Abstract
A study aims to inspect the surface adsorption of Caesalpinia Crista seed (Kanchaki seed) extract as a developing anti-corrosion additive for zinc in acidizing solutions. The study utilizes various experimental approaches like the mass loss, electrochemical impedance spectroscopy (EIS), and potentio-dynamic polarization (PDP). The study achieves high inhibition efficiencies of 99.4%, 85.0%, and 94.8% in mass loss, potentiodynamic studies, and EIS, respectively at a higher concentration (2.0 g/L) of KSE and a temperature of 303 K. Impedance analyses revealed an increase in charge transfer at the zinc-solution interface upon the addition of the KSE. PDP measurements indicated a significant reduction in corrosion current density when using Kanchaki seed extract (KSE), suggesting its role as a mixed-type corrosion inhibitor. The adsorption of the KSE on the zinc substrate followed the Langmuir isotherm, involving a physical adsorption mechanism. Surface analysis, conducted through contact angle and scanning electron microscopy - energy dispersive X-ray (SEM-EDX) and atomic force microscopy (AFM) measurements, provided additional support for the adsorption of the inhibitor on the zinc substrate. Fourier transform infrared spectroscopy (FT-IR) was utilized to calculate the KSE’s functional groups. The experimental outcomes obtained from all the applied approaches show good agreement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Zinc (Zn), a significant non-ferrous metal, is widely utilized for metallic coatings. Similar to aluminum, zinc demonstrates amphoteric characteristics when exposed to alkalis and acids. Both the zinc-coated also the zinc goods rapidly corrode when exposed to damp environments, producing white corrosion products known as ‘white rust’ [1]. For the protection of Zn corrosion in acidic and alkaline environments, application-specific organic compounds can be employed. Due to their efficiency in preventing corrosion, organic corrosion inhibitors are often used, and those containing nitrogen have received significant attention in the literature [2,3,4]. Among these, heterocyclic amines, aliphatic, and aromatic have been extensively researched as inhibitors of corrosion [5,6,7,8].
Due to concerns about the toxicity of certain corrosion inhibitors, there was a growing effort to find environmentally friendly alternatives, often referred to as green corrosion inhibitors [9]. Natural substances derived from plants, containing a range of organic molecules like tannins, alkaloids, pigments, amino acids, and organic acids, have been recognized for their inhibitive properties [10, 11]. This class of inhibitors is characterized by being eco-friendly, less harmful to the environment, cost-effective, readily accessible, and sourced from natural products like plant extracts [12]. Many extracts of plants were identified as potential corrosion inhibitors for the non-ferrous metals [13,14,15,16,17]. However, the exploration for environmentally friendly corrosion inhibitors for Zn in HCl has been limited. Several green inhibitors have been discovered for Zn metal in HCl, such as Artichoke [18], Ailanthus altissima [19], Elettaria cadamomum [20], Ferula hermonis plant [21], Bryophyllum pinnatum leaves [22], Avicennia Marina [23], Nictanthes plant [24], and Achillea fragrantissima [25], are recognized for their well-established corrosion inhibitory abilities. The fact that medicinal herbs have been used traditionally to inhibit corrosion in acidic environments demonstrates the early recognition of their effectiveness in protecting metal alloys [26, 27]. Despite their promise, the development of effective plant-based corrosion inhibitors is an ongoing research area [28].
Caesalpinia crista belongs to the Fabaceae family, and, this plant is distributed widely in India and various regions of Southeast Asia [29]. In India specifically, it goes by the name Katikaranja in Hindi, Kanchaki in Gujarati, and Kuberaxa in Sanskrit. It can be found in states such as West Bengal, Kerla, and Maharashtra. The medicinal plant Caesalpinia crista is known for its many advantageous benefits. It has been traditionally used as an antistress drink and as a remedy for conditions like back pain and rheumatism [30, 31]. Research on this plant has identified several bioactive components, including cassane, new cassane-like diterpenoids, flavonoids [32], proteins, alkaloids, and saponins [33]. These compounds are likely responsible for the plant’s anti-inflammatory, antimalarial, antimicrobial, and antioxidant characteristics [34, 35]. Caesalpinia crista fruit demonstrates strong corrosion inhibition on mild steel in HCl, with inhibition efficiency exceeding 90% at 5mM inhibitor concentration in a 1.0 M HCl solution [36]. Hence, our focus catered on exploring Caesalpinia crista as a potential corrosion inhibitor. The objective was to investigate the inhibitive properties of the Caesalpinia crista seeds extract against zinc corrosion in varying HCl concentrations (0.05 M, 0.075 M, and 0.10 M) via electrochemical and non-electrochemical methods. Additionally, the study encompasses an analysis of the morphological changes on the surface of the zinc.
2 Experimental
2.1 Materials
The test specimen consisted of Zinc which was chemically composed of 0.16% Ni (Nickel)and 99.84% Zn. Corrosion rate determination involved using rectangular Zn specimens measuring 5.0 cm × 2.0 cm × 0.10 cm, each featuring a small hole with a diameter of 0.5 cm near one end, resulting in a total exposed area of 0.23343 mg/dm2. Before conducting the test, all specimens were meticulously cleaned the buffing and then carefully wrapped in bags made up of plastic to protect them from atmospheric corrosion. A.R. (Analytical Regent) grade hydrochloric acid (HCl) has been utilized during the experiment.
2.2 Preparation of KSE
The Kanchaki seeds used in this study were purchased from a local market (Surat, Gujarat, India) and underwent a thorough washing process to remove dust. Afterwards, the seeds were left to air-dry outdoors at room temperature. Once dried, the seed materials have been finely ground in a blender to ensure consistent particle size in powdered form. A 25 g sample of the dry powder was subjected to extraction using 350 ml of distilled ethanol in a Soxhlet extraction system for 5 h. After the process of the extraction had been completed, the ethanol was separated from the extract through fractional distillation, resulting in an inhibitor solution. The raw extracts were stored in glass bottles in a cool and dry environment until they were needed for further experiments. KSE solutions with varying content range from 0.4 to 2.0 g per litter (g/L) were prepared for use in subsequent experiments.
Top of Form.
