, moderately resistant (MR) 11 < PDI ≤ 15%, moderately susceptible (MS) 16 < PDI ≤ 45%, and highly susceptible (HS) had a PDI > 45%. The number of plants infected in each entry was recorded to calculate PDI as per the formula given below:
Estimation of ELCV disease severity in Okra genotypes
Data were recorded from 18 genotypes for days to first appearance of ELCV, and Percent Disease Index (PDI) of ELCV. PDI of genotypes was recorded at six stages at an interval of 15 days starting from 30 days after sowing (DAS) to 60 DAS. No further progress of ELCV disease severity beyond 60 DAS was observed. Cupping of leaves and petiole bending of any form in the plant were treated as disease incidence. The PDI was expressed as percentage from all 20 plants using following disease severity scale as suggested by Alegbejo (1997).
Scale | Description of symptom |
|---|---|
0 | No symptom |
1 | No visible disease symptom |
3 | Top leaves curled and slight stunting of plant |
5 | All leaves curled, twisting of petiole and slight stunting of plant |
7 | Severe curling of leaves, twisting of petiole, stunting of plant and proliferation of auxiliary branches |
Okra yield loss assessment due to YVMV
The okra variety Arka Anamika was taken for yield loss assessment of YVMV disease. Sowing was carried out on 04.08.2016 and the crop was raised following standard agronomic practices. Infected ten plants of seven sets and one set of healthy plant were selected and disease severity was recorded at periodical intervals using a 0–4 rating scale. Tagged 10 healthy plants were maintained by covering with 40 mesh nylon net during entire crop period and subsequently applied systemic pesticides against insect vector (whitefly). The corresponding yields on tagged diseased plant as well as healthy plants were taken. Yield loss was calculated on the basis of cumulative yield on diseased plant in comparison to healthy plants.
Statistical analysis
Data recorded was subjected to analysis of variance (ANOVA) for a randomized complete block design (Gomez and Gomez 1984). Correlations between variables (disease severity whitefly population and physical parameters) were tested for significance (Gomez and Gomez 1984). Computationally, K-means clustering method is analogous to “ANOVA in reverse”. The programme starts with K-random clusters and then joins more objects between those clusters with the goal to (1) minimize variability within clusters and (2) maximize variability between clusters. In K-means Clustering, the programme tries to move objects (cases) in and out of groups (clusters) to get the most significant ANOVA results. For computing K-means Clustering, SPSS 13.0 package has been used.
Results
Plant growth and yield parameters of selected okra genotypes
All the plant growth parameters, yield components and fruit yield were found to be significantly different among YVMV resistant and susceptible genotypes of okra (Table 1). The maximum plant height was recorded in genotype IC-029119-A (125.40 cm) followed closely by VRO-6 (125.00 cm) and Arka Anamika (100.24 cm); the minimum plant height was recorded in genotype Pusa Sawani (78.00 cm). BCO-1 took maximum days to 50% flowering (51.00 days) followed by Parbhani Kranti (44.67 days) and IC-029119-A (42.00 days). The genotype VRO-6 (38.00 days), however, recorded the minimum days to 50% flowering. Among the genotypes, maximum fruit length was observed in IC- 045817 (13.59 cm) followed by VRO-6 (12.64 cm) and Pusa Sawani (11.30 cm), while lowest fruit length was recorded in Arka Anamika (9.65 cm). Maximum fruit diameter was recorded in the genotype VRO-6 (1.82 cm) followed by IC-045817 (1.76 cm) and Pusa Sawani (1.39 cm), with least diameter recorded in Arka Anamika (1.37 cm). Average fruit weight was found to be maximum in Pusa Sawani (15.83 g) followed by BCO-1 (15.04 g) and Arka Anamika (10.05 g), while minimum weight was recorded in genotype IC-029119-A (9.55 g). Number of fruits per plant was recorded to be maximum in IC-029119-A (12.35/plant) followed by VRO-6 (12.04/plant) and Pusa Sawani (9.83/plant), while the least number of fruits per plants was recorded in BCO-1 (9.80/plant). Maximum fruit yield (g/plant) was recorded in BCO-1 (148.40) followed by IC-029119-A (138.45) and Parbhani Kranti (112.48) with minimum yield recorded in Pusa Sawani (65.63). Among all the parameters, it is evident that the fruit yield per plant of YVMV resistant genotypes (BCO-1, IC-029119-A and IC-045817) is higher than all YVMV susceptible genotypes (Arka Anamika, Parbhani Kranti, VRO-6 and Pusa Sawani).
