Abstract
Agriculture remains the main occupation in North East India which comprises of eight states. Among the various soil borne pathogens, Fusarium is one of the important pathogens causing diseases with severe yield reduction in crop plants if adequate management practices are not followed. The various species of this pathogen reported in this region are Fusarium oxysporum, F. solani, F. moniliforme, F. equiseti, F. verticillioides, F redolense, F. chlamydosporum, F. Avenaceum, F proliferatum and F. subglutinans. The pathogen causes wilt, rot, seed decay malformation (Pokkah boeng) etc. The pathogen has been characterised using morphological, cultural and molecular methods. Various management options and diagnostic techniques including nanotechnology have been studied. This review summarises the research works carried out on various aspects of Fusarium diseases in North Eastern India and future strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
North Eastern states include Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, Tripura and Sikkim (Barah 2007). Agriculture is the main occupation in eight North Eastern states and the heavy precipitation, soil conditions and climate of the region are well suited for the cultivation of different kinds of crop plants like plantation crops, spices, fruits, vegetables, flowers and herbs. North Eastern states are bestowed with very good soil agro-biodiversity, wetlands, rainfall and climate. However, the agriculture is mainly rainfed, traditional with rice-based cropping system. Besides rice, pulses and maize are the other important crops in this region. Tea is the principal plantation crop of the region and over 95% of the area is under tea cultivation in Assam (Rahman et al. 2009; Dikshit and Dikshit 2014). Not only does the region thrive in cultivated crops but also there is abundance in the pathogens that cause economic losses to the crops. Owing to heavy rainfall and humidity, diseases are the major concern in this region (Nongmaithem et al. 2017). The average annual rainfall of 2000 mm accounting for about 10% of the country’s total precipitation is received here (Roy et al. 2015). Due to greater moisture retention in the soil, the soil-borne pathogens find a greater chance of survival in the soils of these states. The important soil borne pathogens reported from NER states are Pythium, Fusarium, Rhizoctonia, Verticillium, Ralstonia solancearum etc., (Kumar et al. 2012; Gopi et al. 2011; Dutta 2013; Gopi et al. 2016c). These soil borne pathogens rest, survive, sporulate and proliferate in the soil itself and cause serious damage to the crops affecting mostly the underground parts and the collar regions of the plant. Among the soil borne pathogens, Fusarium is one of the important pathogens causing severe yield reduction in crops and vascular wilt caused by Fusarium sp. is soil borne disease of worldwide distribution (Nongmaithem et al. 2017). It is a cosmopolitan fungi with wide host range and affects majority of the cultivated plants (Booth 1971; Woo et al. 1998; Summeral et al. 2003). It causes mainly wilt and root rot diseases in many economically important crops of both agriculture and horticulture. It has been reported as pathogen of all the plant parts. It survives as resistant chlamydospores in soil. It has been reported that F. o. f. sp. cubense survives in soil for up to 30 years as chlamydospores in infested plant material or in the roots of alternative hosts (Ploetz 2000).
Characterisation of Fusarium
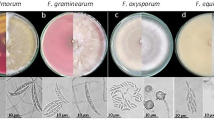
The pathogen Fusarium has been studied and characterized by different workers in North Eastern states. The pathogen Fusarium oxysporum identified from large cardamom was found to produce both micro and macro conidia. The pathogen on PDA produced aerial mycelium which first appeared white, later changed to purple colour. The microconidia were one or two celled and macro conidia were multiseptate gradually pointed and curved toward the ends. The size of macro conidia and micro conidia ranged from 26.91 to 57.64 × 2.01 to 2.59 μm and 5.62 to 8.44 × 1.86 to 2.71 μm, respectively (Anonymous 2015; Gopi et al. 2016a). The Fusarium isolated from Naga King chilli produced pure white mycelia on PDA medium. Out of the two types of conidia, micro conidia were small in size, bicelled equally or unequally, slightly bulged at centre, some are curved and hyaline in colour, whereas macro conidia were large in size, both the ends were pointed with 4–7 septa. The pathogen also produced chlamydospores (Anonymous 2015). Bhattacharjee et al. (2015) reported that the mycelial growth and spore density of F. oxysporum can be enhanced by adding the leaf and root extracts of its respective host plants.
The mycelia of F. verticilloides isolated from the stalk rot disease of maize, were initially white and gradually developed a blackish pigmentation; the microconidia were single-celled, oval to club-shaped, 4.4–11.55 × 1.1–3.3 µm (avg. 6.6 × 1.65 µm) arranged in catenation from monophialides (Borah and Deka 2016). The F. solani causing tea die back disease was tested for its isolation and growth on several media and among them, Czapek dox broth supported maximum vegetative growth of the pathogen (3.74 g) at pH 6.5. PDA and Armstrong media showed moderate growth of the fungal pathogen (Sarmah et al. 2016). Sarmah et al. (2016) reported the formation of nectria (small pink perithecia) by F. solani of tea die back. It also produced white cottony growth on dying tissues that turned brown at its maturity. The Fusarium oxysporum f.sp. cubense isolated from banana exhibited white with purple tinge colony colour, circular shape and smooth margin with abundant aerial cottony mycelia when grown on ¼ strength PDA. Microscopic observation revealed that microconidia (10–12 × 2.4–3.0 µm), one or two celled, oval to kidney shaped (one or no septa), macroconidia (27–30 × 3.4–3.6 µm), four celled (3 septa), sickle-shaped with attenuated tip and borne on foot shaped basal cell. Chlamydospores (7–9 µm dia) both terminal and intercalary with globose shape, formed singly or in pairs in hyphae (Baruah et al. 2018).