2.3 FT-IR
In the investigation, the equipment utilized comprised a Bruker spectrophotometer. This instrument was employed for the characterization of the alcohol extract derived from the Kanchaki seeds extract. The spectra were recorded over a wavelength range spanning from 4000 to 600 cm−1. The purpose is to identify the functional groups within the extract that could potentially contribute to its impact of corrosion inhibiting.
2.4 Mass Loss
The gravimetric analysis is a reliable and extensively used technique for determining how metals will corrode in the inhibitors’ existence. In this investigation, corrosion measurements were made using previously weighed samples exposed to 0.10 M 0.075 M, and 0.05 M HCl solutions, with and without the addition of KSE inhibitor, at a constant temp of 303 K. To examine the inhibitory effect of KSE, the inhibitor concentration was varied at 0.4 g/L, 0.8 g/L, 1.2 g/L, 1.6 g/L, and 2.0 g/L of the acidic solutions. After 24 h, the samples were dried, washed, and reweighted to find the corrosion rate, surface coverage degree, and inhibitor effectiveness for every inhibitor concentration.
2.5 Effect of Temperature
The experiment involved immersing the sample in a 230 ml solution of 0.05 M HCl, containing an inhibitor with concentrations ranging from 0.4 to 2.0 g/L, at temperatures of 313 K, 323 K, and 333 K for three hours. This investigation aimed to assess the influence of temperature on the corrosion rate. Temperature control was maintained using a thermostatic assembly with precision of ± 0.5 °C. The results of the calculations are summarized in Tables 2 and 3, providing data on activation energy (Ea), the free energy of adsorption (ΔG0ads), heat of adsorption (Qads), enthalpy of adsorption (ΔH0ads), entropy of adsorption (ΔS0ads) and Inhibition efficiency (P%).
2.6 Electrochemical Measurements
The working electrode underwent a preparation process involving the use of different grades of emery papers for polishing. It was then washed with the distilled water and degreased by utilizing the acetone. Electrochemical measurements have been conducted by utilizing the CHI 760D Electrochemical impedance analyzer model. Before performing electrochemical experiments, a 30-minute stabilization period was observed, which had been previously established as adequate for achieving a stable OCP (Open Circuit Potential). The experiments were conducted in a 3-electrode cell set up at room temp (303 K). The counter electrode is an electrode of platinum, also the reference electrode has been SCE (Saturated Calomel Electrode). The working electrode has been composed of Zn with a visible surface area of 1 cm2, while the remaining portion was covered with commercially available Teflon. EIS measurements were acquired from the Nyquist plot, utilizing an AC signal with a 0.01 V amplitude. The spectrum of a frequency encompassed ranging from the 100 kHz down to 0.01 Hz. Curves of the potentiodynamic polarization have been recorded by varying the potential within a range of + 300mV from the OCP. The sweep rate for these measurements was set at 0.01 mV/s.
2.7 Surface Analysis
The surface characteristic of the metal sample was documented utilizing the SEM with the model name ‘S3400’. In the conducted investigations, samples of the Zn were submerged in a 0.05 M HCl solution for 24 h, both in the absence and existence of the inhibitor (KSE). After the immersion period, the metal samples were retrieved, rinsed with the use of distilled water, subjected to drying, and then subjected to analysis using the SEM methodology. The acceleration potential of the electron beam utilized in the process was set at 15.0 kV. The protective film presence at a Zn surface under the protected condition has been verified using the AFM method.
3 Results and Discussion
3.1 FT-IR
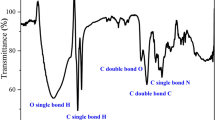
By way of illustrative instance, an FT-IR spectrum of KSE is shown, spanning from 4000 to 600 cm−1, as depicted in Fig. 1. The elucidation of functional groups accountable for the peaks of IR adsorption is delineated as given in Table 1.
3.2 Mass Loss
To evaluate the protective value of KSE as an inhibitor, various concentrations of KSE were added to each HCl concentration (0.05 M, 0.075 M, and 0.10 M). The inhibitor concentration was used 0.4 g/L, 0.8 g/L, 1.2 g/L, 1.6 g/L, and 2.0 g/L. The weight loss experiment data shown in Fig. 2 indicates that the rate of corrosion rises along with an increase in the acid concentration. The rate of corrosion in 0.05 M, 0.075 M, and 0.10 M HCl concentrations have been measured at 1439.9 mg/dm2, 2129.86 mg/dm2, and 2901.24 mg/dm2, respectively over 24 h at 303 K. The results reveal that at a constant concentration acid, the inhibition effectiveness (p%) of KSE in 0.075 M HCl, the inhibition efficiency has been seen to be 89.74%, 97.18%, 98.79%, 99.20%, and 99.40% concerning 0.4 g/L, 0.8 g/L, 1.2 g/L, 1.6 g/L, and 2.0 g/L inhibitor concentration, respectively. This shows that higher concentrations of KSE lead to better corrosion protection. Conversely, at constant inhibitor concentration, the p% reduces and has a rise in the concentration of an acid. At an inhibitor concentration of 2.0 g/L, the p% KSE was measured as 99.40%, 99.40%, and 97.64% with respect to 0.05 M, 0.075 M, and 0.10 M HCl concentration, respectively. This suggests that the effectiveness of KSE decreases with more acidic environments.
Where the inhibition efficiency (P%) is determined by calculating the percentage reduction in corrosion loss achieved through the use of the KSE. The terms MLu and MLi denote the mass loss of corrosion in the absence of and presence of the inhibitor, respectively. The parameter ‘θ’ represents the surface coverage degree, indicating the percentage of the metal surface coated by the inhibitor. The inhibitory impact observed in the KSE can be attributed to the adsorption of its components onto Zn surfaces. The formation of the protective layer, composed of the adsorbed molecules, acts as a barrier, effectively isolating the metal surface from corrosive media. This isolation hinders the dissolution of the zinc surface by safeguarding against corrosion sites, ultimately resulting in a reduced corrosion rate [41].
