Abstract
Aluminium doped nickel ferrite (NiFe2−xAlxO4) with different Al content ranging from 0.3 ≤ x ≤ 1.0 was prepared via modified sol–gel approach. The Al-dopant composition was controlled based on Al/Fe precursor ratio. The structural, morphological and adsorption performance were investigated to understand the influence of non-magnetic Al in the samples. XRD measurement showed the single-phase with inverse spinel ferrite structure. The increment of crystallite size as the Al content increases has been confirmed by XRD analysis. Furthermore, all samples were investigated to determine the CO2 capture efficiency and to analyze the adsorption kinetics model. NiFe2−xAlxO4 (x = 1.0) showed the most efficient CO2 adsorption as it obtained an adsorption capacity of 28.71 mg/g. A recyclability test revealed that CO2 adsorption at 30 ºC exhibits the optimum adsorption with only 8.56% loss in adsorption capacity after five cycles of CO2 adsorption–desorption. The pseudo-second-order kinetics best fit the adsorption with R2 = 0.99981. The relative error between calculated and experimental adsorption capacity is only 0.425%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Doping with various elements is commonly used to alter and enhance existing physical and chemical properties of materials. This helps to design and develop new kinds of structures. Ferrite materials have attracted more attention due to their low cost, good chemical stability, high saturation magnetization and high Curie temperature, making them more applicable in many fields [1]. Nowadays, scientists have studied various types of ferrite materials and their related structures because of their special physical and chemical characteristics which can lead to new kinds of applications including magnetic storage systems, drug delivery, catalytic, optical and biomedical materials [2].
Synthesis methods for ferrites and their composites can produce materials with unique properties. Generally, different types of spinel ferrites have been prepared from different routes including sol–gel, thermal treatment, electrospinning, high energy ball milling and co-precipitation [3,4,5,6,7]. The unique properties of the samples are possessed from all the different synthesis methods. The structural, thermal and adsorption characteristics depend on the synthesis method employed [8]. Currently, the co-precipitation and sol–gel methods are the most widely used methods for the industrial production of ternary materials [9]. However, the co-preparation method is complicated and involves harsh experimental conditions. The method requires the adjustment of pH, stirring rate and temperature accurately as well as incurs high costs and can cause pollution [10,11,12]. The sol–gel synthesis method is more common due to the possibility of controlling the morphology of the final products and the resultant materials have high crystallinity and purity [13]. In addition, the sol–gel method is facile and reasonable in terms of cost [14]. This method also gives a high degree of homogeneity and precise chemical composition. The sol–gel method is proposed to overcome some disadvantages of the co-precipitation method for the preparation of ferrites as discussed in our previous study [15].
Nickel ferrite has a general formula of MFe2O4,which has a cubic close-packed structure. Nickel ferrite is considered one of the most frequently employed soft magnetic materials due to its outstanding physical and chemical properties. This classic inverse spinel structure has gained attention because of its unique physical and chemical properties. Ferrite inverse spinel structure is related to the space group of Fd \(\overline{3}\) m with Ni2+ in octahedral (Oh) sites while Fe3+ is equally distributed between octahedral (Oh) and tetrahedral (Th) sites of the O2− fcc cell. The structure consists of 32 positions in which oxygen atoms occupy, 8 positions in which Fe(Th) atoms occupy while Ni(Oh) and Fe(Oh) atoms are distributed equally in 16 positions. The substitution of Oh sites, in different ferrites by nonmagnetic ions such as Al3+, have been performed using various methods [16, 17]. However, doping of Al into pure nickel ferrite is rare. Doping a metal into inverse spinel can alter the structural, thermal, magnetic and adsorption properties. Previous literature reported that in the inverse spinel ferrite, NiFe2−xCuxO4, Ni2+ ions prefer to occupy [B] sites, while Cu2+ and Fe3+ cations can occupy both [A] and [B] sites [18]. Kumar et., al conducted a study to understand the structural and magnetic properties of Al doped Ni–Zn ferrite prepared by sol–gel auto-combustion method [19]. It was found that the particle size decreased with the Al content and the magnetization curves display no hysteresis with both retentivity and coercivity parameters which were found to be almost zero. In a separate study, Bhujun et al., investigated the potential of Al-doped Ni–Cu ferrite as an electrode material for supercapacitors. The Al-doped Ni-Cu ferrite was synthesized by the sol–gel method [20]. Recently, Sivaprakash et al., reported the change in characteristics of Ni-ferrite upon zinc doping prepared by sol–gel auto-combustion method and annealing at a subsequent temperature [21]. Zinc doping into Ni-ferrite influences the structural, magnetic and dielectric properties. From the structural studies, the phase purity and crystallite size were confirmed to be in the range of 30–41 nm. The analysis indicated that the lattice parameters are directly proportional to the ionic radii and found that the lattice parameters increase because of the increase of ionic radii of Zn. This situation is due to the substitution at the nonmagnetic site.
The present work aims to synthesize Ni-ferrite with various compositions of aluminium doping using the sol–gel method and annealing at subsequent temperatures. Secondly, to study the influence of Al doping in ferrite nanoparticles on the structures in terms of crystallographic properties and crystal defects. Besides, elemental and morphological properties of inverse spinel ferrite nanoparticles were also studied. The gas adsorption property parameters of ferrite nanoparticles were evaluated in detail to understand the influence of Al doping.
2 Materials and Experimental
2.1 Materials
All materials were used as purchased. Nickel (II) nitrate hexahydrate (Ni(NO3)2.6H2O, \(\ge\) 97.0%, Fluka Analytical), iron (III) nitrate nonahydrate (Fe(NO3)3.9H2O, \(\ge\) 98.0%, Bendosen) and aluminium sulphate hydrate (Al2(SO4)3.H2O) \(\ge\) 98.0%, R&M Chemicals) were obtained commercially and used without further purification.
All solvents, such as acetic acid (CH3COOH, \(\ge\) 99.8%, Systerm), isopropanol ((CH3)2CHOH, \(\ge\) 99.7%, R&M Chemicals) and methanol (CH3OH, \(\ge\) 98.0%, R&M Chemicals) were of analytical grade. Citric acid (C6H8O7, \(\ge\) 99.7%, R&M Chemicals) and benzoic acid (C6H5COOH, \(\ge\) 99.5%, Sigma Aldrich) were also obtained commercially.