Earlier, identification of various pathogens were done primarily based on morphological and cultural characters, however, these methods are time consuming and often succumbed to error. In the present scenario, the identification of eukaryotic organisms is based on the nucleotide sequence information from conserved regions using PCR amplification is gaining importance mainly because these methods are very fast in identification. The internal transcribed spacer (ITS) region, intergenic spacer (IGS), translation elongation factor (EF-1a), β-tubulin region and the mitochondrial small subunit (mtSSU) are the important sequences can easily distinquish species of Fusarium (Bayen et al. 2000; O’Donnell et al. 2000; Skovgaard et al. 2001). Nitrate reductase region (NIR), putative reductase, UTP ammonia ligase, trichothecene 3-O-acetyltransferase, and phosphate permease are the other DNA sequences used to study Fusarium species (O’Donnell et al. 2000; Skovgaard et al. 2001). Datta et al. (2011) reported that the isolates of Fusarium oxysporum f. sp. lentis collected from Tripura was moderate in pathogenicity and is different from other isolates collected from the various region of India and formed separate cluster along with isolates of eastern regions in molecular analysis using RAPD, SSR and ITS markers. Thangavelu (2008) studied the races of F. o. f. sp. cubense and found that race 1 Foc isolates (such as 14 RT, 107RT, 132 RA, 127 RKa, 188RN) obtained from various states including North Eastern states of India reacted with ‘Monthan’ isolates of race 2. Thangavelu et al. (2012) isolated Fusarium oxysporum f. sp. cubense from Assam and Nagaland from Rasthali and Karpuravalli varieties and studied genetic diversity by Inter Simple Sequence Repeats (ISSR) analysis along with other isolates collected from various regions of India. Singha et al. (2016) isolated Fusarium from various places in Assam. The isolates were studied both for morphological and molecular characters. Molecular identification of isolates was done by amplifying the internal transcribed spacer (ITS) region of the conserved ribosomal DNA. Based on structures of microconidia, macroconidia and other morphological characters the isolates were identified as F. oxysporum (MTCC8608), F. oxysporum (MTCC9913), F. oxysporum (MTCC8610), Fusarium equisetum, Fusarium subglutinans (MTCC9914), Fusarium proliferatum, F. subglutinans (MTCC9915) and F. subglutinans (MTCC9916). Molecular characterization of Fusarium wilt isolates of Naga king chilli was done using specific primers ITS FU F (5′CAACTCCCAAACCCCTGTGA3′); ITS FU R(5′GCGACGATTACCAGTAACGA3′). Four isolates of F. oxysporum was amplified at a fragment size of 389 bp in PCR analysis and were identified as F. oxysporum (Anonymous 2016). The Fusarium verticillioides the stalk rot pathogen of maize was identified by amplifying ITS1-5.8S-ITS2 regions of the rDNA using the primers ITS1 and ITS4 and sequenced (GenBank Accession No. KF031434). The sequence was compared and showed 99% similarity with Gibberella moniliformis strain SA3 (Borah and Deka 2016). Similarly, a total of 35 Foc isolates of six banana cultivars (Banria, Balhlakual, Balhlathur, Kawrmawt, Lawngbalhla and Malbhog) grown in 19 different regions of Mizoram and Assam were isolated. The genomic DNA isolated from all these 35 Foc isolates were subjected to genetic diversity analysis using five different ISSR primers viz., (GAC)5, (GTG)5, (ACC)6, CCA(TG)5TG and (AC)8YG and VCGs were generated by (GTG)s. The results of the study clearly indicated that there is existence of wide genetic diversity among the Foc isolates obtained particularly from Mizoram indicating the polyphyletic nature of the Foc isolates. However, the ISSR analysis carried out could not differentiate the Foc isolates based on the cultivars/genomic status or geographical origin (Anonymous 2017).
Important diseases and new records of Fusarium diseases in North Eastern states
Fusarium causes various diseases like wilt, rot, blight, dry rot, inflorescence rot etc., in most of the important crops of North Easter states. The various diseases reported by different workers in North eastern states are summarised in Table 1.
Large cardamom stem lodging
Stem lodging, inflorescence rot, root rot are the symptoms caused by F. oxysporum in large cardamom (Gopi et al. 2015). The symptoms initially appear as small brownish lesions on the stem especially on the leaf sheath attached to the stem. The lesions increase in size and eventually turn black. The infected tillers break at the point of infection and the partially broken tillers bent downwards hanging from the point of breakage. The leaves and leaf sheaths of affected tillers give dried up appearance. The infected flowers and capsules appear black due to rotting and emit unpleasant smell. Roots also show discolouration in the infected plants. The disease can be seen throughout the year affecting stem, leaf sheath, inflorescence, capsule and also root. This disease can be seen from June to November or until the harvest of large cardamom capsules (Anonymous 2015; Gopi et al. 2016a). This disease is locally called as ‘agulta’. Continuous rainfall, water stagnation and old neglected plantations with degenerated clumps are favourable for the spread of the disease (Rao et al. 1993).
Wilt of large cardamom
Wilt caused by Fusarium oxysporum Schlecht is one of the most important diseases of large cardamom and occur in nursery as well as in main field. In nurseries, maximum damage occurs in February and March, whereas in plantations the severity of the disease can be seen from October to February. Sudden wilting of the plant or individual leaf is the characteristic symptom of the disease. The symptoms can be seen as chlorosis of the older leaves at the junction of petiole with pseudostem or their collapse while still green. The emerging heart leaf commonly shows necrosis and the pseudostem also may split at the base and eventually the entire clump will dry. Internally the vascular discolouration (brown to black) is seen in the outer leaf sheath, throughout the pseudostem (Srivastava 1991).
Fusarium die back of tea
It is one of the most important diseases in tea caused by F. solani. Blackening of leaf petioles generally occurs that gradually affects the aerial parts of the tea bushes and the primaries will wilt. Tea seeds are also severely affected by this disease. Blackening of fruit carp, immature cracking, dropping of tea seeds are the other symptoms seen on the infected plant (Sarmah et al. 2012, 2016). It has been observed that succulent young tissues of the tea plants (leaves and shoots) were generally more susceptible to the infection (Sarmah et al. 2016).
Tree bean decline
Tree bean decline is a complex malady due to association with insect and fungi. In the initial stage, plant weakens due to attack of stem borer i.e. Coptops aedificator (Fabricius) and fungus by root infection and then at later stage shot hole borer infection perpetuates. Fusarium along with Colletotrichum and Botryodiplodia, species have been isolated from the freshly infected galleries made by the stem borer and shot hole borer. In marshy/waterlogged areas Fusarium species has been reported to cause the root decay and wilting of the tree. The infected/infested trees lose their vigour and leaves of the infected branches become yellow. Gummosis also can be seen in the infected/infested trees. Heavy infestation leads to the death of the tree (Raj et al. 2017).
Patchouli wilt
The disease was reported in Sikkim and is caused by Fusarium solani [Haematonectria haematococca]. The disease symptoms start with yellowing of the older leaves which advances to the middle and terminal leaves. This is followed by drying of green parts and wilting. The diseased plant shows brown to black discolouration of stem and roots along with disintegration of secondary root surface. The infected plants wilt completely at a premature stage and can be pulled off easily from the soil (Kalita et al. 2012).