3.3 Effect of Temperature
The experimental results presented in given Table 2 illustrate the effect of the temp on the Zn corrosion. It is evident that rise in a temperature leads to a greater rate of corrosion, indicating thermal-activated kinetics is a contributing factor [42]. The inhibition efficiency of KSE reduces with increasing temperature, with inhibition efficiencies of 98.36%, 96.00%, and 94.50% observed at 313 K, 323 K, and 333 K, correspondingly, at an inhibitor concentration of 2.0 g/L (Table 2). The mean activation energy (Ea) values have been determined by utilizing Eq. (3) for Zn in 0.05 M HCl, resulting in a value of 16.32 kJ/mol. However, in the presence of an acid-containing KSE, it has been observed that the mean activation energy (Ea) values are higher compared to those in an uninhibited environment (Table 3). The greater Ea values in the inhibitors’ presence suggest stronger interaction among the inhibitor molecules and also the metal, indicating physical adsorption [43]. This adsorption layer acts as a shield, improving the energy barrier for the procedure of corrosion and thus reducing the overall rate of corrosion [44, 45]. The consistency in Ea values from the Arrhenius graph (Fig. 3) and Eq. (4), validates their reliability in studying the inhibitory behaviour of KSE.
The alteration in entropy and alteration in enthalpy information can be determined using the subsequent formula:
Wherein K denotes “the corrosion rate (CR), h symbolizes Plank’s constant, Avogadro’s number is denoted as N, ΔS0ads stands for the activation entropy, and ΔH0ads signifies the activation enthalpy. A graphical representation of Log (K/T) v/s /T, as displayed in Fig. 4, is expected to yield a linear plot. This line should have a gradient of \(\left(\frac{-{\varDelta {H}^{0}}_{ads}}{2.303R}\right)\), and a y-intercept of \(Log \left(\frac{R}{Nh}\right)+\left(\frac{{\varDelta {S}^{0}}_{ads}}{2.303 R}\right)\). Utilizing these values, the ΔS0ads and ΔH0ads details can be determined and exhibited in the given Table 3. The negative ΔS0ads values for the inhibitor show that the activated complex is involved in the step of rate-determining, performing an association instead of a dissociation action. This suggests a reduction in the disorder at the time of transition through the reactant to the activated complex” [46]. Furthermore, evident from Table 3, is the noticeable increase in ΔH0ads values when in comparison to the uninhibited scenario. These values are positive and represent the characteristics of endothermic Zn dissolution throughout the corrosion procedure [47, 48], these sign of ΔH0ads shows that the inhibitor molecules’ adsorption is an endothermic process.
The additional thermodynamic parameter that provides further insight into the adsorption mechanism involved in the procedure of corrosion inhibition is the adsorption heat, denoted as Qads. This parameter is linked to the extent of coverage on the surface, represented as θ, and the relationship is expressed as follows (Eq. 5):
Here, θ1 and θ2 correspond to the degrees of surface coverage at the given temp which are T1 and T2 respectively. The determined Qads values, as also presented in the given Table 3, are all negative and fall ranging from 6.40 to -77.03 kJ/mol. The presence of negative Qads values indicates that as temperature increases, the adsorption process as well as inhibition efficiency experience a reduction [49].
3.4 Adsorption
The analysis of surface coverage (θ) data plays a crucial role in understanding the intricacies of adsorption characteristics. By examining how the proportion of the surface covered changes with concentration under constant temperature conditions, we can gain insight into equilibrium scenarios for various adsorption isotherms. Several models, including Freundlich, Langmuir, Frumkin, and Temkin isotherms, have been employed to match experimental results. However, the most compelling agreement was achieved with the Langmuir and Freundlich isotherms. Equation (6) [50] and (7) [51] below, representing the Langmuir and Freundlich adsorption isotherms respectively, establish the relationship between inhibitor concentration and the extent of surface coverage. The exceptionally high linear correlation coefficient of the fitted data suggests a robust alignment of inhibitor molecule adsorption with Langmuir adsorption isotherm (Fig. 5).
Here, θ represents the surface coverage degree, n is the coefficients, Cinh signifies “the inhibitor concentration in g/L, and Kads represents the equilibrium constant governing the equilibrium desorption/adsorption procedure of molecules of an inhibitor on the surface of the metal. The Kads values have been derived by the adsorption process plot intercept and are interconnected with the free energy alteration of adsorption, denoted as ΔG0ads, through the equation (Eq. 8) [52, 53]:
The Langmuir and Freundlich adsorption parameters and ΔG0ads values, observed in the presence of KSE, are meticulously detailed in Table 4. Scrutiny of Table 4 unveils the negative nature of ΔG0ads, which signifies the tenacity of the layer that is adsorbed at a surface of Zn and the inherent propensity of the adsorption procedure to foster the creation of an adsorbed film on the metal’s surface. Across the range of a concentration is 0.4 to 2.0 g/L for KSE, the ΔG0ads values consistently fall within − 11.91 to -14.05 kJ/mol, indicative of a prevailing physisorption mode. ΔG0ads values lower than − 20 kJ/mol are typically associated with physisorption, while the values higher than − 40 kJ/mol are indicative of chemisorption [54, 55].
The observed linear relationship between surface coverage and inhibitor concentration for KSE (Fig. 6) strongly supports the hypothesis that corrosion inhibition arises from the formation and preservation of protective film on the Zn surface. This additional layer proficiently covers both anodic and cathodic sites through a consistent process of adsorption. The alignment of experimental data with these isotherms gives compelling substantiation for the pivotal role that adsorption plays in the discernible inhibitive impact of the KSE. The linear regression derived through the graph of log θ against log C yields a correlation coefficient of 0.9081 (Fig. 6).
3.5 OCP
In an OCP experiment involving Zn corrosion, a Zn specimen immersed in a 0.05 m HCl solution exhibited a stable potential of – 796 mV compared to a standard SCE. When varied concentration (0.4 to 2.0 g/L) of KSE was introduced, the maximum corrosion potential shifted to – 783 mV for 2.0 g/L. The use of KSE indicated a slightly positive shift in the potential of the corrosion, by proposing the polarization for the local anodes (Fig. 7).