2.2 Synthesis of Al-Doped Nickel Ferrite, NiFe2−xAlxO4 (0.3 ≤ x ≤ 1.0)
NiFe2-xAlxO4 (x = 0.3) is prepared by dissolving 0.001 mol (0.291 g) Ni(NO3)2.6H2O in a mixture of 20 mL isopropanol and 0.3 mL acetic acid. Then, 0.0003 mol (0.113 g) Al2(SO4)3.H2O was added to the mixture before the addition of 0.002 mol (0.808 g) Fe(NO3)3.9H2O to obtain Al-doped NiFe2O4 analogue. After that, citric acid was dissolved in the mixture at the mole ratio of citric acid to total metal-ions 1.2: 1. As much as 50 mL of deionized water was added to the solution. The mixture was stirred for 4 h at 80 ºC. The obtained as-prepared gel of Al-doped NiFe2O4 was dried and then calcined at 500 ºC for 4 h to facilitate crystallization and formation of Al-doped NiFe2O4. The remaining Al-doped NiFe2O4 were synthesised by varying the amounts of Al2(SO4)3.H2O to 1.71 g (x = 0.5) and 3.42 g (x = 0.5) to prepare respective NiFe2−xAlxO4. Other parameters were kept constant.
2.3 Characterization
The crystal structure of Al-doped NiFe2O4 was obtained using a Siemens D5000 X-ray diffractometer (United States) with monochromatic Cu-Kα radiation (λ = 1.5418 Å) operated at 40 kV and 40 mA. The model was used to generate X-ray diffraction (XRD) diffractograms from the samples in the 2θ range of 20–70º at room temperature (RT). The powdered samples are prepared by hand grinding using a mortar and pestle to produce pressed pellet samples for analysis. An FTIR from Perkin-Elmer System 2000 (United States) was used to analyze the functional group of the materials. The materials were prepared as pellets using KBr and scanned in the range of 1400–400 cm−1. The surface area and porosity of the samples were investigated using a NOVA 2200e, Quantachrome (United States) with nitrogen at a temperature of -196 ºC. The materials were degassed at 80 ºC overnight. Brunauer–Emmett–Teller (BET) model was used to calculate the surface areas. Meanwhile, pore size distributions and pore volumes were measured by the Barrett–Joyner–Halenda (BJH) technique. Scanning electron microscope (SEM) analysis was conducted using a Quanta 650 field emission gun SEM (Holland) to study the morphological characterization of the doped ferrite samples. The analysis was conducted at a magnification of 30,000× with an applied voltage of 5.0 kV to obtain the images of the desired materials. Energy-dispersive X-ray (EDX) was carried out to investigate the qualitative and quantitative analysis of the materials.
2.4 Basicity Analysis
The basic strength of the materials was tested using Hammett indicators. Bromothymol blue (H_ = 7.2); phenolphthalein (H_ = 9.8); 2,4-dinitroaniline (H_ 15.0); and 4-nitroaniline (H_ = 18.4) were indicators used in the study. As much as 25 mg of the materials was shaken with 1 mL of Hammett indicator solution diluted with methanol and left to equilibrate for 2 h. The basicity strength was determined from the changes in the colour of the solution. The mixture was titrated with benzoic acid while stirring at a fixed rate. The colour gradually changed, and the titration was stopped. The basicity can be calculated using Eq. 1:
where V is the volume of titrant, N is the concentration of benzoic acid and W is the weight of the samples used.
The basicity analysis of respective materials was also determined using the benzoic acid titration method by Tanabe and Yamaguchi [22]. As much as 1.0 M of benzoic acid was prepared by diluting it with methanol. Then 0.5 g of the samples were placed in 10 mL of the respective indicators (i.e., bromothymol blue, phenolphthalein, 2,4-dinitroaniline, 4-nitoraniline). Color changes concluded the titration end point.
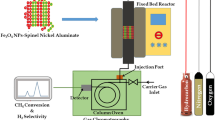
2.5 Thermogravimetrically CO2 Adsorption
Thermogravimetric gas adsorption analysis of CO2 was performed using a Perkin Elmer STA 6000 analyser [23]. 3 mg of gently ground samples were spread out in 100 µL Pt pans. The samples were subjected to a cleaning process from 30 to 300 °C under N2 gas. Then, the temperature was held at 300 °C for 30 min and switched to CO2 for adsorption. Weight increase due to CO2 adsorption was recorded. The experimental data for CO2 adsorption of the samples were analysed with kinetic models such as pseudo-first-order and pseudo-second-order kinetic models. The experiment was repeated with different parameters such as varying reaction temperature, total gas flow rates and adsorbent loadings to optimize the reaction conditions.
3 Results and Discussion
3.1 XRD Analysis
The X-ray diffraction pattern of NiFe2O4 and NiFe2-xAlxO4 (0.3 ≤ x ≤ 1.0) are shown in Fig. 1a and b–d. For the NiFe2O4 in Fig. 1a, the strong peaks at 2θ = 30.30º, 35.67º, 37.22º, 43.27º, 53.81º, 57.40º and 62.96º match the (220), (311), (222), (400), (422), (511) and (440) lattice planes of the cubic inverse spinel structure of NiFe2O4 (JCPDS 01-087-2336). No peaks corresponding to impurities are observed. Upon doping with Al3+, the crystal phase of NiFe2O4 remains intact. As observed in the diffraction pattern (Fig. 1b–d), the effect of increasing the Al3+ doping amount (0.3 ≤ x ≤ 1.0) on NiFe2O4 does not cause alteration of the original NiFe2O4 crystal planes. Al-doped NiFe2O4 revealed more intense diffraction peaks as the Al content was increased. This is due to the increasing crystallite size with the increasing of Al content. All the parameters such as crystallite size, lattice parameter, cell volume, bulk density, X-ray density and porosity are tabulated in Table 1. The crystallite size of the samples was calculated from the most intense peak (311) in the XRD pattern using the Scherrer formula as in Eq. 2.
where D is the crystallite size, β = FWHM (full width at half maximum of the peak), λ = 1.5406 Å (Cu–Kα), θ is the Bragg’s angle for the main peak.