Leaf rot of large cardamom
The disease leaf rot is caused by Fusarium avenaceum (Er.) Sacco and is quite severe during June–September. There is sudden onset of a greyish green colour in the leaves which increases very fast during humid weather. The centre of the affected portion becomes brown and finally the whole leaf becomes water soaked and black in colour and ultimately rots. White cottony growth appears on the surface of infected leaves. If sudden dry weather prevails the disease does not spread and remains restricted to a certain portion of the leaf only and the remaining leaf becomes yellow or dull coloured (Srivastava 1989).
Dry rot of ginger
Rhizome rot or yellow rot or dry rot is a complex disease caused by Fusarium oxysporum f.sp. zingiberi. The diseased rhizomes become discoloured and rotting of root takes place which leads to ceased rhizome growth (Sarma and Jackson 2004). The damage has been commonly observed under storage conditions as well. The maximum field loss reported was about 50% (Srivastava et al. 1995). Though the disease is caused by F. oxysporum f.sp. zingiberi but other species of Fusarium namely, F. solani, F. equiseti, F. moniliforme, F. graminearum and F. roseum was reported to be associated in Sikkim (Srivastava 1995). The lesion nematode, Pratylenchus coffeae was reported to be associated with dry rot in Sikkim and lead to loss in storage(Srivastava 1995; Sarma and Jackson 2004).
Stalk rot of maize
The symptoms of stalk rot were first observed on maize cultivar PAC 740 in a field in Tinsukia, Assam, North East India caused by Fusarium verticillioides. External symptoms included softening and reddish coloration of the stalks near the first three internodes from the bottom. The pith was soft, disintegrating, and light brown to reddish. Lodging was observed in severely infected plants and the leaves were dried in lodged plants (Borah and Deka 2016).
Disease incidence
One of the first attempts to evaluate the disease incidence due to Fusarium of ginger in Sikkim was done by Srivastava et al. (1998) who surveyed different ginger growing areas of Sikkim and found Fusarium oxysporum was one of potent pathogens causing yellow and dry rot in ginger. Nath et al. (1999) also recorded highest incidence of (26%) Pokkah boeng at sugarcane Research Station, Buralikson in Assam. Blackening and die-back of tea twigs due to Fusarium infection has become a common scenario at most of the tea gardens (Bhattacharjee et al. 2015) and causes huge crop loss. A survey was conducted in one-year-old plantation of patchouli (Pogostemon patchouli [P. cablin]) during 2010-11 at different places in Sikkim to determine the incidence of wilt disease. The wilt incidence was: 6.8% at ICAR Reasearch farm, 11.1% at Assam Lingzey, 13.0% at Rey Mindu, and 15.1% at Lingding Basti (Kalita et al. 2012). The incidence of pea and broad bean wilt was ranged from 5 to 15% in Imphal and Chandel district of Manipur (Nongmaithem et al. 2017). Laishram et al. (2017) conducted a survey during 2015 and 2016 for the incidence of soft rot of ginger caused by Fusarium oxysporum f. sp. zingiberi in ginger growing districts of Manipur viz. Imphal- East (Nongpokheirok and Thayong), Imphal West (New Keithelmanbi and Keithelmanbi Namching), Bishnupur District (Yumnam Khunou and Oinam) and Churachandpur District (Vaging village and Khenjang village). During 2015, disease incidence ranged from 14.00 to 47.00% and during 2016, it ranged from 14.47 to 53.30%. The mean maximum per cent disease incidence (53.30%) was observed in Keithelmanbi Namching of Imphal-West district, followed by Yumnam Khunou (51.0%) of Bishnupur district whereas, the least per cent disease incidence (14.00%) was observed in New Keithelmanbi of Imphal-West district. A similar study conducted by them revealed the maximum yield loss of 50% due to dry rot caused by F. o. f. sp. zingeberi (Srivastava 1995).
Disease management
The options for the control of Fusarium wilt are very much limited, since no chemical control measures are more effective so far, as the pathogen is soil borne and survives in the soil for prolonged period as hard resistant spores like chlamydospores. Hence, the Fusarium diseases can be managed by integrating different methods like cultural, biological, physical, chemical and host plant resistance.
Biological control
Biological control of soil plant pathogens by antagonistic microorganisms has proved to be a potential nonchemical means (Baruah et al. 2018). In recent times, biological control has become popular among the scientists and farmers because of its efficiency in managing pathogens and also due its eco-friendly nature. With the increasing interest among the people about organic food, the best alternative in disease management will be biological agents. Several workers have reported that application of biocontrol agents effectively controlled the Fusarium diseases (Marois et al. 1981; Sivan and Chet 1986; Larkin and Fravel 1998). Isolation of native biocontrol is very much essential for the success of biological control using antagonistic microbes in plant disease management (Williams and Asher 1996). Various biocontrol agents like Trichoderma, Pseudomonas, Bacillus and Actinomycetes were isolated and tested against various Fusarium diseases (Rojo et al. 2007; Khan and Khan 2002; Gupta and Bansal 2006; Shanmugam et al. 2013).
In a pot culture studies about the effects of four isolates of endophytic bacteria on growth of ginger cv. Bhaise and its suppressiveness against Pythium sp., F. oxysporum and Pratylenchus coffeae revealed that the endophytes enhanced tillering, overall growth of the plants and suppressed disease incidence (Rajan et al. 2002). Bhat and Srivastava (2003) had isolated 23 isolates of Trichoderma from different places of Sikkim and were tested against F. solani by standard dual culture and cellophone layer technique. Of the 23 isolates, the effective isolates, identified were T. viride (B 6 and B 8) and T. koningii (B 7) and T. harzianum (B 5). Although the potential of T. harzianum was demonstrated in pot culture trials, field studies at Mangalbaria and Maniram revealed that T. harzianum was not highly effective (ISPS 2005).
Kamala and Devi (2012) reported that among the total Trichoderma species isolated from the soils of Manipur 80% shows high degree of antagonism against Fusarium oxysporum. Based on their relative biocontrol potency, three indigenous Trichoderma isolates (T10, T17 and T83) were selected for pot culture experiment for testing their biocontrol efficacy against wilting and damping off diseases of common beans. Among all the treatments, T83 showed better biocontrol efficacy against the two test fungi as compared to the exotic Trichoderma harzianum (ITCC No. 6276) strain.