3.6 Potentiodynamic Polarization
The study involved determining corrosion current densities using extrapolation of cathodic as well as anodic Tafel lines to find their intersection (Fig. 8). The finding indicated that the effectiveness of corrosion protection increased as the concentration of KSE in the treatment solution 0.05 M HCl. This was evident from the shift of the corrosion potential (Ecorr) towards the more noble direction and also a reduction in the corrosion current values (icorr). Cathodic curves exhibited a gradual shift towards the lower current density regions with increasing KSE concentration, while the anodic curves showed no significant shift. The most significant corrosion protection was observed at 2.0 g/L KSE concentration. In the 0.05 M HCl, the substantial KSE concentration caused a potential shift in the direction of an anode, though the shift was modest at 46 mV. This value fell below the generally accepted threshold of ± 85 mV, which is commonly employed to categorize inhibitors as either anodic or cathodic [56]. This outcome might be attributed to the organometallic protective film forming presence at the surface of an electrode, leading to a reduction in the effectiveness of a “rate of electron transfer at the site of the interface. The marginal alteration in both βc & βa values suggests a lack of variation in the inhibition mechanism, both in the absence and presence of the inhibitor [57, 58]. Table 5 provided data on corrosion potential (Ecorr), Corrosion current (icorr), cathodic and anodic Tafel slopes (βc & βa), and inhibition” effectiveness (p%) of KSE. The P% has been determined by utilizing a specified relation (Eq. 9), that involves comparing corrosion densities with and without the inhibitor icorr(inh) and icorr(u) respectively [59].
The higher concentration of KSE results in reduced corrosion current densities, indicating improved corrosion protection for the Zn. This behaviour implies that the presence of KSE on the surface of the Zn electrode leads to enhanced resistance against the corrosion process, making it a favourable choice for corrosion prevention.
3.7 EIS
The EIS experimental data was thoroughly evaluated by fitting it to the similar electrical circuit depicted in the given Fig. 9. The outcome of this analysis was visually represented in Fig.10, where dotted lines indicate the fitted circuit. These indicate two-time constants, each contributing to different processes. The time constant observed in the Nyquist plot represents the behaviour of the electrochemical double layer formed at the interface of zinc electrolyte and was identified as an outcome of passive layer formation on the zinc surface. The passive layer works as a protective barrier, shielding the metal from further corrosion [60,61,62]. When KSE was introduced into the system as an inhibitor, the plot showed an inductive loop at low frequencies. This observation suggests that the KSE extract effectively adsorbed on the zinc surface, functions as an inhibitor to hinder corrosion processes.
Key parameters related to impedance diagrams, such as CPE (Constant Phase Element), charge transfer resistance (Rct), CPE exponent (n), and double layer capacitance (Cdl) along with inhibition effectiveness (p%) were documented in Table 6. The calculation of p% involved the comparison between charge transfer resistances of uninhibited & inhibited cases. Quantifying the inhibition efficiency involves utilizing Eq. (10) [63] with the following expression:
In the context of Eq. (10), Rct(i) & Rct(u) denote the charge transfer resistances in the KSE’s existence & absence, correspondingly. Interestingly, the p% values obtained through both the measurements of a weight loss & electrochemical impedance spectroscopy exhibited a consistent sequence. Further investigation revealed that Rct values increased with rising inhibitor concentration, while Cdl values decreased. This shift was attributed to the increased surface coverage by KSE, leading to higher inhibition efficiency. In this study, the n value encompasses a spectrum from 0.924 to 0.713, signifying the KSE extracted molecule’s adsorption onto the most active sites of the zinc and showcasing substantial surface heterogeneity. The time of a relaxation (measured in seconds) signifies the brief timespan essential for reaching charge distribution equilibrium, as outlined in the following Eq. (11). The adsorption process of the KSE undergoes a rapid equilibration, as evident from Table 6. Noteworthy is the elongation of relaxation time with increasing KSE concentration., indicative of heightened KSE extracted constituent’s adsorption at a surface of Zn [64]. This effectively diminishes the exposed electrode area within corrosive surroundings.
The estimation of Cdl values is derived from impedance values through the Nyquist plot. The estimation is achieved using Eq. (12) [65]. Here, fmax corresponds to the frequency where the max imaginary component of the impedance denoted as (− Z’ max) is attained. The calculation of Rct values is established by the subsequent Eq. (13) [66]:
Figure 11a–b exhibit the Bode curves, indicating a positive relationship between the rise in the linear slope section and the amount of KSE. This implies that KSE adsorbate is present on Zn before oxidation occurs during sliding [67]. Additionally, the increase in phase angles is indicative of enhanced surface coverage [68] (Fig. 11b).
3.8 SEM
SEM micrographs in Fig. 12a–b exhibit Zn samples with and without 2.0 g/L of the investigated KSE. The absence of KSE (Fig. 12a) [69] displays a severely corroded and rough surface. However, in the KSE existence, the damage to the Zn surface significantly reduces due to the KSE presence, resulting in a much smoother surface (Fig. 12b).
3.8.1 EDX
To collect an understanding of the zinc sample’s surface composition, we conducted EDX analysis in both inhibitor-free and inhibitor-treated environments, immersed in a 0.05 M HCl solution for three hours, the KSE employed at a concentration of 2.0 g/L, showcasing the highest inhibition efficiency (Fig. 13) [69]. Table 7 outlines the Atomic (At %) and Weight (Wt %) percentages of elements obtained from EDX analysis. Figure 13 examination unveils a conspicuous decrease in O and Cl peaks, juxtaposed with an ascent in the C peak in the presence of KSE. In HCl solutions, heightened O peck arises from zinc oxide formation on the metal surface [70]. The mitigation of O and Cl peaks, coupled with the augmentened C peak, indicates the KSE efficacy in shielding the surface from corrosion products. This observation aligns with EDX data, affirming the robost adsorption of inhibitor molecules on the Zn surface, cuminnating in the formation of a protective film [71].