From the XRD diffractograms, the crystallite size of the NiFe2−xAlxO4 containing x = 0.3, 0.5 and 1.0 are 30.41, 33.42 and 39.67 nm respectively. It can be seen that the crystallite size increases with increasing amounts of Al3+ in the NiFe2O4. Internal strain is produced in NiFe2O4 due to the mismatch of ionic radii of Al3+ and Fe3+. Diffusion rate is increased in Al-doped NiFe2O4 due to the smaller size of Al3+ ions. Grain boundaries migrate and leads to the growth of nanocrystals with the increase in amount of dopant [24]. The different sizes of Al3+ and Fe3+ can lead to grain anisotropy as the substitution of ions generates strain inside the crystal volume with increasing Al3+ doping amount. The balance between crystal anisotropy and volume strain is required to maintain an equilibrium state.
Furthermore, the Fe3+ takes up either Th or Oh sites. Meanwhile, Al3+ ions tend to occupy the Oh site in the crystal structure of NiFe2−xAlxO4 [25]. It is consistent with previous studies which mentioned that Al3+ preferentially replaces Fe3+ in Oh sites because of favorable crystal field effects [26].
The lattice parameter, a and volume also decrease as the amount of Al3+ ion increases. Lattice parameters of all the samples were calculated based on the cubic inverse spinel structure as in Eq. 3.
where d is the inter-planner spacing, a is the lattice parameter and h k l are the Miller indices of the ferrite crystal planes. Cell volume is calculated from Eq. 4.
The trend observed can be explained similarly to the explanation given for the decrease in crystallite size observed. The size of Al3+ is smaller than Fe3+ which decreases the cell volume of the structure as the amount of doping increases. The same trend was also reported in literature elsewhere [27, 28]. Rather and Lemine [29] explained this trend as due to the difference in ionic radii of Fe3+ ions when substituted with small Al3+ ions. The following equations were used to calculate the bulk density (ρm), X-ray density (ρX-ray) and porosity (P) using XRD data.
where m is the mass of the pellet, r is the radius of the pellet, h is the height of the pellet as obtained from XRD, Z = 8 which is the number of formula units in a unit cell, M is the molecular mass of the sample and N is the Avogadro’s number.
It can also be seen that the bulk density and X-ray density decreases with increasing Al3+ doping. This is directly related to the decrease in ionic radii of the samples. This trend is also reported in other literature [19, 27, 28]. Other than that, the percentage of porosity increases in doped samples as the Al3+ content increases. This can be due to the increasing vacancies created upon replacement of Fe3+ by Al3+ ions. The incorporation of Al3+ into the structure creates crystal imperfections and distortion, which results in the increasing vacancy observed. The following equation calculates the defects in terms of average strain, \(\varepsilon\).
The relationship between Eqs. 2 and 8 simply sums up as Eq. 9. The relationship assumes that crystallite size and average strain are not dependent on each other.
Rearranging Eq. 9, gives
Williamson-Hall (W–H) equations as in Eqs. 9 and 10 are also used to calculate crystal size and strain. The graph can be plotted with \({\beta }_{hkl}{\text{cos}}\theta\) as the y-axis and \(4{\text{sin}}\theta\) along the x-axis. The crystallite size is calculated from the intercept while strain is the slope of the graph as shown in Fig. 2.
The crystallize size has been calculated using the Scherrer method and Williamson–Hall method. The results are tabulated in Table 2. The data confirmed that both crystallite size and lattice strain calculated from the two methods are not in good agreement with each other and the values are inconsistent over the range. As can be seen in Fig. 2, the W–H method results in graphs with points scattered away from the linear expression. Hence, the results for crystallite size and strain may not be accurate. This may be because the strain in the crystallite formed, may not be uniform in all the crystallographic directions as Eq. 10 is based on the uniform deformation model (UDM) which makes the assumption that strain is supposed to be uniform. As such, this study will follow the Scherrer’s formula for the determination of the crystallite size of NiFe2-xAlxO4 (0.3 ≤ x ≤ 1.0).
3.2 FTIR Analysis
Figure 3 shows the IR absorption spectra of as-grown nanoparticles of Al3+ doped NiFe2O4 ferrites in the range 1400–400 cm−1. Generally, two broad metal–oxygen bands appear for all samples in the IR spectra. The most intense band, \(\nu\) 1 was observed at around 600 cm−1. The band corresponds to the intrinsic stretching vibrations of the metal at Fe(Th) \(\leftrightarrow\) O. In contrast, the lower intensity band, \(\nu\) 2, is seen at 400 cm−1 and is assigned to the octahedral-metal stretching Fe(Oh)\(\leftrightarrow\) O [30, 31]. The Fe–O bond in the Th occurs at a higher wavelength compared to the Oh. This is because it has a shorter bond length which results in more energy required to vibrate.
Upon incorporation of higher amounts of Al, the higher frequency band (\(\nu\) 1) is discovered to shift from 597 to 589 cm−1. In comparison, the lower frequency band (\(\nu\) 2) shifts from 404 to 412 cm−1 as shown in Fig. 3. It is found that, by increasing the Al dopant content, the peak of \(\nu\) 1 is shifted to the lower wavenumber while the peak \(\nu\) 2 is shifted to the higher wavenumber. The shifting in the band position is due to the differences in the distance between the Fe3+–O2− bonds for both Oh and Th sites. A decrease in particle size after doping with Al3+ ions change the environment which will lead to a change in the characteristic vibrational frequencies.
3.3 SEM Analysis
SEM micrographs were investigated to understand the morphology of doped Ni-ferrite samples. Figure 4 depicts the SEM images of the doped samples with their respective size distribution histograms. Here, images (a–c) are of the ferrite-doped samples where x = 0.3, 0.5 and 1.0. Based on the images, it shows the surface morphology of all compositions of Al-doped NiFe2O4 is about the same. It is observed that the particles are spherically shaped when Al is incorporated. The SEM images of NiFe2O4 from the previous study also illustrates spherical shaped structures [32]. These results confirm the similarity of morphologies of undoped and Al-doped of NiFe2O4 in terms of shape. However, doping Al into NiFe2O4 decreases the average particle sizes with the increase of dopant composition. The average particle size was found to be 355.2, 351.4 and 287.9 nm for x = 0.3, 0.5 and 1.0 correspondingly. A similar trend was seen in a previous study which reported that the average size of the Mg doped ZnO nanoparticles decreases as the Mg concentration increases [33]. Likewise, another study also revealed that the average size of the Eu-doped TiO2 nanoparticles decreases with the increase in dopant concentration [34]. The result suggests that the incorporation of dopant suppresses the growth of the samples to a certain extent.