Mishra et al. (2013) obtained a total of 74 Trichoderma isolates from different regions of Mizoram and dual plate assay was performed against Fusarium oxysporum f. sp. pisi (MTCC-2480) and found out that the Trichoderma BPS-1 was showing the maximum antagonistic activity against the pathogen. Similarly, Kumhar et al. (2015) found Trichoderma virens as an effective biocontrol agent against this pathogen and utilized it for the management of die-back in tea caused by F. solani.
The rhizosperic soil of tomato was used to isolate a total of 11 actinomycetes strains from Mayang Imphal area of Manipur,out of which seven isolates showed strong antagonism to Fusarium oxysporum. Isolates RCM-SSR-5, RCM-SSR-9 and RCM-SSR-11 recorded more than 50% colony growth inhibition (Anonymous 2015). In another study involving bacterial endophyte, the isolate KEB5 showed maximum mycelia inhibition of 69.26% over control followed by KEB2 on Fusarium of Naga King chilli. The crude antibiotic isolated from KEB11 and KEB2 showed maximum inhibition area of 35.14 mm and 34.16 mm, respectively against F. oxysporum (Rajesha et al. 2015). Deb et al. (2017) studied antagonistic potential of insect fungi Beauveria spp. against major soil borne pathogens like Fusarium sp. The screened isolates were further evaluated for their antagonistic potential against viz., Fusarium oxysporum, by employing dual culture assay. In their study, the isolate BP1.1 of Beauveria spp. showed significantly highest inhibition percentage of 68.30% against F. oxysporum.
Singh et al. (2017) evaluated native Trichoderma isolates of Manipur against Fusarium oxysporum f.sp. zingiberi (soft rot of Ginger) under in vitro conditions and observed maximum inhibition (88.21%) by Trichoderma harzianum–CAUNCIPM-61 isolate followed by T. viride and T. harzianum obtained from Bangalore. Kripalini et al. (2017) tested fifteen isolates of Trichoderma collected from different districts of Manipur namely Imphal East, Imphal West, Thoubal, Bishnupur, Ukhrul and Tamenglong for their ability to produce volatile and non-volatile compounds (in two concentration i.e. 7.5% and 15% v/v) against F. oxysporum f. sp. pisi. The inhibitory effect of volatile compounds produced by different Trichoderma isolates against test pathogen ranged from 20.77% (TUK-1, Litan of Ukhrul district) to 57.77% (TTH-1, Lilong of Thoubal district). It was observed that out of 37 isolates, in 10 isolates of rhizospheric microbes the per cent reduction in growth of Fusarium oxysporum f. sp. cubense after 120 h of inoculation was found more than 70%, of which 3 rhizospheric microbes performed better with a per cent reduction in growth above 80% (Baruah et al. 2018). It was observed that biopesticide T. viride significantly suppressed dry rot pathogen (Fusarium o. f. sp. zingeberi) of ginger when inoculated after the hot water treatment of ginger rhizomes at 51 °C for 10 min. The result of integration of hot water treatment and application of biopesticide was at par with carbendazim treatment (Daiho and Tato 2016). Kshetri et al. (2017) screened twelve actinobacterial strains of Streptomyces spp. for plant growth promoting and bio-control activity against the major phytopathogens. Among twelve, RCM-SSR-1, -2, -5, -6, -9 and -11 showed antagonistic activity against the Fusarium oxysporum. Maximum colony growth inhibition (67%) of Fusarium oxysporum was observed by RCM-SSR-5; followed by RCM-SSR-6 and -11 (62%).
Botanicals
Many plants and their products have been reported to possess pest control properties and these have been exploited to be used for managing pathogens. These are good alternatives to chemical pesticides, as they are readily biodegradable in nature (Singh et al. 2011). Crude plant extracts of 44 medicinal plants/weeds was tested in vitro against F. oxysporum, F. moniliforme and F. solani which were isolated from ginger filed/stored ginger (Bhat 2001). Among the plants, Schima wallichii showed maximum inhibition i.e. 66.7% against F. moniliforme and 50% inhibition against F. oxysporum and F. Solani. In the green house studies It was observed that 1% (w/w) amendment of crude chloroform extract of Piper betle L. (PbC) in soil was more efficient in reducing the Fusarium population in soil than carbendazim and the combined amendment of carbendazim and PbC (Singh et al. 2011).
Cultural control
Generally, cultural practices are recommended to prevent the introduction of the pathogen into the field and to reduce the inoculum level of the pathogen. These practices are economical and easy to adopt. Among the various methods, cultural method is one of the important one practiced by the farmers from time immemorial. Crop rotation is one of the most important one recommended for various soil borne diseases including Fusarium diseases. Most of the farmers practice crop rotation and very common among the farmers growing ginger to manage the rhizome rot diseases complex in North Eastern states (Rahman et al. 2009). Incidence of Fusarium root rot in garden pea (7.17%) was lower in poultry manure @ 5 t/ha + bio-fertilizers as compared to other treatments (Anonymous 2015). Date of sowing plays a very important role in the management of various diseases. In pea, it was found that sowing in the month of November, December and January did not show any incidence of wilt in pea in Sikkim. Mulching with maize straw also reduced the incidence of wilt by 73.5% (Gopi et al. 2016b).
Chemical control
In India, chemical fungicides are widely used by the farmers for the disease management. Bhat and Srivastava (2003) carried out in vitro studies on the efficacy (inhibitory effects) of 14 fungicides (250–1000 ppm) and four neem formulations (5000 ppm) against F. solani, F. oxysporum and F. moniliforme. The results showed that Saaf (mixture of carbendazim 12% and Mancozeb 63%) was highly effective against all the three pathogens even at lower concentration (250 ppm), while Dithane M-45 have completely inhibited F. solani and F. oxysporum at 500 ppm. In vitro evaluation of five fungicides viz. Bavistin (carbendazim 50WP), Antracol (propineb 75 WP), Sectin (fenamidone 10% + mancozeb 50% WG), Monceren 250SC (pencycuron 22.9 SC) and Folicur 250 SC (tebuconazole 25.9 EC) was done against Fusarium solani, Folicur and Monceren gave 100% control of Fusarium solani, followed by Bavistin as compared to control (Anonymous, 2010). Fungicides namely Copper oxychloride @1:400, Carbendazim @1:400, Copper hydroxide @1:400, Mencozeb @1:400, Hexaconazole @1:1000, Propiconazole @1:1000 were found as effective in controlling the Fusarium dieback disease in tea caused by F. solani (Sarmah et al. 2010). Devi et al. (2017a, b) studied prevalence and organic management of emerging fungal diseases in some important horticultural crops of Manipur and found the association of Fusarium with Soft rot of ginger (10.00–2.00%), wilt of chilli (13.85–73.04%), wilt of pea (33.00–77.00%). Among the treatments, copper oxychloride found better in suppressive effect against Fusarium soft rot of ginger.