Leaf morphological characters
The variations in leaf morphological parameters like adaxial and abaxial pubescence density, leaf thickness and leaf color of okra genotypes is presented in Table 2. The means of both adaxial and abaxial pubescence density per cm2 leaf area and leaf thickness varied significantly among all the genotypes of okra studied in the investigation. Genotype BCO-1 possessed the highest adaxial (25.00) and abaxial (21.67) pubescence density followed by IC-029119-A (20.33, adaxial) and IC-045817 (19.67, abaxial), while the genotype Pusa Sawani recorded the lowest. The leaf lamina thickness of different YVMV resistant and susceptible genotypes okra also varied (0.51–0.92 mm) significantly. The genotype Pusa Sawani recorded the maximum thickness (0.92 mm) followed by Parbhani Kranti (0.81 mm), Arka Anamika (0.78 mm), while BCO-1 recorded the minimum (0.51 mm).
Population of whitefly in different okra genotypes
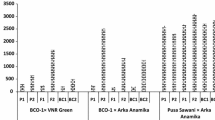
Some of the morphological traits act as mechanical barriers to the pest, such as the presence of pubescence or hairs on leaf lamina, while others influence the general growing habit and appearance of the plant, such as leaves or red pigmentation of the plant (Jenkins and Wilson 1996; Wilson and Sadras 1998) or even the microclimatic conditions of leaves (Wilson 1994). A varietal reaction in terms of incidence of whitefly was observed (Fig. 1). The population of whitefly increased significantly over growth stages of the crop among all the genotypes. The attack of whitefly commenced from 20 days after sowing during 3rd week of August, 2016 followed by a drastic increase in number with the advancement of growth stages and reached its peak at 62 DAS in all the seven YVMV resistant and susceptible genotypes of okra. The population of whitefly was found to be minimum in YVMV resistant genotypes (BCO-1) as compared to YVMV susceptible genotypes (Parbhani Kranti, VRO-6, Pusa Sawani and Arka Anamika). The maximum whitefly infestation was recorded in Pusa Sawani (16.08/leaf) followed by VRO-6 (13.24/leaf) and IC-029119-A (5.46/leaf) with minimum infestation recorded in BCO-1 (5.10/leaf) at 62 DAS.
YVMV disease severity (PDI %) at different day intervals
The YVMV disease severity (PDI %) increased significantly over growth stages of the crop among all the genotypes. First appearance of YVMV disease was observed at 20 DAS (3rd week of August, 2016) in all the genotypes except BCO-1 (Table 3). The infection of YVMV commenced from 20 days after sowing of crop during 3rd week of August, 2016 which further increased with the advancement of growth stages and reached its peak at 60 DAS. The minimum PDI % of YVMV disease ranged from 0.00% (BCO-1) to 35.00% (IC-029119-A and IC-045817). The maximum disease severity was recorded in Arka Anamika (75.00%) followed by Pusa Sawani (70.00%), VRO-6 (65.00%) and Parbani Kranti (60.00%) at 60 DAS (Table 3).
Percent disease index (PDI) of ELCV
Out of 565 screened genotypes, only 18 genotypes were infected by ELCV disease. Eighteen genotypes were found to be disease free at 20 DAS, but moderately susceptible to ELCV based on the scale of PDI values (Table 4). The range of disease severity varied from 5.47% (BCO-1) to 30.11% (IC417885) at 40 DAS and 15.87% (IC111551, IC433616) to 40.11% (IC417885) at 60 DAS.
Correlation study
Correlations between leaf morphological parameters and whitefly population per leaf are presented (Table 5). A highly significant negative correlation was obtained between both adaxial and abaxial pubescence density and the number of adult whiteflies. (r = − 0.829** to − 0.936**; − 0.757** to 0.951**) throughout the growth stages of plant. Whereas, leaf lamina thickness showed positive correlation with the number of whitefly population (r = 0.700–0.824) at different growth stages. Similar trend of correlations were also observed between leaf morphological parameters and YVMV disease severity (Table 5). Considering the combined effect of above three leaf morphological characters, it was found that, lower the adaxial and abaxial pubescence density and higher leaf lamina thickness resulted higher whitefly population which lead to a higher disease severity in okra.