The composition of Al-doped NiFe2O4 samples was analyzed by EDX analysis as in Fig. 5. The peaks shown in profile (a–c) correspond to elements Ni, Fe, Al, and O. The Al content increases with the amount of x in NiFe2−xAlxO4 samples. The chemical composition investigated from EDX analysis was found to be close to the expected stoichiometry. The presence of the carbon peak is due to the carbon coating employed during the measurement of EDX.
3.4 N2 Adsorption Desorption
Physical adsorption studies were also performed to characterize the surface textures of the samples. The BET isotherm of NiFe2O4 is shown in Fig. 6. According to the International Union of Pure and Applied Chemistry (IUPAC), pore size distribution between 2 and 50 nm is categorized as mesopores [35, 36]. Results of pore size distribution in Fig. 6 (inset) show that all the samples have large amounts of mesopores. Thus, they can be classified as mesoporous materials. The type of isotherm shown is Type IV according to IUPAC classification scheme. The initial part of this type of isotherm is attributed to monolayer-multilayer adsorption [37]. The overlaps of adsorption and desorption curves indicate that the adsorption–desorption reaction is fully reversible [38]. The presence of the H3 type hysteresis loop further explains the capillary condensation of mesopores. Previously, it was reported that the BET surface area of undoped NiFe2O4 is 12.296 m2 g−1 [15]. However, when the amount of Al3+ doping in NiFe2O4 was increased from x = 0.3–1.0, the BET surface area increased from 22.066 to 28.038 m2 g−1. The increase in the surface area of the samples, as shown in Fig. 6, may be attributed to the formation of smaller particle size. This trend is similar to another study which showed an increase in the BET surface area when Ni2+ was introduced into CoFe2O4 [39]. In this study, the increasing BET surface area is also consistent with the increase in pore volume and pore size, from 0.04915 to 0.08724 cm3 g−1 and 89.10–94.46 nm, respectively, when the Al3+ doping in NiFe2O4 was increased. All values are tabulated in Table 3. Generally, BET surface area depends on particle size, pore size, type of pores and distribution of pores. The increase in the values is due to the distortion in the arrangement of the structure. The substitution of small Al3+ ions in the NiFe2O4 contributes to more porosity in the arrangement of the samples. It involves more imperfection as the doping composition increases. Such imperfections cause more surface roughness and disorder, increasing the surface area, pore volume and pore size. In this study, this can be beneficial to achieve high CO2 adsorption in the application.
3.5 Basicity Study
The basicity strength of the samples was determined by the Hammett indicator method. The basic strength and total basicity values obtained from titration are tabulated in Table 4. Analysis of the Al3+ doped NiFe2O4 samples using the Hammett indicator, demonstrated a colour change when 2,4 dinitroanaline was employed. However, no change was seen in the presence of 4-nitronaline. Hence basicity strength of the Al3+ doped in NiFe2O4 is in the range of 15.0 < H- < 18.4. Total basicity of all dopant samples is in the range of 1.025–1.038 mmol g−1. In contrast, the NiFe2O4 has a basic strength in the range of 7.2 < H- < 9.8 with a total basicity of 0.545 mmol g−1. Hence it can be summarized that upon Al-doping, Al-O basic sites covered the surfaces of NiFe2O4 which caused an increase in the basicity of oxides. Additionally, a previous study revealed that doping with Na+ ions increased the strength of basicity. This was attributed to the formation of a dense layer of doped metal on the oxides that plugged pores [40].
3.6 Thermogravimetrically CO2 Adsorption
Figure 7 demonstrates the CO2 adsorption results for all three samples at 30 \(^\circ\)C. The cleaning process in the nitrogen atmosphere was conducted to ensure all the water moisture and organic solvent were removed from the pores of the samples. The adsorption capacity was obtained from the weight gain as in Fig. 7. At the beginning of the adsorption, it is observed that a steep increase indicates that CO2 adsorption occurs on NiFe2−xAlxO4 (0 ≤ x ≤ 1.0). The adsorption is almost stagnant when the adsorption process reaches equilibrium. For the initial steep increase, a strong binding force occurs between CO2 and the samples. The higher composition of Al doping (x = 1.0) gives a higher CO2 adsorption capacity. This may be due to the higher basic properties in samples containing higher Al compositions. This is shown in Table 5. The surface of the samples provides a basic active site which is mainly responsible for the formation of carbonate species and enhancing the adsorption capacity. The formation of the carbonate species by the CO2 chemisorption can be simplified as in Eq. 11.
The oxygen species on the surface of the samples, exchange electrons with CO2 during the chemisorption process. Hence, carbonate species are formed. The lower CO2 adsorption capacity for the NiFe2−xAlxO4 (x = 0.3) might be ascribed to the lower amount of oxygen species on the surface of the samples as the amount of Al-O is not as much as in the sample with composition (x = 1.0). Hence, it contributes to the weak adsorption capacity of the sample. All the CO2 adsorption capacity for NiFe2−xAlxO4 (0 ≤ x ≤ 1.0) are tabulated in Table 5.
Subsequently, the surface area, pore volume and pore size also influence the CO2 adsorption capacity for the samples. In Table 3, NiFe2-xAlxO4 (x = 1.0) has the highest BET surface area, pore volume, and size. The data is in good agreement with the adsorption capacity. A higher surface area, pore volume and pore size allow more CO2 molecules to bind to the active sites of the sample. A similar trend was also reported previously [41].
3.7 Parameters Affecting the CO2 Adsorption Capacity
3.7.1 Effect of the Adsorption Temperature on CO2 Adsorption
Based on the previous discussion, the best performance for CO2 adsorption is NiFe2−xAlxO4 (x = 1.0). Hence, this sample was used for further studies. Firstly, the effect of different CO2 adsorption temperatures, (30 °C, 50 °C and 70 °C) by NiFe2−xAlxO4 (x = 1.0) are presented in Fig. 8 and summarized in Table 6. The adsorption capacity at 30 °C is 28.71 mg/g. This value decreased to 26.02 mg/g and 24.02 mg/g when the temperature was increased to 50 °C and 70 °C, respectively. The trend observed can be explained as due to the higher kinetic energy of the CO2 molecules as temperature is increased. The CO2 molecules move faster causing less adsorption time on the surface of the adsorbent. This leads to the reduced adsorption capacity at higher adsorption temperature [42]. The trend observed is consistent with literature, where gas adsorption decreases when temperature increases [43]. When CO2 gas molecules come in contact with the adsorbent, heat is produced when the adsorbent adsorbs the gas molecules. Hence the adsorption is an exothermic process. Previous work have reported that the nature of the adsorption would be chemisorption [44].