Host plant resistance
Resistant varieties are the one of most important and easiest eco-friendly methods for the management of plant diseases. Only a few reports on development resistant varieties and evaluation against various Fusarium diseases in North East India are available. Sarmah et al. (2016) reported that the varieties of tea such as TV 1, TV 9, TV 17, TV 18, TV 20, TV 22, TV 23, TV 25, TV 26, TV 28, TV 29, TV 33, Teenali17, P 126, TS 446, T3E3, TA 17, P-38, Nokhroy, Betjan, S3A3, NP4 were found to be susceptible to tea die back caused by F. solani. Occurrence of Fusarium wilt was noticed in Malbhog and Vim kol varieties of banana in Mizoram (Anonymous 2015).
Nanotechnology and disease management
Nanotechnology is an emerging science and it is proved to be as good in resources management of agricultural field, pest and disease management and maintaining fertility of the soil. Boruah and Pranab (2017) demonstrated encouraging result of combined use of nanoparticles and biocontrol agent for the management of soil borne plant pathogens. Biosynthesized chitosan nanoparticle improved the biocontrol potentiality of Trichoderma asperellum and superior in inhibiting the mycelial growth of the Fusarium oxysporum as compared to the recommended chemical at 0.1%. Nanochitosan based liquid formulation of T. asperellum also showed significantly effective results against the pathogen alone with increasing result of plant growth parameters. Kaman and Dutta (2016) synthesized biogenic silver nanoparticles (AgNP) using Trichoderma aspereluum and the efficacy was tested against various soil borne plant pathogens including Fusarium oxysporum and AgNP was found very effective under in vitro condtions even at very low concentration of 10 ppm. So induction of biocontrol agents to form nano particles will definitely help in the management of various diseases including Fusarium and also reduce the cost of cultivation as it was found that nanoform was very effective against various pathogens. The efficacy of combination of T. asperallum and chitosan NP at different concentrations (0.01 ppm, 0.02 ppm) was tested against Fusarium sp. and was found very effective in comparison to carbendazin @ 0.3 ppm (Boruah et al. 2016).
Future strategies
From the above discussion on Fusarium diseases, it is clearly evident that the research on Fusarium diseases of North East India is still in initial stages. A greater amount of work for diagnosis and management of Fusarium diseases needs to be taken and hence the future strategies should focus on following,
-
Identification of various Fusarium species in different crops and studying the morphological, cultural, pathogenic and molecular variability with other isolates collected from other parts of India which will be helpful in identification of prominent and possibly different races of Fusarium from the North East India.
-
Breeding strategies should be initiated to develop varieties with resistant potential against various Fusarium diseases prevailing in this region of India considering the unique climate which is entirely different from other parts of India.
-
Development of molecular diagnostic tools for early detection and development, characterization, studying and managing diseases caused by Fusarium.
-
Identification and development of biocontrol formulations is also very much needed for the effective management of Fusarium diseases and the formulations should be properly evaluated in farmers field and demonstrated to the farmers.
-
Integrated management using cultural methods, bio agents, plant products and resistant cultivars should be adopted. The pathogen Fusarium being a soil borne pathogen can be managed effectively by cultural methods like crop rotation which should be encouraged among the farmers.
-
Documentation of ITKs used by the farmers for the management of Fusarium diseases and subsequent validation is also very crucial. In recent years organic agriculture is gaining popularity all over the world and most importantly in all NE states. Therefore it is essential to test various organic management practices and organically approved chemicals for the management of fusarium diseases.
-
Farmers also should be trained on identification and management of various diseases caused by Fusarium species by experts, scientists and extension functionaries of state and central government organizations.
References
Anonymous (2009) ICAR-NEH, 2009, Annual Report, 2008–09, p 236
Anonymous (2010) ICAR-NEH, 2010, Annual Report, 2009–10, p 252
Anonymous (2015) ICAR-NEH, 2015, Annual Report 2014–15, p 202
Anonymous (2016) ICAR-NEH, 2016, Annual Report, 2015–16, p 150
Anonymous (2017) ICAR-NRCB, 2017, Annual Report 2016–17, p 174
Baiswar P, Chandra S, Rajiv Kumar (2008) Evaluation of different bio-control agents against Botrytis gladiolorum and Fusarium oxysporum f.sp. gladioli of gladiolus under in vitro conditions. J Ornam Horticult 11(1):79–80
Barah BC (2007) Strategies for agricultural development in the North-East India: challenges and emerging opportunities. Ind J Agri Econ 62(1):13–31
Barthakur BK (2011) Recent approach of Tocklai to plant protection in tea in North-east India. Sci Cult 77:381–384
Baruah N, Ashok B, Thangavelu R, Puzari KC (2018) In vitro screening of native banana rhizospheric microbes and endophytes of assam against Fusarium oxysporum f. sp. cubense. Int J Curr Microbiol App Sci 7(6):1575–1583
Bayen RP, O’Donnell K, Bonants PJM, Cigelnik E, Kroon LPNM, Roebrock EJA, Walwijck C (2000) Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology 90:891–907
