Abstract
This study assessed the contamination and human and ecological risk of potentially toxic elements in soils from automobile workshops in Asante Mampong, Ghana. Concentrations were determined using a Varian Spectra AA220 Zeeman atomic absorption spectrophotometer. Mean potentially toxic elements concentrations in soils from the automobile workshop Centre had the order: Hg < Cd < Cr < As < Pb < Mn < Cu < Zn < Fe. The mean concentrations of Cd and Pb exceeded the EU guideline limit for Cd (0.35 mg/kg) and Pb (7.2 mg/kg) in soils whereas the mean Hg content was lower than the soil quality criteria limit of 0.3 mg/kg set to protect fauna and flora. The study revealed that the automobile workshop centre is moderately polluted with Cd and highly contaminated with Zn. The Cd levels recorded could pose considerable risks to the ecological environment whilst the other PTEs pose no ecological risks. Cd showed a strong correlation with Pb, Mn and As. Principal Component analysis classified the PTEs into two groups with their sources being the natural geology and vehicle repair activities undertaken at the centre. The concentrations of PTEs measured are not likely to pose non-carcinogenic adverse health effects as the hazard quotients and hazard index computed are all less than unity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Heavy metal pollution in air and soil is of great concern because of the dangers posed to workers, inhabitants and the environment [1,2,3]. Therefore, the inhabitants, including children and numerous workers residing and working in these polluted environments, are at serious risk of heavy metals toxicity. Greater awareness of this issue is needed to help reduce the incidence of heavy metal intoxication among inhabitants [4,5,6]. Heavy metals do not contaminate only the surface environment but also contribute to air pollution, as they may become airborne and also enter drainage systems to affect aquatic ecosystems [7].
In general, the presence of heavy metals in high concentrations in the environment has resulted in health hazards such as adverse effects on the nervous system, blood formation, and renal and reproductive systems [8]. Other effects include; reduced intelligence, attention deficit and behavioural abnormality. Heavy metals have also contributed to cardiovascular diseases in adults and children [9]. For children, ingestion of contaminated soil is found to be the most significant pathway into the body [10].
Soils may become contaminated by heavy metals due to accumulation from auto-mechanic workshops during their daily operations [11, 12]. The activities at auto-mechanic workshops include electrical repair of automobiles, repair of brakes and steering, automatic or standard transmission engine, spray painting of vehicles, recharging of auto batteries, welding and soldering among others. Where such activities are not properly monitored and regulated, they may lead to higher levels of heavy metals in the environment that may affect quality of food production. Therefore, there is a need to continually monitor their nature, volume, harmful effects and current methods of disposal as well as potential impacts on the environment [13].
Gyimah et al. [12] and Asamoah et al. [28] have studied potentially toxic elements contamination in soils of vicinities of auto-mechanic workshops in two different regions of Ghana. Auto-mechanic workshops have sprung up in many parts of Asante Mampong as clusters in open ground. Wastes such as metal scraps, used batteries, worn-out vehicle parts generated by auto-mechanic workshops and their activities are dumped indiscriminately into the environment. Asante Mampong is a major contributor to food production in Ghana. No study has investigated the extent of potentially elements contamination in the soils of vicinities of auto-mechanic workshops in the Asante Mampong municipal and their human health risks. The present study aims to determine the levels of toxic elements in soil from different artisan locations within the Asante Mampong auto-mechanic workshop centre; identify the possible sources of toxic elements as well as assess the environmental and human health risk of the toxic elements from the investigated area.
This research is intended to raise awareness of the issue of heavy metal contamination at auto-mechanic workshop sites and find out the contributions from known activities such as vehicular body works and welding, repair of lead-acid batteries, vehicle paint spraying, vehicle engine repair and food vending locations within the auto-mechanic workshop center. Assessing the levels of heavy metal pollution at the auto-mechanic workshops would also help establish the environmental and health impact of these metals at the workshops.
2 Materials and Methods
2.1 Study Area
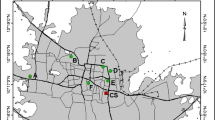
The study was carried out at the Asante Mampong auto-mechanic workshop center in the Ashanti Region of Ghana. Asante Mampong is the administrative capital of the Asante-Mampong Municipality and it is about 57 km north of Kumasi. The municipality is located within longitudes 0° 05″ W and 1° 30″ W and latitudes 6° 55″ N and 7° 30″ N, covering a total land area of about 23.9 km2. The population of the Municipality according to the 2021 Population and Housing Census stands at 116,632 with 56,965 (48.84%) males and 59,667 (51.16%) females [14].