3.7.2 Effect of the Adsorbent Loading on CO2 Adsorption
The effects of different NiFe2−xAlxO4 (x = 1.0) loadings on CO2 adsorption were also investigated as shown in Fig. 9. Three amounts of the sample which are 3, 6 and 9 mg were selected for CO2 adsorption. According to the data presented in Table 6, increasing the adsorbent loading did not increase the adsorption capacity of CO2. The highest adsorption capacity of 28.71 mg/g was found for the lowest loading of 3 mg and other loadings of 6 and 9 mg did not increase the CO2 adsorption capacity. Values of 22.18 and 19.33 mg/g, were observed for the 6 and 9 mg loadings, respectively. Therefore, the adsorption capacity gradually decreases with the increase in the adsorbent dose. The increased adsorbent amount provided additional active sites, however at high adsorbent dosage, it is possible that the adsorbent underwent agglomeration. Therefore, this decreases the unoccupied adsorption active sites and effective surface area [45, 46]. This situation led to lower adsorption capacity at high adsorbent loading.
3.7.3 Effect of Total Gas Flow Rate on CO2 Adsorption
The influence of gas flow rates (10, 20 and 30 mL/min) towards the adsorption of CO2 on NiFe2-xAlxO4 (x = 1.0) was also investigated. The gas flow rate at 10 mL/min produced a higher adsorption capacity compared to 20 and 30 mL/min as shown in Fig. 10. The higher adsorption capacity of 28.71 mL/min (10 mL/min) in comparison with 27.52 and 25.92 mg/g for 20 and 30 mL/min, respectively is because of the slower CO2 adsorption diffusion at the lower flow rates. The high flow rate of gases saturated the adsorption column very quickly, which can be associated with higher mass transfer coefficients [47]. All the data are tabulated in Table 6.
3.8 Recyclability Test of Adsorbent
Five cycles of CO2 adsorption–desorption were conducted to investigate the recyclability of the NiFe2-xAlxO4 (x = 1.0), generally as shown in Fig. 11. The curve for the cyclic CO2 adsorption is shown in Fig. 12a. Experimental data is presented in Fig. 12b. Based on the curve, the adsorption capacity of the fresh sample was 28.71 mg/g and the adsorption capacity later decreased to 27.89 mg/g and 27.22 mg/g at the second and third cycles. A similar decreasing trend continued until five cycles and the end of the cycle shows an adsorption capacity of 26.25 mg/g. The loss in adsorption capacity was approximately 8.56%. This is due to the fact that the pores of the sample were chemically bonded to the CO2 molecules with aluminium on the surface of the adsorbent. Even so, this study suggests that the adsorbents can be reused for several cycles successfully under these optimum conditions.
3.9 Kinetic Analysis
The kinetics of CO2 adsorption for NiFe2−xAlxO4 (x = 1.0) at optimum condition for the first cycle was studied using two kinetic models, namely pseudo-first-order and pseudo-second-order kinetic model. The pseudo-first-order rate equation may be represented as in Eq. 12.
where k1 is the pseudo-first-order constant (min−1), qe is the amount of CO2 adsorbed onto the ferrite-based oxides at equilibrium (mg/ gads), and qt is the amount of CO2 adsorbed onto the samples at any particular time t (mg/gads). The integration of Eq. 12 will give Eq. 13. A linear graph of log(qe–qt) against t is plotted to estimate the value of the gradient from the slope while the theoretical qe can be determined from the y-intercept of the graph. As for the pseudo-second-order rate, it can be expressed as in Eq. 14.
where k2 is the pseudo-second-order rate constant (g mg−1 min−1). Integrating Eq. 14 gives Eq. 15. The equation can be expressed into a linear form as in Eq. 16. Table 7 describes the equations for both order kinetics.
The plots for both kinetic models are shown in Fig. 13. The kinetic parameters are tabulated in Table 8. The pseudo-first-order kinetic model describes the initial phase and the progress of adsorption. It explains the adsorption in solid–gas systems based on the adsorption capacity of solids [48]. It is clear that the calculated adsorption capacity significantly deviates from the actual adsorption capacity which is similar to findings for this model with previous studies [49, 50]. Therefore, the data shows that the pseudo-first kinetic model does not support this study. The kinetic data achieved in this study failed to fit the model with a low R2 value of 0.55856.
This study was further treated using the pseudo-second-order kinetic model. This model describes that chemisorption controls the system. The graph \(\frac{t}{{q}_{t}}\) is plotted against \(t\) as shown in Fig. 13b. The slope and intercept can be used to calculate \({k}_{2}\). Based on the data in Table 8, the result shows high correlation coefficient values of R2 which is 0.99981. Overall, this kinetic model gives a low relative error between actual and calculated CO2 adsorption capacity compared to the first-pseudo kinetic model. The relative error calculated for NiFe2-xAlxO4 (x = 1.0) samples is 0.426%.
Therefore, based on the data of R2 values and relative errors, the pseudo-second-order kinetic model is the most suitable model for CO2 adsorption onto the NiFe2−xAlxO4 (x = 1.0). Based on Table 8, high correlation coefficient values of R2, 0.99981, are seen, which support the adsorption capacity of CO2 towards the sample. It can be concluded that CO2 adsorption is dominant towards chemisorption. This situation results in the adsorption process mainly governed by monolayer adsorption. This is because chemisorption takes place as a result of the reaction between adsorbent and adsorbate.