Yield loss assessment due to YVMV disease
Variety Arka Anamika of okra was selected for the experiment of yield loss assessment for YVMV disease (Table 6). Infected ten plants of seven sets and one set of healthy plant were selected and disease severity was recorded at periodical interval using 0–4 rating scale. The corresponding yields on tagged diseased plants as well as healthy plants were taken and yield loss was calculated on the basis of cumulative yield on diseased plants in comparison to healthy plants. First disease appearance was observed at 16 DAS when PDI was 87.5% but disease appearance was late at 53 DAS when PDI was 15.2%. The maximum marketable yield (1.227 kg/10 plants) was found at 15.2% PDI whereas, the marketable yield was 1.48 kg/10 plant for healthy plant. The lowest yield (0.052 kg/10 plants) was found when PDI was 87.5%. At this stage whole plants were infected and fruits became yellowish in colour which rendered them unfavorable for marketing. The marketable yield loss ranged from 17.09 to 96.49%. Therefore, the regression equation was developed for yield loss assessment which is as follows: y = − 0.010x + 1.519 with R2 = 0.998 (Fig. 2).
Discussion
The result showed that all the yield components, plant growth parameters and fruit yield were significantly differed among YVMV resistant and susceptible genotypes of okra. The maximum fruit yield (g/plant) was recorded in BCO-1 (148.40) followed by IC-029119-A (138.45). In a previous study, the genotype BCO-1 was found to be most promising with respect to fruit yield per plant in the Gangetic plains of West Bengal (Seth et al. 2016). The result also showed that the means of both adaxial and abaxial pubescence density per cm2 leaf area and leaf thickness varied significantly among the genotypes of okra, with genotype BCO-1 possessing the highest adaxial and abaxial pubescence density. The results agreed well with the findings of Seth et al. (2016).
The population of whitefly increased significantly over growth stages of the crop among all the genotypes. The population was found to be minimum in YVMV resistant genotype (BCO-1) as compared to YVMV susceptible genotypes (Arka Anamika, Pusa Sawani, VRO-6 and Parbhani Kranti). Maximum whitefly infestation was observed in genotype Pusa Sawani (16.08/leaf), while minimum infestation was recorded in BCO-1 (5.10/leaf). In our previous study, BCO-1 was found to be less susceptible to whitefly attack (Acharya et al. 2019). Hasan et al. (2008) observed that the population of whitefly reached its peak at 60 DAS with a mean population of 3.2–6.7 adult/leaf during kharif season.
Maximum YVMV disease severity was found in Arka Anamika (75.00%) followed by Pusa Sawani (70.00%) and VRO-6 (65.00%), while no severity was observed in BCO-1 at 60 DAS. The genotype BCO-1 was also found to be promising with respect to disease severity in the Gangetic plains of West Bengal as reported by Seth et al. (2016). The leaf lamina thickness showed positive correlation with the number of whitefly population (r = 0.700–0.824) at different growth stages. Similar trend of correlations was also observed between leaf morphological parameters and YVMV disease severity. Considering the combined effect of above three leaf morphological characters, it was found that the lower adaxial and abaxial pubescence density and higher leaf lamina thickness resulted higher whitefly population which lead to higher disease severity in okra. Many workers reported association of several morphological traits with partial resistance to whitefly. Okra shaped leaves and very smooth (glabrous) or very hairy leaves harbor less whiteflies than moderately hairy leaves (Butler et al. 1991; Chu et al. 2002; Miyazaki et al. 2013). Very high level of resistance against whitefly has been reported in the wild diploid species Gossypium thurberi (Walker and Natwick 2006) which has both okra and glabrous leaf traits. The previous study (Seth et al. 2016) clearly depicted that PDI of YVMV disease was positively and significantly correlated with whitefly population and leaf thickness. It was found that the less preferred okra genotypes possessed thinner leaf lamina than the susceptible ones, which harbored a lower whitefly population than other varieties. The possible reason for not being attractive to the