Bhat N (2001) In vitro cultivation of some leaf extracts against Fusarium spp. causing dry rot/yellows of ginger in Sikkim. Plant Dis Res 16(2):259–262
Bhat MN, Srivastava LS (2003) Evaluation of some fungicides and neem formulations against six soil borne pathogens and three Trichoderma spp. in vitro. Plant Dis Res 18(1):56–59
Bhattacharjee J, Bhattacharjee D, Borkar SG (2015) Performance of fungal platn pathogens in the culture media in presence of host plant parts. In: The abstracts of National Seminar on Sustaining Hill Agriculture in Changing Climate held December, 05–07, 2015, Agartala, Tripura, pp 363
Booth C (1971) The genus Fusarium. Common wealth Mycological Institute, Kew
Vijayan AK, Gudade B A, Bora, SS, Bhat S (2015) Fungal and viral diseases problems in large cardamom and their management. In: The abstracts of International Symposium on Next Generation Approaches for Sustainable Development o f Hill and Upland Horticulture held on 05–07, November, 2015 at Gangtok, p 140
Borah SN, Deka S (2016) First report of Fusarium verticillioides causing stalk rot of maize in Assam, India. Plant Dis 100(7):1501
Boruah S, Dutta P, Puzari KC (2016) Combined effect of nanoparticles and biocontrol agent has higher efficacy against plant pathogens. In: The abstracts of Symposium on Plant health Management in North East India held on 15–16, 2016 at ICAR Research Complex for NEH Region, Umiam, Meghalaya, p 55
Chattopodhyay SB, Sengupta SK (1967) Twig blight disease of Orange. Sci Cult 33:129
Daiho L, Tato N (2016) Biopesticide as important component in integrated plant disease management. In: The abstracts of Symposium on Plant health Management in North East India held on 15–16, 2016 at ICAR Research Complex for NEH Region, Umiam, Meghalaya, p 55
Datta S, Choudhary RG, Shamim MD, Singh RK, Dhar V (2011) Molecular diversity in Indian isolates of Fusarium oxysporum f.sp. lentis inciting wilt disease in lentil (Lens culinaris Medik). Afr J Biotechnol 10(38):7314–7323
Deb L, Rajesh T, Devi RKT, Majumder D, Studies on antagonistic potential of Beauveria spp. against major soil borne pathogens (2017) Ngachan SV, Prakash N, Sharma SK, Singh IM, Roy SS, Ningombam A, Punitha P, Ansari MA, Singh RK, Sailo B, Singh TB, Sonia Ch, Anand YR, Singh TS and Singh YH (eds) 2017. In: Souvenir and Abstracts of National Symposium on Emerging and Reemerging Plant Diseases in North East India: Challenges and Strategies. 10–11 October, 2017, Imphal, Manipur, India, p 144
Devi PS, Sinha B, Devi HC, Devi KS Chanu WT. Prevalence and organic management of emerging fungal diseases in some important horticultural crops of Manipur (2017) Ngachan SV, Prakash N, Sharma SK, Singh IM, Roy SS, Ningombam A, Punitha P, Ansari MA, Singh RK, Sailo B, Singh TB, Sonia Ch, Anand YR, Singh TS and Singh YH (eds) 2017. Souvenir and abstracts of national symposium on emerging and reemerging plant diseases in north east india: challenges and strategies 10–11 October, 2017, Imphal, Manipur, India, p 144
Devi TJ, Devi RKT, Singh NI, Sinha B. Detection of seed borne fungi of black gram (2017) Ngachan SV, Prakash N, Sharma SK, Singh IM, Roy SS, Ningombam A, Punitha P, Ansari MA, Singh RK, Sailo B, Singh TB, Sonia Ch, Anand YR, Singh TS and Singh YH (eds) 2017. Souvenir and Abstracts of National Symposium on Emerging and Reemerging Plant Diseases in North East India: Challenges and Strategies. 10–11 October, 2017, Imphal, Manipur, India, p 144
Dikshit KR, Dikshit JK (2014) Agriculture in North-East India: past and present. In: North-east india: land, people and economy. Advances in Asian Human-Environmental Research. Springer, Dordrecht
Dutta P (2013) Efficacy of bioformulations against bacterial wilt of tomato caused by Ralstonia solanacearum. J Biol Control 27(1):64–66
Ghosh SP, Singh RB (1993) Citrus in South Asia. FAO/RAPA Publication No. 1993/24. Bangkok, Thailand, p 70
Gopi R, Borah Tasvina R, Kalita H (2011) A new record of head rot of cabbage by Rhizoctonia solani in Sikkim. J Mycol Plant Pathol 41(4):642–643
Gopi R, Avasthe RK, Kalita H, Ashish Yadav, Kapoor C, Poudyal C (2015) A new record of Fusarium oxysporum causing root rot, inflorescence and capsule rotting in large cardamom. In: The abstracts of National Seminar on Sustaining Hill Agriculture in Changing Climate held December, 05–07, 2015, Agartala, Tripura, p 363
Gopi R, Avasthe RK, Kalita H, Yadav A, Kapoor C, Poudyal C (2016a) A new record of Fusarium oxysporum causing stem lodging, inflorescence and capsule rot in large cardamom. Indian Phytopath 69(3):316–317
Gopi R, Kalita H, Avasthe RK (2016b) Organic management of soft rot of ginger (Zingiber officinale) in Sikkim Himalayan region. Indian J Agr Sci 86(12):1586–1590
Gopi R, Avasthe RK, Singh R, Yadav A, Kalita H, Kapoor C, Singh NJ (2016b) Effect of mulching and date of sowing on pea diseases in Sikkim. In: The abstracts of Symposium on Plant health Management in North East India held on 15–16, 2016 at ICAR Research Complex for NEH Region, Umiam, Meghalaya, p 55
Gupta RK, Bansal RK (2006) Comparative efficacy of Trichoderma viride and fungicides against Fusarium oxysporum schlecht inducing fenugreek wilt under poly house conditions. Ann Biol 19:35–37
ISPS (Indo Swiss Project Sikkim) (2005) Experience in Collaboration-Ginger Pests and Diseases, Inter-cooperation India Programme. Series 1. Indo-Swiss Project Sikkim, Hyderabad, India, p 57
Kalita H, Borah TR, Gopi R, Helim R, Das B (2012) New record of patchouli wilt from Sikkim Himalayas. J Mycol Plant Pathol 42(2):254–255
Kamala Th, Devi SI (2012) Biocontrol properties of indigenous Trichoderma isolates from North-east India against Fusarium oxysporum and Rhizoctonia solani. Afr J Biotechnol 11(34):8491–8499