The Asante Mampong auto-mechanic workshop center (Fig. 1) is made up of a cluster of small-scale workshops with a specialty in vehicle body works and welding, vehicle upholstery, repair of lead-acid battery, vehicle paint spraying, vehicle engine repair, sale of vehicular spare-parts, vulcanizing work and women who sell food items in the vicinity of these workshops.
2.2 Sampling
Forty-eight (48) composite soil samples were collected from three workshops belonging to each of the following categories: Vehicle Body Works (VBW), Upholstery Work (UPW), Battery Repair Work (BRW), Vehicle Spraying Work (VSW), Food Vending Area (FVA), Engine Repair Work (ERW), Sale of Automobile Parts (SAP), and Vulcanizing Work (VCW). Two composite soil samples were collected from each of the two parts of three workshops belonging to a particular category. The two parts in each workshop are the activity area and rest area. Five different samples were collected from each area and combined to form a composite. The control was collected at the AAMUSTED campus for data comparison and to evaluate the extent of heavy metal pollution and enrichment in soils at the workshops studied. The soil samples were collected at (0–0.20 m depth) at the selected location. The soil samples were collected with a plastic spoon, placed in sealed polythene bags and transported to the laboratory of the Soil Research Institute of the Council for Scientific and Industrial Research (CSIR-SRI), Kumasi, Ghana for elemental analysis. The plastic spoon was washed with distilled water and wiped dry after every sample to prevent cross-contamination.
2.3 Preparation and Analysis of Samples
2.3.1 Preparation of Samples
The soil samples were air-dried for 6 days at room temperature, homogenized and made lump free by gently crushing repeatedly using an acid-pre-washed mortar and pestle. The samples were then sieved using a 2 mm plastic nylon mesh sieve to remove coarse soil fraction prior to the analysis.
2.3.2 Analysis of Soil Samples
One gram of the dried fine soil sample was weighed and transferred into an acid washed 100 mL volumetric flask. A 10 mL mixture of HNO3, H2SO4 and HClO4 (9:4:1 v/v) was added. The mixture was slowly evaporated at 90 °C for one hour on a hot plate. The flask was placed on a hot plate in the fume hood and heated at the temperature range of 90–200 °C until the fumes of HClO4 were completely evaporated. The mixture was allowed to cool to room temperature and then filtered using Whatman No.42 filter paper into a 50 mL volumetric flask. The reacting vessels were rinsed to recover any residual metal. The filtrate was then made up to the mark by adding deionized water. Heavy metals concentrations were determined using a Varian Spectra AA220 Zeeman Atomic Absorption Spectrophotometer (ZAAS) [15] at the Council for Scientific and Industrial Research-Soil Research Institute (CSIR-SRI), Kumasi, Ghana.
The instrument was calibrated using standard solutions of the respective metals. Blanks were analyzed after every 10 samples. Certified Reference Materials (Channel BCR-320R Sediment) provided by Institute for Reference Materials and Measurements, Belgium) was used to validate the method. Replicate analysis of the reference material showed good accuracy and recovery rates which ranged from 85 to 112%.
2.4 Quantification of Soil Pollution
2.4.1 Geoaccumulation Index
Igeo, a geochemical criterion introduced by [16], was used to evaluate soil contamination by comparison with current and preindustrial concentrations. Unlike other methods of pollution assessment, Igeo takes the natural diagenetic process into account, which makes its assessments more realistic. Igeo is calculated using the following equation:
where Cn is the measured concentration of the potentially toxic elements in soil (mg/kg), Bn is the geochemical background value of the corresponding potentially toxic element (mg/kg), and the coefficient 1.5 is used due to potential variation in the baseline data [17]. According to [16], Igeo values fall into seven classes. The corresponding relationships between Igeo and the pollution level are listed (Table 1).