3.9 AFM
Atomic force microscopy (AFM), stands out as an exceptional high-resolution scanning probe method, widely acclaimed for its prowess in delving into surface morphology investigation. AFM is potent for analyzing surface characteristics at the scale of micro to nano. It has emerged as a valuable method to explore how inhibitors influence corrosion at the interface of metal and solution [72]. The research delved into the topography of the surface using 2D and 3D imaging, extracting parameters like surface roughness (Rmax), average roughness (Ra), and maximum peak-to-valley height. The study presented diverse AFM parameters for Zn surfaces subjected to different environments. Results demonstrated that immersion without KSE led to the creation of zinc oxides, evidenced by elevated Ra (38.0 nm), Rmax (557.0 nm), and peak valley height (164.3 nm) values, signifying increased roughness. In contrast, when immersed with an inhibitor formulation containing 2.0 g/L KSE, the Zn surface exhibited decreased Ra (10.6 nm) and Rmax (110.0 nm) values, along with a peak-to-valley height 27.9 nm. indicating the protective film formation at surface of the metal. This film contributed to smoother and more homogeneous surfaces, devoid of corrosion product deposits. Assessing the alterations in symmetry around surfaces’ mean line involves measuring roughness skewness, a parameter that demonstrates remarkable sensitivity to irregular high peaks or deep valleys [73]. The attainment of a negative skewness value is instrumental in creating an exceptional bearing surface, as indicated by previous studies [74]. In terms of statistical analysis, roughness kurtosis offers insights into spike distribution above and below the mean line. Regardless of the existence or else the absence of the inhibitor, kurtosis values exceeding 3 denote a prevalence of spiky surfaces, while a value below 3 indicates surfaces with bumps. A value close to 3 suggests a random surface texture [69]. These findings strongly affirm the corrosion resistance capabilities of the KSE inhibitor (Fig. 14, Table 8).
3.10 Mechanism
In the corrosive environment of an HCl electrolyte, Zn undergoes an anodic reaction, where it oxidizes to form zinc ions (Zn+2) and releases two electrons ( \(Zn \to {Zn}^{+2}+ {2e}^{-})\). Being a metallic conductor, Zn readily facilitates the electronic transfer to its cathodic regions. Within this electrolyte, hydrogen ions are also present due to the dissociation of HCl. These hydrogen ions can interact with the free electrons in Zn, leading to the formation of hydrogen gas at the cathodic regions, as expressed by the equation \({2 H}^{+}+ {2e}^{-}\to {H}_{2}\) [75]:
Many plant extracts contain organic molecules that are rich in sulphur (S), nitrogen (N), and oxygen (O) atoms, and the aromatic rings at the chemical structures. The preliminary phytochemical screening of the alcoholic and aqueous extract of C. crista revealed existence of various compounds, including: Tannins, Flavonoids, alkaloids, proteins, carbohydrates, coumarins, and triterpenoids [76]. Furthermore, the plant seeds of Crista were found to be contain the 49% carbohydrate including starch (6.1%), water soluble mucilage (4.4%), and pentosan (16.8%). In addition to these compounds, 4-O-methyl myoinositol hydrate has been isolated through the C. crista that grows in the China as per the study conducted by Chopra in 1993 [77]. According to Umoren et al. [78] The primary confines associated through the utilization of the inhibitors that is derived through the extracts of plants are the complex nature of these extracts, which contain a wide variety of heteroatoms. The complexity makes it challenging to identify a specific molecule which is responsible for the process of the inhibition [79]. KSE naturally comprises a multitude of organic compounds, including the above-mentioned molecules, which can enhance inhibition effectiveness. This is because extracts of the plant are rich in organic compounds that contain heteroatoms [80]. These heteroatoms have the capacity to donate electrons, and these molecules activities are linked to their capability to adsorb onto metal surfaces through the utilization of unshared pair of electrons from O atoms [81]. Moreover, these atoms or the π (pi) electrons that exists at aromatic ring can coordinate with empty orbital of the corroding Zn metal creating a protective layer at the metal’s surface.
4 Conclusion
By “the above forgoing experiments, the mentioned conclusions could be drawn regarding the corrosion inhibition of Zn in 0.05–0.1 M HCl solutions by KSE.
-
KSE effectively inhibits the zinc corrosion in solutions of 0.05-0.10 M HCl, demonstrating its potential as a corrosion inhibitor.
-
The corrosion inhibition is achieved through the adsorption of KSE onto the Zn surface, generating a protective layer that hinders the destructive attack.
-
The inhibition efficiency of KSE rises with the greater inhibitor concentrations, suggesting the possibility of tailoring its performance for corrosion protection needs.
-
KSE works as the mixed-type inhibitor for Zn surface corrosion in 0.05 M solutions of HCl, inhibiting both the cathodic & anodic processes.
-
The KSE adsorption at a surface of zinc from the 0.05 M HCl solutions adheres to the Langmuir and Freundlich adsorption isotherms, implying monolayer adsorption and uniform coverage.
-
SEM micrographs provide graphic validation of the formation of a protective layer on the Zn surface, supporting the inhibitive properties of KSE and its role in protecting the metal from further corrosion reactions”.
-
Additionally, AFM and SEM have been utilized to confirm and support the findings that are attained through mass loss, EIS, and polarization approaches.
-
Consideration of Kanchaki seeds extract as a corrosion inhibitor is recommended, due to their low cost and environmentally friendly attributes. The advantages, including easy application and minimal disruption to industrial processes make plant-based inhibitors, like Kanchaki seeds, an environmentally sustainable and economically viable solution for corrosion control. Before widespread implementation, it is recommended to conduct comprehensive research and testing to ascertain the full potential and effectiveness of Kanchaki seeds in specific industrial applications.