Kaman P, Dutta P (2016) Biogenic synthesis of myconanoparticle (Ag nanoparticle) and management of soil borne plant pathogen. In: The abstracts of Symposium on Plant health Management in North East India held on 15–16, 2016 at ICAR Research Complex for NEH Region, Umiam, Meghalaya, p 55
Khan MR, Khan SM (2002) Effect of root-dip treatment with certain phosphate solubilizing microorganisms on the Fusarium wilt of tomato. Bioresour Technol 85:213–215
Kripalini N, Biswas MK, Devi PS, Sinha B, Chanu WT, Sarda K, Laishram S and H Chandrajini (2017) Effect of Volatile and Non-Volatile compounds of potent Native Trichoderma isolates against Fusarium oxysporum f.sp. pisi (Wilt of Pea). Ngachan SV, Prakash N, Sharma SK, Singh IM, Roy SS, Ningombam A, Punitha P, Ansari MA, Singh RK, Sailo B, Singh TB, Sonia Ch, Anand YR, Singh TS and Singh YH (eds) 2017. Souvenir and Abstracts of National Symposium on Emerging and Reemerging Plant Diseases in North East India: Challenges and Strategies. 10–11 October, 2017, Imphal, Manipur, India, p 144
Kshetri P, Roy SS, Sharma SK, Singh TS, Ansari MA, Sailo B, Prakash N, Ngachan SV (2017) Screening of native actinobacteria of Manipur for phytostimulating activity, production of agroactive compounds and biocontrol potential. Ngachan SV, Prakash N, Sharma SK, Singh IM, Roy SS, Ningombam A, Punitha P, Ansari MA, Singh RK, Sailo B, Singh TB, Sonia Ch, Anand YR, Singh TS and Singh YH (Eds.) 2017. Souvenir and Abstracts of National Symposium on Emerging and Reemerging Plant Diseases in North East India: Challenges and Strategies. 10–11 October, 2017, Imphal, Manipur, India, p 144
Kumar R, Tapwal A, Borah RK (2012) Identification and controlling verticillium wilt infecting Parkia roxburghii seedlings in Manipur India. Res J For 6:49–54
Kumhar KC, Babu A, Bordoloi M, Banerjee P, Dey T (2015) Biological and chemical control of Fusarium solani, causing dieback disease of tea Camellia sinensis (L): an in vitro study. Int J Curr Microbiol Appl Sci 4:955–963
Laishram S, Mandal NC, Devi PS, Sinha B, Chanu WT, Devi KS, Kripalini N. Survey for the incidence of soft rot of ginger caused by Fusarium oxysporum f. sp. zingiberi in different districts of Manipur(2017) Ngachan SV, Prakash N, Sharma SK, Singh IM, Roy SS, Ningombam A, Punitha P, Ansari MA, Singh RK, Sailo B, Singh TB, Sonia Ch, Anand YR, Singh TS and Singh YH (Eds.) 2017. Souvenir and Abstracts of National Symposium on Emerging and Reemerging Plant Diseases in North East India: Challenges and Strategies. 10–11 October, 2017, Imphal, Manipur, India, p 144
Larkin R, Fravel D (1998) Efficacy of various fungal and bacterial biocontrol organisms for the control of Fusarium wilt of tomato. Plant Dis 82:1022–1028
Marak SR, Tiameren AO (2016) Study on seed-borne mycoflora on rice grains (Oryza sativa). In: The abstracts of Symposium on Plant health Management in North East India held on 15–16, 2016 at ICAR Research Complex for NEH Region, Umiam, Meghalaya, p 55
Marois JJ, Mitchel DJ, Somada RM (1981) Biological control of Fusarium crown and root rot of tomato under field condition. Phytopathology 12:1257–1260
Mishra RR, Sharma GD, Tiwari BK (1981) Report on Fusarium wilt in Pinus Kesiya Royle. Curr Sci 50(1):36
Mishra VK, Mishra AK, Passari BP, Singh AK, Passari Singh BP (2013) Identification of trichoderma BPS-1, a biological control agent against pea wilt pathogen Fusarium Oxysporum f.sp. pisi. Int J Biotechnol Bioeng Res 4(4):375–382
Mustaffa MM, Thangavelu R (2011) Status of fusarium wilt in india. Acta Hortic 897:323–329
Nath P, Saikia AK, Singh SN (1999) On the occurrence of sugarcane diseases in Assam. Indian Phytopath 52(4):420
Nongmaithem N, Basudha Ch, Sharma SK (2017) Incidence of rust, powdery mildew and wilt in pea and broad bean plant of Manipur, India. Int J Curr Microbiol Appl Sci 6(8):2611–2616
O’Donnell K, Kistler HC, Tachke BK, Casper HH (2000) Gene genealogies reveal global phylogeographic structure and reproductive isolation among lineages of Fusarium graminearum, the fungus causing wheat scab. Proc Natl Acad Sci 97:7905–7910
Ploetz RC (2000) Panama disease: a classic and destructive disease of banana. Plant Health Prog 10:1–7
Pradhan P (2015) Preliminary checklist of phytopathogenic fungi in Sikkim, India. PeerJ PrePrints 3:e953v1. https://doi.org/10.7287/peerj.preprints.953v1
Rahman H, Karuppaiyan R, Kishore K, Denzongpa R (2009) Traditional practices of ginger cultivation in Northeast India. Indian J Tradit Knowl 8(1):23–28
Raj H, Borah RK, Yadav S. Preliminary investigations of Parkia roxburghii decline in North East India (2017) Ngachan SV, Prakash N, Sharma SK, Singh IM, Roy SS, Ningombam A, Punitha P, Ansari MA, Singh RK, Sailo B, Singh TB, Sonia Ch, Anand YR, Singh TS and Singh YH (eds) 2017. Souvenir and Abstracts of National Symposium on Emerging and Reemerging Plant Diseases in North East India: Challenges and Strategies. 10–11 October, 2017, Imphal, Manipur, India, p 144
Rajan PP, Gupta SR, Sarma YR, Jackson GVH (2002) Diseases of ginger and their control with Trichoderma harzianum. Indian Phytopath 55(2):173–177
Rajesha G, Bendangsenla, Deka BC, Ngachan SV (2015) Isolation and screening of bacterial endophytes against the fungal pathogesn of Naga King Chilli. In: The abstracts of National Seminar on Sustaining Hill Agriculture in Changing Climate held December, 05-07, 2015, Agartala, Tripura, p 363
Rao YS, Kumar A, Chatterjee S, Naidu R, George CK (1993) Large cardamom (Amomum subulatum Roxb.)—a review. J Spices Aromatic Crops 2(1&2):1–15
Reddy P (2015) Plant protection in tropical root and tuber crops. Springer, Berlin, p 331