2.4.2 Contamination Factor (CF)
The contamination factor reflects the extent of anthropogenic input in elemental pollution [18]. It is widely used to measure the overall PTE contamination of soil. Mathematically, it is expressed as:
where Cmetal is the concentration of the metal examined in soil samples and Cbackground value is the geochemical background concentration of the metal. The index ranges from CF < 1 refers to low contamination; 1 < CF < 3 indicates moderate contamination; 3 < CF < 6 indicates considerable contamination and CF > 6 indicates very high contamination [19, 20].
2.4.3 Ecological Risk Assessment Method
The method introduced by Hakanson was adopted to assess the ecological risks posed by potentially toxic element pollution in the topsoil of the study area. Hakanson [20] developed the following quantitative approach, and this method was used in many other studies to assess the ecological risks of potentially toxic elements in soils [19, 21]. The potential ecological risk factor of a given contaminant (E(i)) is defined as:
where Ti is the toxic-response factor for a given substance, Ci represents potentially toxic element content in the topsoil, and C0 is the regional background potentially toxic element content of topsoil [22]. The sum of the individual potential risk factors (EI(i)) is the potential ecological risk index (RI), which represents the potential risk in a region. RI can be expressed as:
2.5 Human Health Risk Assessment
Human Health Risk posed by Potentially Toxic Elements (PTEs) was estimated using the model recommended by the United States Environmental Protection Agency [23]; non-carcinogenic risk and carcinogenic risk were considered in this method. Non-carcinogenic risks for adults and children were considered in this study. Ingestion, inhalation, and dermal contacts are three pathways by which humans can be exposed to risk. The average daily exposure dose (ADD) of PTE is considered was calculated using Eqs. 5, 6 and 7 respectively.
where ADDing, ADDinh, and ADDderm are the average daily exposure doses of PTEs for the three exposure pathways (ingestion of soil and food, air inhalation and dermal absorption respectively); C is the PTE concentration in surface soils; IngR is the ingestion rate (mg/d); InhR is the inhalation rate (m3/d); CF is a conversion factor (10–6, kg/mg); EF is the exposure frequency (d/a); ED is the exposure duration (a); BW is the average body weight (kg); SA is the exposed skin area (cm2); SL is the skin adherence factor (mg/(cm2 d)); PEF is the particle emission factor (m3/kg); ABS is the dimensionless dermal absorption factor; AT is the average time (d); and ED × 365 days for non-carcinogenic risk and 72 × 365 days for carcinogenic risk. The detailed reference values of these parameters are given in Table 2.
For non-carcinogenic risk, the hazard index (HI) is the summation of the hazard quotients (HQ), the total hazard index (THI) is the summation of the HI of potentially toxic elements considered, and HQ is the quotient of ADD to its associated toxicity threshold (dose (RfD, mg/(kg d)).
When HQ < 1 or HI < 1, the non-carcinogenic risk is negligible. HI and HQ were calculated using Eqs. 7 and 8 respectively.
For carcinogenic risk, the total carcinogenic risk (TCR) is the summation of the carcinogenic risk (CR) of PTEs, and the CR and TCR were calculated using Eqs. 10 and 11 respectively
where CSF represents cancer slope factor (mg/(kg d)) [7]. If CR < 1 × 10–6, carcinogenic risk is negligible. Reference values of RfD and CSF are indicated in Table 3. This study considered Cd, Cr, Pb and As due to their potential carcinogenicity.
3 Results and Discussion
3.1 Potentially Toxic Elements (PTEs) Distribution
The levels of PTEs assessed in this study varied across the various locations in the study area (Table 4).