whiteflies may be the thinner leaf lamina which is less succulent and less preferred for oviposition and feeding by the whiteflies than the thicker leaf lamina. Similar results were found in green gram [Vigna radiata (L.) R. Wilczek] (Lakshminarayan et al. 2008), black gram (Butter and Vir 1989), cotton (Taggar and Gill 2012) and cucumber (Shibuya et al. 2009) where whitefly population was positively correlated with leaf thickness. But Ayyasamy and Baskaran (2005) reported a reverse trend wherein leaf thickness was negatively correlated with the occurrence of B. tabaci on eggplant. So, if it was found that some varieties had lower leaf thickness but their whitefly attractiveness was high, which could be caused by other leaf characters like pubescence density and pubescence length. For this reason, traits conferring constitutive morphological resistance, such as a high leaf hair density or thickness often are initial targets in resistance breeding programs. Other traits conferring constitutive host plant resistance, such as constitutive chemical compounds are relatively easier to target. However, the initial identification of the specific compounds involved in the resistance is often more a challenging task than identification of morphological traits of the host plant conferring resistance. From the study, BCO-1 has been identified as the most resistant genotype of okra against whitefly and YVMV disease, while Arka Anamika has been recognized to be the most susceptible lines among all. Thus, the BCO-1 is a promising genotype to be used as donor parent in future resistance breeding programme against the most destructive insect pests and YVMV disease of okra in the Gangetic plains of eastern India.
The regression equation indicates that with every 1% increase in severity of disease, the yield loss will be 0.010 kg/10 plants (0.66%). Yellow vein mosaic virus disease transmitted by whitefly (Bemisia tabaci Genn.) is the most destructive disease that infects all stages of plant growth causing heavy economic losses. The losses caused are both qualitative and quantitative. Cent percent infection of plants in a field is common in the Gangetic plains of West Bengal. Sastry and Singh (1974) reported yield losses ranging from 50 to 94% depending on stage of crop growth at which infection occurs.
The present investigation showed that except few okra genotypes BCO-1, IC029119-A and IC045817, most of the genotypes were found to be highly susceptible to YVMV disease in the Gangetic plain of West Bengal and this indicates that Gangetic plain of West Bengal will serve as one of the hot spots for screening of YVMV disease in okra. The local check (BCO-1) exhibited consistently superior performance with respect to YVMV and ELCV disease severity over different growth stages and identified as most promising among the tested genotypes of okra. Thus, BCO-1 could be utilized in dual disease resistant breeding programme. We have identified certain physico-chemical traits which exhibited negative correlations with YVMV disease suggesting their implication as selection indices for identification of genotype tolerant to this disease. Okra production is threatened in the Gangetic plains of West Bengal as marketable yield loss may go up to 96.49%.
References
Acharya J, Chatterjee S, Konar A, Chattopadhyay A, Mandal AK, Dutta S (2019) Host plant resistance through physico-chemical characters against major insect pests of okra occurring in the Gangetic plains of eastern India. Intl J Pest Manage 65(2):137–146
Alegbejo MD (1997) Evaluation of okra genotype for resistance to okra mosaic virus. In: Abstract of papers delivered at the 15th Annual conference of the Horticultural society of Nigeria held at the National Horticultural Research Institute. Ibadan, p 60
Ali MI, Khan MA, Rashid A, Ehetisham-ul-haq M, Javed MT, Sajid M (2012) Epidemiology of Okra Yellow Vein Mosaic Virus (OYVMV) and its management through tracer, mycotal and imidacloprid. Am J Plant Sci 3:1741–1745
Anonymous (2017) National Horticulture Board. Indian Horticulture Database. In: Ministry of Agriculture and Farmers Welfare, Gurgaon, India
Ayyasamy R, Baskaran P (2005) Influence of certain leaf characters of brinjal accessions with incidence of Bemisia tabaci. J Food Agric Environ 3:333–334