References
Ogoko EC, Odoemelam SA, Ita BI, Eddy NO (2009) Adsorption and inhibitive properties of clarithromycin for the corrosion of Zn in 0. 01 to 0. 05 M H2SO4. Port Electrochim Acta 27:713–724. https://doi.org/10.4152/pea.200906713
Junaedi S, Al-Amiery A, Kadihum A, Kadhum A, Mohamad A (2013) Int J Mol Sci 14(6):1915
Kadhum A, Mohamad A, Hammed L, Al-Amiery A, Hooi San N, Musa A (2014) Materials 7(6):4335
Fouda AS, Abdallah M, Atwa ST, Salem MM (2010) Mod Appl Sci Canada 4(12):41
Hackerman M, Sudbery JD (1950) J Electrochem Soc 97:109
Unni VKV, Ramachar JC (1965) J Electrochem Soc (Japan) 33:557
Vashi RT, Desai SA, Desai PS (2008) Ethylamines as corrosion inhibitors for zinc in nitric acid. Asian J Chem 20:4553
Desai SA, Vashi RT, Desai PS (2008) Toluidines as corrosion inhibitors for zinc in nitric acid. J Ultra Chem 4:27–34
Al-Sehaibani H (2000) Evaluation of extracts of Henna leaves as environmentally friendly corrosion inhibitors for metals. Mat-wiss u Werkstofftech 31:1060–1063. https://doi.org/10.1002/1521-4052(200012)31:12%3c1060::AID-MAWE1060%3e3.0.CO;2-K
Abiola OK, Oforka NC (2003) Corrosion inhibition effect of Cocos nuciferal juice on mild steel in 5% hydrochloric acid solution. Scientia Africana 2:82–90
Ekpe UJ, Ebenso EE, Ibok UJ (1994) Inhibitory action of Azadirachta indica leaves extract on the corrosion of mild steel in H2SO4. JW Afri Sci Assoc 37(3):13–30
Avwiri GO, Igho FO (2003) Inhibitive action of Vernonia amygdalina on the corrosion of aluminium alloys in acidic media. Mater Lett 57:3705–3711. https://doi.org/10.1016/S0167-577X(03)00167-8
Sithuba T, Masia ND, Moema J et al (2022) Corrosion inhibitory potential of selected flavonoid derivatives: electrochemical, molecular⋅⋅⋅Zn surface interactions and quantum chemical approaches. Results Eng 16:100694. https://doi.org/10.1016/J.RINENG.2022.100694
Desai PS, Desai F, Patel A, Parmar B (2023) Anticorrosive properties of Eucalyptus (Nilgiris) leaves extract on 2S grade aluminium in acid solutions. Appl Surf Sci Adv 16:100414. https://doi.org/10.1016/j.apsadv.2023.100414
Sassi W, Msaadi R, Hihn J-Y, Zrelli R (2021) Effect of pyridine as advanced polymeric inhibitor for pure copper: adsorption and corrosion mechanisms. Polym Bull 78:4261–4280. https://doi.org/10.1007/s00289-020-03311-3
Desai PS, Desai FP (2023) An overview of sustainable green inhibitors for aluminum in acid media. AIMS Environ Sci 10:33–62. https://doi.org/10.3934/environsci.2023003
Abdel-Gaber AM, Rahal HT, El-Rifai MS (2021) Green approach towards corrosion inhibition in hydrochloric acid solutions. Biointerface Res Appl Chem 11:14185–14195. https://doi.org/10.33263/BRIAC116.1418514195
Elabbasy HM, Mohamed OA, Fouda AEAS (2021) Artichoke extract as an eco-friendly corrosion inhibitor for zinc in 1 M hydrochloric acid solution. Lett Appl NanoBioScie 10:2655–2679. https://doi.org/10.33263/LIANBS104.26552679
Fouda AS, Rashwan SM, Darwish MM, Arman NM (2018) Corrosion inhibition of Zn in a 0.5 M HCL solution by Ailanthus altissima extract. Port Electrochim Acta 36:309–323. https://doi.org/10.4152/pea.201805309
Sobhi M (2012) Naturally occurring Elettaria cardamomum extract as a corrosion inhibitor for the dissolution of Zinc in 1.0 M HCl. ISRN Corros 2012:1–6. https://doi.org/10.5402/2012/971650
Fouda AS, Mohamed OA, Elabbasy HM (2021) Ferula Hermonis plant extract as safe corrosion inhibitor for Zinc in hydrochloric acid solution. J Bio-Tribo-Corrosion. https://doi.org/10.1007/s40735-021-00570-z
Kolo AM, Idris S, Bamishaiye OM (2018) Corrosion inhibition potential of ethanol extract of Bryophyllum pinnatum leaves for Zinc in acidic medium. Edelweiss Appl Sci Technol 2:18–25. https://doi.org/10.33805/2576.8484.108
Fouda AS, Rashwan SM, Kamel MM, Arman NM (2017) Adsorption and inhibition behavior of Avicennia marina for Zn metal in hydrochloric acid solution. Int J Electrochem Sci 12:11789–11804. https://doi.org/10.20964/2017.12.95
Chauhan JS, Dixit A, Gupta DK (2013) Corrosion Inhibit Zn HCL Nictanthes Plant Extract 1:58–61
Ali AI, Megahed HE, El-Etre MA, Ismail MN (2014) Zinc corrosion in HCl in the presence of aqueous extract of Achillea fragrantissima. J Mater Environ Sci 5:923–930
Mary Anbarasi C, Divya G (2017) A green approach to corrosion inhibition of aluminium in acid medium using azwain seed extract. Mater Today Proc 4:5190–5200. https://doi.org/10.1016/J.MATPR.2017.05.026
Kumpawat N, Chaturvedi A, Upadhyay RK (2012) Study on corrosion inhibition efficiency of stem alkaloid extract of different varieties of holy basil on aluminium in HCl solution. J Korean Chem Soc 56:401–405. https://doi.org/10.5012/jkcs.2012.56.4.401
Zhang M, Guo L, Zhu M et al (2021) Akebia Trifoliate Koiaz peels extract as environmentally benign corrosion inhibitor for mild steel in HCl solutions: integrated experimental and theoretical investigations. J Ind Eng Chem 101:227–236. https://doi.org/10.1016/J.JIEC.2021.06.009
Mandal N, Mandal S, Hazra B et al (2011) Assessment of the antioxidant and reactive oxygen species scavenging activity of methanolic extract of Caesalpinia crista leaf. Evid-based Complement Altern Med 2011:1–11. https://doi.org/10.1093/ecam/nep072
Cheenpracha S, Srisuwan R, Karalai C et al (2005) New diterpenoids from stems and roots of Caesalpinia crista. Tetrahedron 61:8656–8662. https://doi.org/10.1016/J.TET.2005.06.109
Vaidya B (1999) Nighantu part:2. Gujarat Pustakalaya Sahayak Sahkari Mandali Ltd., Vadodara, India, p 767