The concentration of Cd ranged from 0.14 mg/kg in the FVA to 0.92 mg/kg in the VBW. The maximum Cd level in the soil exceeds the world average Cd level of 0.41 mg/kg [24]. The Cd levels in this study are higher than levels of Cd recorded in automobile mechanic workshops in Benin City (0.00–0.05 mg/kg), Nigeria [25]. Cadmium levels recorded in VBW (0.92 ± 0.31 mg/kg), BRW (0.74 ± 0.15 mg/kg), VSW (0.56 ± 0.08 mg/kg) and ERW (0.43 ± 0.14 mg/kg) are comparable with levels (0.38–0.91 mg/kg) reported in [12] in soils from auto-mechanic workshops in Tarkwa, Ghana. These levels also exceed the EU standard for soils of 0.35 mg/kg [12]. The concentration of Hg was lowest (0.08 mg/kg) in the FVA and highest (0.31 mg/kg) at BRW. It is worthy of note that the lowest level exceeded the average background value (0.004 mg/kg) of mercury in Ghana [26]. The lowest value of Pb (4.22 mg/kg) in this study was recorded at the VCW whereas the highest value (40.63 mg/kg) occurred at the BRW. The Pb levels far exceed the range of values reported by [25] in Nigeria (0.21–13.60 mg/kg) and [27] in Asafo auto-mechanic workshop in Kumasi, Ghana (3.65 mg/kg). The Pb levels are however lower than levels (0.54–501.10 mg/kg) reported by [28] in soils from auto-mechanic workshops from Sunyani in Ghana. The mean and maximum levels of Pb in this study are higher than the EU standard of Pb (7.2 mg/kg) permitted in soils [12]. The minimum Fe concentration (105.61 mg/kg) occurred at the BRW while the highest (508.68 mg/kg) at the VBW. Although Fe is naturally abundant in the Earth’s crust, levels of Fe recorded in all the sampled sites were significantly higher than in the control sample. Irrespective of the possible geogenic source of Fe in soils at Mampong, activities such as metal casting, welding and fabrication could be responsible for the elevated Fe levels. The levels of Fe in this study are below that reported in Tarkwa (39,031–52,084 mg/kg) [12] and Suame Magazine (2050–2350 mg/kg) [29]. In this study, the concentration of Cu ranged from 36.84 mg/kg in VCW soil to 114.39 mg/kg in ERW soil and showed a marked difference with the control sample. The levels recorded are higher than Cu levels in soils of auto mechanic workshops in Benin City, Nigeria (0.00–10.00 g/kg) [25] and Asafo auto mechanic workshop in Kumasi, Ghana (1.84 mg/kg) [27]. Again, this study reports high Cu levels than what is reported by [28]. This may be due to the release of copper connecting wires and rusted materials that contains Cu into the soil.
Zinc showed its lowest level (126.44 mg/kg) at the vulcanizing workshop whereas the highest (467.55 mg/kg) appeared at the vehicle body workshop. The maximum Zn content in this study exceeds the permissible guideline value by the Canadian Council of Ministers of the Environment (200 mg/kg) [30] and Dutch Target (140 mg/kg) [31]. Zinc in auto-mechanic workshops may come from mechanical abrasion of vehicular parts, motor oils and worn-out vehicle body parts. Zinc showed higher levels in this study than Cu reported by [25] in Nigeria and [28] in Ghana. Zinc and copper are essential elements however, elevated levels can cause important side effects such as abdominal cramps, anaemia, headaches, metabolic disorders, and liver and kidney damage [32].
The lowest Mn level (9.34 mg/kg) occurred at the area automobile spare parts shop whereas the highest (154.12 mg/kg) occurred at the vehicle body workshop. The Cr content is lowest (0.61 mg/kg) at the vulcanizing workshop and highest (1.52 mg/kg) at the vehicle body workshop. The levels are far lower than the permissible Cr content in soils set by international agencies. The auto-mechanic workshops and others dealing with steel and metal plating in the area could serve as a possible source of the metal. Chromium is a metal with low mobility especially close to neutral pH values where there are moderate oxidizing and reducing conditions. Arsenic was lowest (0.68 mg/kg) at the sales area and highest (2.13 mg/kg) at the battery repair workshop. The As contents could originate from other anthropogenic activities observed around the study area and were associated with metallurgical and chemical activities as indicated by [24]. Most of the metals analysed recorded higher mean concentrations at the VBW station. This is expected because the body of vehicles is mostly made of metallic substances. The repair works on the body of vehicles and worn-out body parts normally results in the release of metals into the soil.
3.2 Pollution Estimation
The extent of soil pollution was estimated using pollution indices (Table 5). The Index of geoaccumulation assesses the level of elemental accumulation in soils against a background concentration. The Igeo measured for Cd were below 1. This indicates that the soils are unpolluted with Cd except those sampled from VBW (Igeo of 1.032) were moderately polluted with Cd. The study area is unpolluted with respect to Pb, Cu, Mn, Cr, As and Hg. The estimated Igeo values in respect of Pb, Cu, Mn, Cr, As and Hg are all less than 1. Zn recorded Igeo of 1.17, 1.14 and 1.10 at VBW, BRW and FVA sites respectively. These show VRW, BRW and FVA environments were moderately polluted with Zn. The other sites viz VPW, VSW, ERW, SAP and VCW are unpolluted with Zn. Cadmium and Zn recorded the highest CF of 3.06 and 4.92 respectively for VBW. Thus, with respect to Cd and Zn, the site (VBW) was highly contaminated.