Butler GDJ, Wilson FJ, Fishler G (1991) Cotton leaf trichomes and populations of Empoasca lybica and Bemisia tabaci. Crop Prot 10:461–464
Butter NS, Vir BK (1989) Morphological basis of resistance in cotton to the whitefly Bemisia tabaci. Phytoparasitica 17:251–561
Chu CC, Natwick ET, Henneberry TJ (2002) Bemisia tabaci (Homoptera: Aleyrodidae) biotype B colonization on okra and normal leaf upland cotton strains and cultivars. J Econ Entomol 95:733–738
Das S, Chattopadhyay A, Chattopadhyay SB, Dutta S, Hazra P (2013) Breeding okra for higher productivity and yellow vein mosaic tolerance. Int J Veg Sci 19:58–77
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research, 2nd edn. Willey, New York
Hasan W, Ansari MS, Haidar A (2008) Distribution pattern of white fly, Bemisia tabaci under natural condition on okra cultivars. Entomol J 33(2):113–117
Jellis GJ (2009) Crop plant resistance to biotic and abiotic factors: combating the pressures on production systems in a changing world. In: Feldmann F, Alford DV, Furk C (eds) Crop plant resistance to biotic and abiotic factors, pp 15–20
Jenkins JN, Wilson FD (1996) Host plant resistance. In: King EG, Phillips JR, Coleman RJ (eds) Cotton insects and mites: characterization and management. Cotton Foundation Publisher, Memphis, pp 563–597
Lakshminarayan S, Singh PS, Mishra DS (2008) Relationship between whitefly population, YMV disease and morphological parameters of green gram germplasm. Environ Ecol 26:978–982
Miyazaki J, Stiller WN, Wilson LJ (2013) Identification of host plant resistance to silverleaf whitefly in cotton: implications for breeding. Field Crops Research 154:145–152
Sanwal SK, Singh M, Singh B, Naik PS (2014) Resistance to yellow vein mosaic virus and okra enation leaf curl virus: challenges and future strategies. Meeting Report-indian institute of vegetable research. Curr Sci 106(11):1470–1471
Seth T, Chattopadhyay A, Chatterjee S, Dutta S, Singh B (2016) Selecting parental lines among cultivated and wild species of okra for hybridization aiming at YVMV disease resistance. J Agr Sci Tech 18:751–762
Shastry KMS, Singh SJ (1974) Effect of yellow vein mosaic virus infection on growth and yield of okra crop. Indian Phytopathol 27(3):294–297
Shibuya T, Hirai N, Sakamoto Y, Komuro J (2009) Effects of morphological characteristics of Cucumis sativus seedlings grown at different vapour pressure deficits on initial colonization of Bemisia tabaci (Hemiptera: Aleyrodidae). J Econ Entomol 102:2265–2267
Singh Y, Jha A, Verma S, Mishra VK, Singh SS (2013) Population dynamics of sucking insect pests and their natural enemies on okra agro-ecosystem in chitrakoot region. African J Agric Res 28(8):3814–3819
Solankey SS, Singh AK, Singh RK (2013) Genetic expression of heterosis for yield and quality traits during different growing seasons in okra (Abelmoschus esculentus). Indian J Agr Sci 83(8):815–819
Taggar GK, Gill RS (2012) Preference of whitefly, Bemisia tabaci, towards black gram genotypes: role of morphological leaf characteristics. Phytoparasitica 40:461–474
Venkataravanappa V, Reddy CNL, Jalali S, Reddy MK (2013) Molecular characterization of a new species of begomovirus associated with yellow vein mosaic of bhendi (Okra) in Bhubaneswar, India. Eur J Plant Pathol 136:811–822
Walker GP, Natwick ET (2006) Resistance to silverleaf whitefly, Bemisia argentifolii (Hem. Aleyrodidae), in Gossypium thurberi, a wild cotton species. J Appl Entomol 130:429–436
Wilson LJ (1994) Resistance of okra-leaf cotton genotypes to two-spotted spider mites (Acari: Tetranychidae). J Econ Entomol 87:1726–1735
Wilson LJ, Sadras VO (1998) Host plant resistance in cotton to spider mites. In: Halliday RB, Walter DE, Proctor HC, Norton RA, Colloff MJ (eds) International congress of acarology, VIC. CSIRO Publishing, Clayton, pp 314–327
Acknowledgements
The authors are thankful to ICAR-NBPGR for financial support and for providing genetic material of okra for this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jamir, I., Mandal, A.K., Devi, A.P. et al. Screening of genotypes against viral diseases and assessment of yield loss due to yellow vein mosaic virus in okra grown in the eastern part of India. Indian Phytopathology 73, 125–133 (2020). https://doi.org/10.1007/s42360-019-00183-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-019-00183-0