Jakabová S, Vincze L, Farkas Á et al (2012) Determination of tropane alkaloids atropine and scopolamine by liquid chromatography–mass spectrometry in plant organs of Datura species. J Chromatogr A 1232:295–301. https://doi.org/10.1016/J.CHROMA.2012.02.036
Upadhyay P, Joshi BC, Sundriyal A, Uniyal S (2019) Caesalpinia crista L.: a review on traditional uses, phytochemistry and pharmacological properties. Curr Med Drug Res 3:1–6. https://doi.org/10.53517/cmdr.2581-5008.312019191
Banskota AH, Attamimi F, Usia T et al (2003) Novel norcassane-type diterpene from the seed kernels of Caesalpinia crista. Tetrahedron Lett 44:6879–6882. https://doi.org/10.1016/S0040-4039(03)01722-2
Linn TZ, Awale S, Tezuka Y et al (2005) Cassane- and norcassane-type diterpenes from Caesalpinia crista of Indonesia and their antimalarial activity against the growth of Plasmodium falciparum. J Nat Prod 68:706–710. https://doi.org/10.1021/np0401720
Desai NS, Malik GM (2016) Inhibition study of Caesalpinia crista on corrosion of mild steel in sulphuric acid. J Appl Chem (Lumami India) 5:1226–1235
Guillen MD, Cabo N (1998) Methods for monitoring composition and flavor quality of cheese using a rapid spectroscopic method. Food Chem 46(5):1788–1793
Yang H, Irudayaraj J, Paradkar MM (2005) Discriminant analysis of edible oils and fats by FTIR, FT-NIR and FT-Raman spectroscopy. Food Chem 93:25–32. https://doi.org/10.1016/J.FOODCHEM.2004.08.039
Ahmed MK, Daun JK, Przybylski R (2005) FT-IR based methodology for quantitation of total tocopherols, tocotrienols and plastochromanol-8 in vegetable oils. J Food Compos Anal 18:359–364. https://doi.org/10.1016/J.JFCA.2003.12.008
Coates J, Meyers RA (2000) Encyclopaedia of analytical chemistry. John Wiley & Sons, Chichester
El-Katori EE, Al-Mhyawi S (2019) Assessment of the Bassia muricata extract as a green corrosion inhibitor for aluminum in acidic solution. Green Chem Lett Rev 12:31–48. https://doi.org/10.1080/17518253.2019.1569728
Sanyal B, Nigam RS (1970) Labdev J Sci Tech 8:120
Putilova IN (1960). In: Barannik VP, Balezin SA (eds) Metallic corrosion inhibitors. Pergamon Press, Oxford, pp 30–32
Muthukrishnan P, Jeyaprabha B, Prakash P (2017) Adsorption and corrosion inhibiting behavior of Lannea coromandelica leaf extract on mild steel corrosion. Arab J Chem 10:S2343–S2354. https://doi.org/10.1016/J.ARABJC.2013.08.011
Noor E (2008) Comparative study on the corrosion inhibition of mild steel by aqueous extract of Fenugreek seeds and leaves in acidic solution. J Eng Appl Sci 3:23–30
Saliyan VR, Adhikari AV (2008) Inhibition of corrosion of mild steel in acid media by N’-benzylidene-3- (quinolin-4-ylthio) propanohydrazide. Bull Mater Sci 31:699–711. https://doi.org/10.1007/s12034-008-0111-4
Yadav DK, Chauhan DS, Ahamad I, Quraishi MA (2013) Electrochemical behavior of steel/acid interface: adsorption and inhibition effect of oligomeric aniline. RSC Adv 3:632–646. https://doi.org/10.1039/C2RA21697C
Li X, Deng S, Fu H (2011) Three pyrazine derivatives as corrosion inhibitors for steel in 1.0 M H2SO4 solution. Corros Sci 53:3241–3247. https://doi.org/10.1016/J.CORSCI.2011.05.068
Bhajiwala HM, Vashi RT (2001) Ethanolamine, diethanolamine and triethanolamine as corrosion inhibitors for zinc in binary acid mixture [HNO3 + H3PO4]. Bull Electrochem 17:441–448
Delahy P (1965) Double layer and electrode kinetics. Interscience Publishers, Division of John Wiley & Sons, Inc, New York
Ekemini B, Ituen, Udo UE (2012) Phytochemical profile, adsorptive and inhibitive behaviour of Costus afer extracts on aluminium corrosion in hydrochloric acid. Der Chem Sin 3:1394–1405
Khaled KF, Hackerman N (2003) Investigation of the inhibitive effect of ortho-substituted anilines on corrosion of iron in 1 M HCl solutions. Electrochim Acta 48:2715–2723. https://doi.org/10.1016/S0013-4686(03)00318-9
Nnanna LA, Nwadiuko O C, Ekekwe D et al (2012) Adsorption and inhibitive properties of leaf extract of Newbouldia leavis as a green inhibitor for aluminium alloy in H2SO4. Am J Mater Sci 1:143–148. https://doi.org/10.5923/j.materials.20110102.24
Ating EI, Umoren SA, Udousoro II et al (2010) Leaves extract of Ananas sativum as green corrosion inhibitor for aluminium in hydrochloric acid solutions. Green Chem Lett Rev 3:61–68. https://doi.org/10.1080/17518250903505253
Ebenso EE (2003) Effect of halide ions on the corrosion inhibition of mild steel in H2SO4 using methyl red, part 1. Bull Electrochem 19(5):209–216
Oguzie EE, Li Y, Wang FH (2007) Effect of 2-amino-3-mercaptopropanoic acid (cysteine) on the corrosion behaviour of low carbon steel in sulphuric acid. Electrochim Acta 53:909–914. https://doi.org/10.1016/J.ELECTACTA.2007.07.076
Laamari MR, Benzakour J, Berrekhis F et al (2016) Adsorption and corrosion inhibition of carbon steel in hydrochloric acid medium by hexamethylenediamine tetra(methylene phosphonic acid). Arab J Chem 9:S245–S251. https://doi.org/10.1016/j.arabjc.2011.03.018
Hamani H, Douadi T, Daoud D et al (2017) 1-(4-Nitrophenylo-imino)-1-(phenylhydrazono)-propan-2-one as corrosion inhibitor for mild steel in 1 M HCl solution: weight loss, electrochemical, thermodynamic and quantum chemical studies. J Electroanal Chem 801:425–438. https://doi.org/10.1016/j.jelechem.2017.08.031
Emregül KC, Abdülkadir Akay A, Atakol O (2005) The corrosion inhibition of steel with Schiff base compounds in 2 M HCl. Mater Chem Phys 93:325–329. https://doi.org/10.1016/J.MATCHEMPHYS.2005.03.008