Except UPW, the other locations in the study area recorded CF values between 1 and 3 for Cu. This indicate that these sites were moderately contaminated with Cu. Similarly, Pb recorded the highest CF of 2.03 and 1.14 at BRW and VBW respectively. These also indicate sites BRW and VBW are moderately contaminated with Pb.
The ecotoxicological impacts were assessed using the potential ecological risks factor and index [33, 34]. Cadmium concentrations detected pose a varied risk to the ecological environment. Approximately 13% of the sites sampled pose considerable risk to the ecological environment with ER value of 92. Thirty-seven per cent of the investigated sites would experience moderate ecological risk. Half of the sites would experience low ecological risk with respect to Cd. All the other elements would pose a low risk to the ecological environment as they all recorded ER values of less than 40.
3.3 Correlation Analysis of PTEs
Correlation analysis when applied to environmental studies helps to reveal the relationship that exists between multiple variables. The correlation matrix is presented in Table 6. In this study, correlation coefficients > 0.8, 0.6 < r < 0.8 and < 0.6 were considered strong, moderate and weak correlations respectively as indicated in a study by [35]. Generally, the geology of an area plays a significant role in defining its environmental chemistry [36]. Chemical ions including many metallic ions in an environment are attributed to geogenic origins. However, the levels of potentially toxic elements in the control sample used in this study suggest that the heavy metals could not be linked to geogenic origins, as there are no geologic deposits rich in the analysed PTEs. Cd correlated strongly with Mn and As. Though Cr has a strong correlation with Cu and Zn it had a moderate correlation with As and Mn. Fe also had a moderate correlation with Cr and weak correlations with Hg, Pb, Cu, Zn, Mn and As. Pb showed a strong correlation with Cd and As whereas it moderately correlated with Hg. Although Fe contents of the soils were low, its weak correlation with Hg, Pb, Cu, Mn, and As except Zn and Cr suggests that its presence in the soil did not originate from the same source as that from where Hg, Pb, Cu, Mn, and As originated. The origins of the potentially toxic elements in the soils may be attributed to activities at the automobile mechanic workshops such as engine repairs, industrial plants, Pb-acid batteries, car body spraying and air conditioning coolants replacements.
3.4 Principal Component Analysis of Potentially Toxic Elements
Principal Component Analysis (PCA) was employed to investigate the association between the analysed PTEs and the sampled sites. This multivariate analytical tool helped to identify the possible sources of PTEs contamination in the soils at the studied environment. Principal components were extracted with eigenvalues > 1 through Varimax rotation. Two principal components with a total variance of 85.8%. As is indicated in Fig. 2 and Table 7, PC1 is explained by high loadings of Fe, Cu, Zn, Cr and Cd contributed a variance of 69.4% to the total variance. This indicates that Fe, Cu, Zn, Cr and Cd emerged from the local geology. Very high Fe loading suggest that it may have originated from a source other than that from where Hg, Pb, Cu, Zn, Mn, Cr and As may have been into the environment. These sources could be scrap metal and metal fabrication activities done at the auto-mechanic workshops. PC2 is positively loaded with Pb, Hg, As, Mn and Cd. This account for 16.4% of the variance. Pb and Hg had high loadings and could be released from Lead-acid battery recharging and repair activities observed in the study sites. Cd, Mn and As also had positive loadings in the PC2. Thus vehicular repair, maintenance, welding, and fabrication, waste electrical and electronic equipment may be responsible for elevated levels of Cd, Mn and As recorded.
3.5 Human Health Risk Assessment of Toxic Metals
Carcinogenic and non-carcinogenic risks estimated are presented in Tables 8 and 9. The assessment revealed that none of the PTEs poses health risk to workers as well as the inhabitants near the auto-mechanic workshops. The hazard quotient and hazard index computed were all below unity Table 8. However, this does not eliminate all risk of the exposure to heavy metals. Thus, the disposal of used vehicle parts should be properly managed and activities regulated to minimize release of PTEs into the soil. In all instances, children are exposed to higher risks than adults because the hazard quotients and indices computed were all higher for children than adults in all the pathways considered. The dermal contact pathway is the largest contributor to the overall risk followed by the ingestion pathway. This leads to an additional concern for children as they are more likely to play in the soil as well as practice hand to mouth activities.