3.10 Adsorption Mechanism
The adsorption process of CO2 onto porous material can be divided into five successive steps as in Fig. 14. The five steps are (i) CO2 molecule diffusion from the bulk gas phase to the exterior of gas film (bulk diffusion), (ii) CO2 molecule diffusion through the gas film (film diffusion), (iii) CO2 molecule diffusion in the pore among the agglomerate particles (interparticle diffusion), (iv) CO2 molecule diffusion in the pore among the crystalline grains (intraparticle diffusion) and (e) CO2 molecule interacts with the adsorbent (surface adsorption). Generally, the surface adsorption is very rapid. It is assumed to be negligible. Hence, the adsorption process is controlled by one of the four diffusion process or the combination of them.
Based on Fig. 7, it was found that porous NiFe2−xAlxO4 (0 ≤ x ≤ 1.0) showed obvious two-stage adsorption process with a rapid initial CO2 uptake and subsequent slow adsorption process under the CO2 adsorption temperature. The narrow pores in the samples could cause strong diffusion resistance of CO2 molecules. Hence, the synthesized sample would display sluggish adsorption stage after the initial rapid stage due to the intense pore diffusion resistance.
Pseudo-second-order kinetic model suggests the CO2 adsorption is inclined towards chemisorption for NiFe2−xAlxO4 (0 ≤ x ≤ 1.0). It is known that for chemisorption, CO2 molecules have to diffuse into the pore of adsorbent and interact with the internal surface of the adsorbent. NiFe2−xAlxO4 particles are surrounded by CO2 molecules and react to form NiFe2−xAlxO4–CO3. The measured CO2 adsorption on the NiFe2−xAlxO4 (0 ≤ x ≤ 1.0) is from chemisorbed CO2 in the form of surface carbonate species. It is reasonable to postulate that the surface properties of the samples play a key role in the capture of CO2. The surface properties of the doped samples are determined by three factors: (i) the surface basicity (ii) the surface of oxygen vacancies and (iii) the surface segregation of the dopants. It was shown that the surface basicity of the samples can be enhanced by the presence of oxygen vacancies. Hence, it will bind the CO2 more strongly. Meanwhile, the surface oxygen vacancies can participate directly in the binding of CO2 to form carbonate species. The number of oxygen defect sites increased with the amount of doping. Therefore, more bonded CO2 is expected for NiFe2−xAlxO4 (x = 1.0) with more defect sites than other samples. This explains why the CO2 adsorption capacity for the NiFe2−xAlxO4 (x = 1.0) is the highest whereas the NiFe2−xAlxO4 (x = 0.3) gives the lowest CO2 capacity. This result is also consistent with the basicity study in Sect. 3.5. The effect of dopants on the CO2 adsorption behavior is also controlled by the total surface area. Higher surface area should give higher CO2 adsorption capacities as shown for the NiFe2−xAlxO4 (x = 1.0) sample. This suggests the enhancement of the CO2 interaction with the incorporation of dopants into NiFe2O4.
4 Conclusion
In summary, Al-doped NiFe2O4 (NiFe2−xAlxO4 (0.3 ≤ x ≤ 1.0)) were successfully synthesized via a modified sol–gel method. The non-magnetic Al-dopant content in NiFe2O4 was controlled by the Al/Fe precursors ratio. The influence of Al3+ composition on structural, morphological and CO2 adsorption has been investigated. XRD revealed the formation of a single-phase with a cubic inverse spinel ferrite structure. Additionally, Al3+ doping induced decreasing lattice parameters and increase in crystallite size. SEM images show the morphology remains unaltered after doping. The agglomerated spherical morphology is due to the higher surface area and indicates the pore present in the material. This is in good agreement with BET analysis. As the composition of Al-dopant increases, the BET surface area, pore volume and pore size increase. Isotherms for NiFe2−xAlxO4 (0.3 ≤ x ≤ 1.0) show Type IV, indicating the presence of mesopores. Further, the basicity analysis was analyzed and showed the highest composition of Al3+ in the sample has the highest basicity compared with the composition of 0.3 and 0.5. All samples were studied for CO2 adsorption capacity under different experimental conditions including flow rate, adsorption temperature and adsorbent loading. The adsorption experiment revealed that the feed flow rate of 10 mL/min, adsorbent loading of 3.0 mg and adsorption temperature of 30 °C were optimum for CO2 adsorption. NiFe2−xAlxO4 (x = 1.0) offers good properties regarding high CO2 capacity and recyclability.
Data Availability
The data that support the findings of this study are available within the article.
References
Sharifi I, Shokrollahi H, Amiri S (2012) Ferrite-based magnetic nanofluids used in hyperthermia applications. J Magn Magn Mater 324:903–915. https://doi.org/10.1016/j.jmmm.2011.10.017
Pham TN, Huy TQ, Lee AT (2020) Spinel ferrite (AFe2O4)-based heterostructured designs for lithium-ion battery, environmental monitoring, and biomedical applications. RSC Adv 10:31622–31661. https://doi.org/10.1039/d0ra05133k
Chae KP, Lee JG, Kweon HS, Lee YB (2004) The crystallographic, magnetic properties of Al, Ti doped CoFe2O4 powders grown by sol-gel method. J Magn Magn Mater 283:103–108. https://doi.org/10.1016/j.jmmm.2004.05.010
Zhuo M, Yang T, Fu T, Li Q (2015) High-performance humidity sensors based on electrospinning ZnFe2O4 nanotubes. RSC Adv 5:68299–68304. https://doi.org/10.1039/c5ra09903j
Wang M, Ai Z, Zhang L (2008) Generalized preparation of porous nanocrystalline ZnFe2O4 superstructures from zinc ferrioxalate precursor and its superparamagnetic property. J Phys Chem C 112:13163–13170. https://doi.org/10.1021/jp804009h