Rojo FG, Reynoso MM, Sofia M, Chulze IN, Torres AM (2007) Biological control by Trichoderma species of Fusarium solani causing peanut brown root rot under field conditions. Crop Prot. 26:549–555
Roy AK (1984) Two new Fusarium diseases in Karbi anglong, Assam India. Curr Sci 53(24):1303–1304
Roy A, Singh NU, Dkhar DS, Mohanty AK, Singh SB, Tripathi AK (2015) A Study on the performance of agricultural sector in India. Agric Econ Res Rev 28:259–266
Sahay G, Sarma BK, Gupta HS, Pathak KA, Prasad MS (1999) Biotic stresses of pulses in North Eastern Hill Region of India. Indian J of Hill Farmg 12(1–2):6–8
Sarma YR, Jackson GH (2004) Disease and pest management in Ginger with special reference to North East Region of India, pp 46–52. In: Souvenir-National Consultative Meeting for Improvement in Productivity and Utilization of Ginger, May 12–13, Aizwal, Mizoram
Sarmah SR, Baruah PK, Das SC (2010) Effect of different fungicides on die back disease of tea plant. J Adv Plant Sci 5:92–96
Sarmah SR, Boruah PK, Das SC (2012) Pathogenicity study of Fusarium solani, isolated from Fusarium die back of tea [Camellia sinensis (L.) O. Kuntze] on its host plant. Two Bud 59:91–94
Sarmah SR, Dutta P, Bhattacharyya PN, Payeng B, Tanti AJ (2016) Growth habit of tea pathogens and evaluation of relative susceptibility of selected tea cultivars. Int Res J Biol Sci 5(7):1–9
Shakywar RC, Krishna S, Tomar and Pathak M (2012) Panama wilt disease of banana and its management. http://www.krishisewa.com/articles/disease-management/80-panama-wilt-banana.html
Shanmugam V, Gupta S, Dohroo NP (2013) Selection of a compatible biocontrol strain mixture based on co-cultivation to control rhizome rot of ginger. Crop Prot 43:119–127
Singh R (2016) Aerobiology in north-east India in the context of epidemiology and forecasting for fungal diseases of major crop plants. Int J Mendel 33(1–2):63–68
Singh R, Sunder S (1997) Foot rot and bakanae of rice:retrospects and prospects. Intern J Trop Pl Dis 15:153–176
Singh TS, Devi PS, Sinha B, Singh NG, Sharma SK, Linda RK, Singh TN, Singh Chanu NT, Singha IM, Kakoty Y, Unni BG, Kalita MC, Naglot J, Naglot A, Wann SB, Singh L (2011) Control of Fusarium wilt of tomato caused by Fusarium oxysporum f. sp. lycopersici using leaf extract of Piper betle L.—a preliminary study. World J Microbiol Biotechnol 27:2583–2589
Singh TS, Devi PH, Sinha B, Singh NG, Sharma SK, Linda RK, Singh TN, Chanu NT and Singh YH (2017) Development of a biocontrol based management module for Fusarium oxysporum f.sp. zingiberi causing soft rot of ginger. Ngachan SV, Prakash N, Sharma SK, Singh IM, Roy SS, Ningombam A, Punitha P, Ansari MA, Singh RK, Sailo B, Singh TB, Sonia Ch, Anand YR, Singh TS and Singh YH (eds) 2017. Souvenir and abstracts of national symposium on emerging and reemerging plant diseases in North East India: challenges and strategies, 10–11 October, 2017, Imphal, Manipur, India, p 144
Singha IM, Kakoty Y, Unni BG, Das J, Kalita MC (2016) Identification and characterization of Fusarium sp. using ITS and RAPD causing fusarium wilt of tomato isolated from Assam, North East India. J Genet Eng and Biotechnol 14(1):99–105
Sivan A, Chet I (1986) Biological control of Fusarium spp. in cotton, wheat and muskmelon by Trichoderma harzianum. J Phytopathol 116:39–47
Skovgaard K, Nirenberg HI, O’Donnell K, Rosendahl S (2001) Evolution of Fusarium oxysporum f. sp. vasinfectum races inferred from multigene genealogies. Phytopathology 91:1231–1237
Srivastava LS (1989) Leaf rot oflarge cardamom. Spice India 2:23
Srivastava LS (1991) Wilt of large cardamom, a new disease. Spice India 4:13
Srivastava LS (1995) Review of ginger diseases. In: Ginger Disease Workshop: proceedings and Strategic Plan, 12–13 September 1995, Gangtok, Sikkim
Srivastava LS, Gupta SR, Basnet CP, Mahato UP, Neopani B (1998) Microorganisms associated with ginger in Sikkim. J Hill Res 11(1):120–122
Summeral BA, Salleh B, Leslie JF (2003) A utilitarian approach to Fusarium identification. Plant Dis 87:117–128
Thangavelu R (2008) Banana wilt. p.266–285. In: Final Technical Report of Network Project on Wilt of Crops with Special Reference to Cultural Morphological Molecular Characterization and Pathogenic Variability of Isolates in India. Indian Institute of Pulses Research, Kanpur, India
Thangavelu R, Muthu Kumar K, Ganga Devi P, Mustaffa MM (2012) Genetic Diversity of Fusarium oxysporum f.sp. cubense Isolates (Foc) of India by Inter Simple Sequence Repeats (ISSR) Analysis. Mol Biotechnol 51:203–211
Vignesh P, Singh LNK, Sinha B. Stem rot of rice present scenario in India (2017) Ngachan SV, Prakash N, Sharma SK, Singh IM, Roy SS, Ningombam A, Punitha P, Ansari MA, Singh RK, Sailo B, Singh TB, Sonia Ch, Anand YR, Singh TS and Singh YH (eds) 2017. Souvenir and Abstracts of National Symposium on Emerging and Reemerging Plant Diseases in North East India: Challenges and Strategies, 10–11 October, 2017, Imphal, Manipur, India, p 144
Williams GE, Asher MJC (1996) Selection of rhizobacteria for the control of pythium ultimum and Aphanomyces cochlioides on sugar-beet seedlings. Crop Prot 15:479–486
Woo SL, Noviello C, Lorito M (1998) Sources of molecular variability and applications in characterization of the plant pathogen Fusarium oxysporum, pp. 187–208. In: Bridge P, Couteaudier Y, Clarkson J (eds) Molecular variability of fungal pathogens. CAB Int. Wallingford Oxon, United Kingdom 319
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gopi, R., Singh, S. & Raj, C. Status of Fusarium diseases of crop plants in North East India. Indian Phytopathology 72, 637–646 (2019). https://doi.org/10.1007/s42360-019-00148-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-019-00148-3