In this study, As, Cd, Cr and Pb were assessed for their carcinogenic risk. As recorded total cancer risk range of 3.53 × 10–6 at SAP to 1.10 × 10–5 at VBW and 2.02 × 10–6 at SAP to 6.34 × 10–6 at VBW for children and adults respectively (Table 9). These levels are likely to pose significant cancer risk to both children and adults near the automobile workshops. From Table 9 it is inferred that Cd, Cr and Pb would pose no significant cancer risk because the total cancer risk recorded for these three metals were all below 1 × 10–6 which indicate acceptable cancer risk. Since children are more susceptible to adverse health effects because of less developed immune system it is not advisable to allow children to play in the soils near the automobile workshops (Table 9).
4 Conclusion
The study investigated concentration of Cd, Hg, Pb, Fe, Cu, Zn, Mn, Cr and As and their pollution profiles in soils from the auto-mechanic workshops in the Asante-Mampong of the Ashanti region of Ghana. Their ecological and human health risk were also evaluated. The study revealed that the PTEs recorded occurred at higher levels than those found in the control soil. Levels of investigated metals also exceeded guideline values prescribed by international regulatory agencies such as the European Union and the World Health Organization. Mean concentration of Pb and Cd were higher than their European Union limit of 7.2 mg/kg and 0.35 mg/kg respectively. The study revealed that the automobile workshop Centre is moderately polluted with Cd (contamination factor of 3.06) and highly contaminated with Zn (contamination factor of 4.09). Cadmium poses high risk to the ecological environment with risk factor greater than 92 whereas all other analysed PTEs pose low risk to the ecological environment. The levels of PTEs analysed are not likely to pose adverse health effects to humans at the time of the study because the HQs are less than unity. However, these PTEs are persistent and as such regular monitoring is necessary to ensure they do not accumulate to toxic levels. PTEs in soils can move to other spheres of the environment through leaching, run-off and evasion where they may enter aquatic ecosystem and the food chain to cause adverse health effects on organisms. The District Assemblies and the Environmental Protection Agency should regulate the activities of the artisanal auto-mechanics in order to institute measures to reduce pollution to the environment.
Data Availability
The data is found in the manuscript or immediately available upon request from the authors.
References
Gong C, Lu H, Zhang Z et al (2022) Spatial distribution characteristics of heavy metal (loid) s health risk in soil at scale on town level. Sci Rep. https://doi.org/10.1038/s41598-022-20867-4
Ghaderi A, Mohammadzadeh M, Banafshe HR et al (2023) The carcinogenic and non-carcinogenic risk assessment of heavy metals from the butts of smoked and non-smoked cigarettes. Hum Ecol Risk Assess Int J 29:187–201. https://doi.org/10.1080/10807039.2022.2150598
Mahmudiono T, Hoseinvandtabar S, Mehri F et al (2023) Potentially toxic elements ( PTEs ) in coastal sediments of Bandar Abbas city, North of Persian Gulf: an ecological risk assessment. Int J Environ Health Res. https://doi.org/10.1080/09603123.2023.2173154
Gong C, Wang S, Wang D et al (2022) Ecological and human health risk assessment of heavy metal (loid) s in agricultural soil in hotbed chives hometown of Tangchang, Southwest China. Sci Rep. https://doi.org/10.1038/s41598-022-11397-0
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem 2019:14. https://doi.org/10.1155/2019/6730305
Yuan C, Wang X (2023) Source apportionment and health risk assessment of heavy metals in soils of old industrial areas—a case study of Shanghai, China. Int J Environ Res Public Health 20:17. https://doi.org/10.3390/ijerph20032395