El Maalam K, Fkhar L, Mahhouti Z, Mounkachi O, Aitali M, Hamedoun M, Benyoussef A (2016) The effects of synthesis conditions on the magnetic properties of zinc ferrite spinel nanoparticles. J Phys Conf Ser. https://doi.org/10.1088/1742-6596/758/1/012008
Xin S, Liu S, Wang N, Han X, Wang L, Xu B, Tian Y, Liu Z, He J, Yu D (2011) Formation and properties of SrB6 single crystals synthesized under high pressure and temperature. J Alloys Compd 509:7927–7930. https://doi.org/10.1016/j.jallcom.2011.05.037
Kafshgari LA, Ghorbani M, Azizi A (2019) Synthesis and characterization of manganese ferrite nanostructure by co-precipitation, sol-gel, and hydrothermal methods. Part Sci Technol 37:900–906. https://doi.org/10.1080/02726351.2018.1461154
Yuan X, Xu QJ, Wang C, Liu X, Liu H, Xia Y (2015) A facile and novel organic coprecipitation strategy to prepare layered cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 with high capacity and excellent cycling stability. J Power Sources 279:157–164. https://doi.org/10.1016/j.jpowsour.2014.12.148
Ren D, Shen Y, Yang Y, Shen L, Levin BDA, Yu Y, Muller DA, Abruna HD (2017) Key parameter optimization for the scalable synthesis of uniform, high-energy, and high stability LiNi0.6Mn0.2Co0.2O2 cathode material for lithium-ion batteries. ACS Appl Mater Interfaces 9:35811–35819. https://doi.org/10.1021/acsami.7b10155
Xu L, Zhou F, Kong J, Chen Z, Chen K (2017) Synthesis of Li(Ni0.6Co0.2Mn0.2)O2 with sodium DL-lactate as an eco-friendly chelating agent and its electrochemical performances for lithium-ion batteries. Ionics 24:2261–2273. https://doi.org/10.1007/s11581-017-2363-8
Zhou F, Xu L, Kong J (2017) Co-precipitation synthesis of precursor with lactic acid acting as chelating agent and the electrochemical properties of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium-ion battery. J Solid State Electrochem 22:943–952. https://doi.org/10.1007/s10008-017-3837-3
MARSSIM (2000) 7 sampling and preparation for laboratory measurements. Revision 1 Chapter 7 1–28
Dai Z, Meiser F, Mohwald H (2005) Nanoengineering of iron oxide and iron oxide/silica hollow spheres by sequential layering combined with a sol-gel process. J Colloid Interface Sci 288:298–300. https://doi.org/10.1016/j.jcis.2005.02.076
Sulaiman NI, Abu Bakar M, Abu Bakar NHH, Saito N, Thai VP (2023) Modified sol-gel method for synthesis and structure characterization of ternary and quaternary ferrite- based oxides for thermogravimetrically carbon dioxide adsorption. Chem Papers 77:3051–3074. https://doi.org/10.1007/s11696-023-02687-6
Al-Juaid AA, Gabal MA (2021) Effects of co-substitution of Al3+ and Cr3+ on structural and magnetic properties of nano-crystalline CoFe2O4 synthesized by the sucrose technique. J Mater Res Technol 14:10–24. https://doi.org/10.1016/j.jmrt.2021.06.023
Hashim M, Alimuddin KS, Ali S, Koo BH, Chung H, Kumar R (2012) Structural, magnetic and electrical properties of Al3+ substituted Ni-Zn ferrite nanoparticles. J Alloys Compd 511:107–114. https://doi.org/10.1016/j.jallcom.2011.08.096
Batoo KM (2011) Structural and electrical properties of Cu doped NiFe2O4 nanoparticles prepared through modified citrate gel method. J Phys Chem Solids 5:68–77. https://doi.org/10.1016/j.jpcs.2011.08.005
Kumar KV, Paramesh D, Reddy PV (2015) Effect of aluminium doping on structural and magnetic properties of Ni–Zn ferrite nanoparticles. World J Nano Sci Eng 5:68–77. https://doi.org/10.4236/wjnse.2015.53009
Bhujan B, Shanmugam AS, Tan MTT (2016) Aluminium-doped nickel copper ferrites for high-performance supercapacitors. Int J Res Chem Metall Civ Eng. 3:3–7. https://doi.org/10.15242/IJRCMCE.E0316002
Sivaprakash P, Divya S, Parameshwari R, Saravanan C, Sagadevan S, Arumugam S, Esakki Muthu S (2020) Influence of Zn2+ doping towards the structural, magnetic, and dielectric properties of NiFe2O4 composite. J Mater Sci Mater Electron 31:16369–16378. https://doi.org/10.1007/s10854-020-04187-9
Tanabe K, Yamaguchi T (1963) Instructions for use basicity and acidity of solid surfaces. J Res Inst Catal Hokkaido Univ. 11:179–184
Lu CM, Liu J, Xiao K, Harris AT (2010) Microwave enhanced synthesis of MOF-5 and its CO2 capture ability at moderate temperatures across multiple capture and release cycles. Chem Eng J 156:465–470. https://doi.org/10.1016/j.cej.2009.10.067
Sagheer R, Khalil M, Abbas V, Kayani ZN, Tariq U, Ashraf F (2020) Effect of Mg doping on structural, morphological, optical and thermal properties of ZnO nanoparticles. Optik 200:163428. https://doi.org/10.1016/j.ijleo.2019.163428
Yao H, Ning X, Zhao H, Hao A, Ismail M (2021) Effect of Gd-Doping on structural, optical, and magnetic properties of NiFe2O4 as-prepared thin films via facile sol-gel approach. ACS Omega 6:6305–6311. https://doi.org/10.1021/acsomega.0c06097
Shinde BL (2019) Cation distribution study of Al3+ doped Zn–Ni–Cu ferrite. Int J Res Ana Rev. 6:750–753. https://doi.org/10.1729/Journal.20051
Raghavender AT, Shirsath SE, Pajic D, Zadro K, Milekovic T, Jadhav KM, Kumar KV (2012) Effect of Al doping on the cation distribution in copper ferrite nanoparticles and their structural and magnetic properties. J Korean Phys Soc 61:568–574. https://doi.org/10.3938/jkps.61.568
Waghmare SP, Borikar DM, Rewatkar KG (2017) Impact of Al doping on structural and magnetic properties of Co-Ferrite. Mater Today Proc 4:11866–11872. https://doi.org/10.1016/j.matpr.2017.09.105
Rather SU, Lemine OM (2020) Effect of Al doping in zinc ferrite nanoparticles and their structural and magnetic propertie. J Alloys Compd 812:152058. https://doi.org/10.1016/j.jallcom.2019.152058
Hiti El (1996) AC electrical conductivity of Ni–Mg ferrites. J Phys D: Appl Phys 29:501–505. https://doi.org/10.1088/0022-3727/29/3/002