Şafak S, Duran B, Yurt A, Türkoĝlu G (2012) Schiff bases as corrosion inhibitor for aluminium in HCl solution. Corros Sci 54:251–259. https://doi.org/10.1016/J.CORSCI.2011.09.026
Khaled KF (2008) Application of electrochemical frequency modulation for monitoring corrosion and corrosion inhibition of iron by some indole derivatives in molar hydrochloric acid. Mater Chem Phys 112:290–300. https://doi.org/10.1016/J.MATCHEMPHYS.2008.05.056
Behpour M, Ghoreishi SM, Mohammadi N et al (2010) Investigation of some Schiff base compounds containing disulfide bond as HCl corrosion inhibitors for mild steel. Corros Sci 52:4046–4057. https://doi.org/10.1016/J.CORSCI.2010.08.020
Quraishi MA, Rawat J (2002) Inhibition of mild steel corrosion by some macrocyclic compounds in hot and concentrated hydrochloric acid. Mater Chem Phys 73:118–122. https://doi.org/10.1016/S0254-0584(01)00374-1
Raghavendra N, Bhat JI (2016) Natural products for material protection: an interesting and efficacious anticorrosive property of dry arecanut seed extract at electrode (aluminum)–electrolyte (hydrochloric acid) interface. J Bio-Tribo-Corros. https://doi.org/10.1007/s40735-016-0051-2
Galal A, Atta NF, Al-Hassan MHS (2005) Effect of some thiophene derivatives on the electrochemical behavior of AISI 316 austenitic stainless steel in acidic solutions containing chloride ions: I. Molecular structure and inhibition efficiency relationship. Mater Chem Phys 89:38–48. https://doi.org/10.1016/J.MATCHEMPHYS.2004.08.019
Yadav AP, Nishikata A, Tsuru T (2004) Electrochemical impedance study on galvanized steel corrosion under cyclic wet–dry conditions––influence of time of wetness. Corros Sci 46:169–181. https://doi.org/10.1016/S0010-938X(03)00130-6
Haddadi SA, Alibakhshi E, Bahlakeh G et al (2019) A detailed atomic level computational and electrochemical exploration of the Juglans regia green fruit shell extract as a sustainable and highly efficient green corrosion inhibitor for mild steel in 3.5 wt% NaCl solution. J Mol Liq 284:682–699. https://doi.org/10.1016/J.MOLLIQ.2019.04.045
Udensi SC, Ekpe OE, Nnanna LA (2020) Newbouldia laevis leaves extract as tenable eco-friendly corrosion inhibitor for aluminium alloy AA7075-T7351 in 1 M HCl corrosive environment: gravimetric, electrochemical and thermodynamic studies. Chem Afr 3:303–316. https://doi.org/10.1007/s42250-020-00131-w
Desai PS, Desai F, Patel A, Parmar B (2023) Thorn apple (Datura stramonium) extract acts as a sustainable corrosion inhibitor for zinc alloy in hydrochloric acid solutions, Results in Surfaces and Interfaces. https://authors.elsevier.com/tracking/article/details.do?aid=100176&jid=RSURFI&surname=Desai
Preethi Kumari P, Shetty P, Rao SA (2017) Electrochemical measurements for the corrosion inhibition of mild steel in 1 M hydrochloric acid by using an aromatic hydrazide derivative. Arab J Chem 10:653–663. https://doi.org/10.1016/j.arabjc.2014.09.005
Chellouli M, Chebabe D, Dermaj A et al (2016) Corrosion inhibition of iron in acidic solution by a green formulation derived from Nigella sativa L. Electrochim Acta 204:50–59. https://doi.org/10.1016/j.electacta.2016.04.015
Satapathy AK, Gunasekaran G, Sahoo SC et al (2009) Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros Sci 51:2848–2856. https://doi.org/10.1016/j.corsci.2009.08.016
Chaubey N, Singh VK, Quraishi MA (2018) Papaya peel extract as potential corrosion inhibitor for aluminium alloy in 1 M HCl: electrochemical and quantum chemical study. Ain Shams Eng J 9:1131–1140. https://doi.org/10.1016/j.asej.2016.04.010
Rajesh Kumar B, Subba Rao T (2012) AFM studies on surface morphology, topography and texture of nanostructured zinc aluminium oxide thin films. Dig J Nanomater Biostruct 7:1881–1889
Fontana MG (1986) Corrosion engineering, 3rd edn. McGraw-Hill Book Company
Afrin S, Pervin R, Sabrin F et al (2016) Assessment of antioxidant, antibacterial and preliminary cytotoxic activity of chloroform and methanol extracts of Caesalpinia crista L. leaf. Bangladesh J Bot 45:1061–1068
Chopra RN (1933) Indigenous drugs of India, 1st edn. The Art Press, Calcutta
Umoren SA, Solomon MM, Obot IB, Suleiman RK (2019) A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J Ind Eng Chem 76:91–115. https://doi.org/10.1016/J.JIEC.2019.03.057
Onukwuli OD, Anadebe VC, Nnaji PC et al (2021) Effect of pigeon pea seed (isoflavone) molecules on corrosion inhibition of mild steel in oilfield descaling solution: electro-kinetic, DFT modelling and optimization studies. J Iran Chem Soc 18:2983–3005. https://doi.org/10.1007/s13738-021-02250-8
Anadebe VC, Nnaji PC, Okafor NA et al (2021) Evaluation of bitter kola leaf extract as an anticorrosion additive for mild steel in 1.2 m H2SO4 electrolyte. S Afr J Chem 75:6–17. https://doi.org/10.17159/0379-4350/2021/v75a2
Desai PS, Vashi RT (2011) Inhibitive efficiency of sulphathiazole for aluminium corrosion in trichloroacetic acid. Anti Corros Methods Mater 58:70–75. https://doi.org/10.1108/00035591111110714
Acknowledgements
The authors appreciate the lab and library resources provided by the Arts Science and Commerce College’s Chemistry Department in Kholwad, Surat, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Desai, P.S., Parmar, B.B., Desai, F.P. et al. Caesalpinia Crista (Kanchaki) as Green Corrosion Inhibitor for Zinc in Hydrochloric Acid Solutions. Chemistry Africa 7, 2173–2187 (2024). https://doi.org/10.1007/s42250-023-00874-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00874-2