Priyadharsini P, Pradeep A, Rao PS, Chandrasekaran G (2009) Structural, spectroscopic and magnetic study of nanocrystalline Ni-Zn ferrites. Mater Chem Phys 116:207–213. https://doi.org/10.1016/j.matchemphys.2009.03.011
Kesavamoorthi R, Vigneshwaran AN, Sanyal V, Raja CR (2016) Synthesis and characterization of nickel ferrite nanoparticles by sol - gel auto combustion method. J Chem Phar Sci 9:160–162
Shayesteh SF, Dizgah AA (2013) Effect of doping and annealing on the physical properties of ZnO: Mg nanoparticles. Pramana J Phys 81:319–330. https://doi.org/10.1007/s12043-013-0562-z
Pal M, Pal U, Jiménez JMGY, Pérez-Rodríguez F (2012) Effects of crystallization and dopant concentration on the emission behavior of TiO2: Eu nanophosphors. Nanoscale Res Lett 7:1–12. https://doi.org/10.1186/1556-276X-7-1
Sulaiman NI, Abu Bakar M, Abu Bakar NHH, Hussin MH (2019) Sol-gel synthesis of barium hexaferrite and their catalytic application in methyl ester synthesis. IOP Conf Ser Mater Sci Eng 509:012103. https://doi.org/10.1088/1757-899X/509/1/012103
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87:1051–1069. https://doi.org/10.1515/pac-2014-1117
Sing KSW (1982) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 54:2201–2218. https://doi.org/10.1515/iupac.57.0007
Yang Y, Shukla P, Wang S, Rudolph V, Chen XM, Zhu Z (2013) Significant improvement of surface area and CO2 adsorption of Cu-BTC via solvent exchange activation. RSC Adv 3:17065–17072. https://doi.org/10.1039/c3ra42519c
Owolabi TO, Saleh TA, Olusayo O, Souiyah M, Oyeneyin OE (2021) Modeling the specific surface area of doped spinel ferrite nanomaterials using hybrid intelligent computational method. J Nanomater. https://doi.org/10.1155/2021/9677423
Chew KY, Tan WL, Abu Bakar NHH, Abu Bakar M (2017) Transesterification of palm cooking oil using barium-containing titanates and their sodium doped derivatives. Int J Energy Environ Eng 8:47–53. https://doi.org/10.1007/s40095-016-0222-4
Wan-Ab-Karim-Ghani WA, Madzaki H, Yaw TCS, Rashid U, Muda N (2018) Carbon dioxide adsorption on activated carbon hydrothermally treated and impregnated with metal oxides. J Kejuruter 30:31–38. https://doi.org/10.17576/jkukm-2018-30(1)-05
Mori Y, Yamada A (1994) Dynamic behaviour of an adsorption column heat exchanger. Int Heat Trans Conf 10:161–166. https://doi.org/10.1615/IHTC10.5220
Akpasi SO, Isa YM (2022) Effect of operating variables on CO2 adsorption capacity of activated carbon, kaolinite, and activated carbon-kaolinite composite adsorbent. Water-Energy Nexus 5:21–28. https://doi.org/10.1016/j.wen.2022.08.001
Rojahn P, Hessel V, Nigam KD, Schael F (2018) Applicability of the axial dispersion model to coiled flow inverters containing single liquid phase and segmented liquid-liquid flows. Chem Eng Sci 182:77–92. https://doi.org/10.1016/j.ces.2018.02.031
Tan YL, Islam MA, Asif M, Hameed BH (2014) Adsorption of carbon dioxide by sodium hydroxide-modified granular coconut shell activated carbon in a fixed bed. Energy 77:926–931. https://doi.org/10.1016/j.energy.2014.09.079
Lei T, Li SJ, Jiang F, Ren ZX, Wang LL, Yang XJ, Tang LH, Wang SX (2019) Adsorption of cadmium ions from an aqueous solution on a highly stable dopamine-modified magnetic nano-adsorbent. Nanoscale Res Lett. https://doi.org/10.1186/s11671-019-3154-0
Ezzati R (2020) Derivation of pseudo-first-order, pseudo-second-order and modified pseudo-first-order rate equations from Langmuir and Freundlich isotherm for adsorption. Chem Eng J 392:123705. https://doi.org/10.1016/j.cej.2019.123705
Munusamy K, Sethia G, Patil DV, Somayajulu Rallapalli PB, Somani RS, Bajaj HC (2012) Sorption of carbon dioxide, methane, nitrogen and carbon monoxide on MIL-101(Cr): volumetric measurements and dynamic adsorption studies. Chem Eng J 195–196:359–368. https://doi.org/10.1016/j.cej.2012.04.071
Rashidi NA, Yusup S, Hameed BH (2013) Kinetic studies on carbon dioxide capture using lignocellulosic based activated carbon. Energy 61:440–446. https://doi.org/10.1016/j.energy.2013.08.050
Gopal K, Mohd NI, Raoov M, Suah FBM, Yahaya N, Zain NNM (2019) Development of a new efficient and economical magnetic sorbent silicone surfactant-based activated carbon for the removal of chloro- and nitro-group phenolic compounds from contaminated water samples. RSC Adv. https://doi.org/10.1039/C9RA07151B
Acknowledgements
The authors would like to acknowledge the School of Chemical Sciences, Universiti Sains Malaysia for the research facilities and the research university grant which supported this work [1001/PKIMIA/8011071].
Author information
Authors and Affiliations
Contributions
NIS: Writing–original draft, Investigation, Methodology, Data curation, Visualization, Validation, Formal analysis, Conceptualization. NHHAB: Supervision, Writing—review & editing, Project administration, Funding acquisition, Resources, Conceptualization. MAB: Supervision, Writing—review & editing, Conceptualization.
Corresponding authors
Ethics declarations
Conflict of Interest
We wish to draw the attention of the Editor to the following facts which may be considered as potential conflicts of interest and to significant financial contributions to this work. This work has been supported by Universiti Sains Malaysia (USM) under the Research University Grant (RUI) (1001/PKIMIA/8011071).
Human and Animals Statement
This article does not contain any studies involving humans and animals performed by any of the authors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sulaiman, N.I., Abu Bakar, N.H.H. & Abu Bakar, M. Effect of Al-Doping on Structural and Adsorption Properties of NiFe2O4 via Modified Sol–Gel Approach for CO2 Adsorption. Chemistry Africa 7, 2139–2154 (2024). https://doi.org/10.1007/s42250-024-00879-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-024-00879-5