Li Y, Zhou S, Jia Z et al (2021) Temporal and spatial distributions and sources of heavy metals in atmospheric deposition in western Taihu Lake, China. Environ Pollut 284:117465. https://doi.org/10.1016/j.envpol.2021.117465
Gyamfi O, Borgen P, Darko G, Ansah E (2020) Human health risk assessment of exposure to indoor mercury vapour in a Ghanaian artisanal small-scale gold mining community. Chemosphere. https://doi.org/10.1016/j.chemosphere.2019.125014
Christoforidis A, Stamatis N (2009) Heavy metal contamination in street dust and roadside soil along the major national road in Kavala’s region, Greece. Geoderma 151:257–263. https://doi.org/10.1016/j.geoderma.2009.04.016
Moya J, Phillips L (2014) A review of soil and dust ingestion studies for children. J Expo Sci Environ Epidemiol 24:545–554. https://doi.org/10.1038/jes.2014.17
Konwuruk N, Sheringham L, Darko G, Dodd M (2021) Distribution, bioaccessibility and human health risks of toxic metals in peri-urban topsoils of the Kumasi Metropolis. Sci African 11:e00701. https://doi.org/10.1016/j.sciaf.2021.e00701
Gyimah E, Nana G, Gyimah W (2022) Ecological and human risk assessments of heavy metal contamination of surface soils of auto - mechanic shops at Bogoso Junction, Tarkwa, Ghana. Environ Monit Assess. https://doi.org/10.1007/s10661-022-10429-6
Nti F, Gyamfi O, Ansah E et al (2023) Human health risk assessment of potentially toxic elements in soil and air particulate matter of automobile hub environments in Kumasi, Ghana. Toxicol Rep 11:261–269. https://doi.org/10.1016/j.toxrep.2023.09.010
Ghana Statistical Service G (2022) Population of regions and districts. Ghana 2021 population and housing census. General report volume 3A
Mukherjee AG, Renu K, Gopalakrishnan AV (2023) Heavy metal and metalloid contamination in food and emerging technologies for its detection. Sustainability 15:30. https://doi.org/10.3390/su15021195
Muller G (1979) Schwermettalle in den sedimenten des Rheins-Veranderungen seit 1971. Umschau Wissensch Tech 79:778–783
Turekian KK, Wedepohl HK (1961) KARL K. TUREKIAN Dept. Geology, Yale University, New Haven, Conn. KARL HANS WEDEPOHL Mineralogische-Institut der Universitat, Gottingen, Germany Distribution of the Elements in Some Major Units of the Earth’s Crust. America (NY), pp 175–192. https://doi.org/10.1130/0016-7606(1961)72
Akoto O, Bortey-Sam N, Ikenaka Y, Nakayama SM, Baidoo E, Yohannes YB, Ishizuka M (2017) Contamination levels and sources of heavy metals and a metalloid in surface soils in the Kumasi Metropolis, Ghana. J Health Pollut 7(15):28–39. https://doi.org/10.5696/2156-9614-7.15.28
Dartey E, Sarpong K (2016) Assessment of levels of trace elements in sediment and water in some artisanal and small-scale mining sites at Tarkwa—Nsuaem Municipality in the Western Region of Ghana. J Environ Sci Pollut Res 2:129–133
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001. https://doi.org/10.1016/0043-1354(80)90143-8
Gyamfi O, Wireko-Gyebi RS, Ansah E et al (2022) Assessment and awareness of health risks posed by mercury in artisanal gold mining in the Ashanti Region of Ghana. Chem Afr 5:1765–1775. https://doi.org/10.1007/s42250-022-00453-x
Pan L, Fang G, Wang Y et al (2018) Potentially toxic element pollution levels and risk assessment of soils and sediments in the upstream. Int J Environ Res Public Health 15:17. https://doi.org/10.3390/ijerph15112364
US EPA (2009) Risk assessment guidance for superfund volume I: human health evaluation manual (Part F, supplemental guidance for inhalation risk assessment). Off Superfund Remediat Technol Innov Environ Prot Agency I:1–68
Darko G, Dodd M, Nkansah MA et al (2017) Distribution and ecological risks of toxic metals in the topsoils in the Kumasi metropolis, Ghana. Cogent Environ Sci. https://doi.org/10.1080/23311843.2017.1354965
Ajeh AE, Jonathan F, Patrick I (2022) Health risk estimations and geospatial mapping of trace metals in soil samples around automobile mechanic workshops in Benin city, Nigeria. Toxicol Rep 9:575–587. https://doi.org/10.1016/j.toxrep.2022.03.021
Gyamfi O, Sørensen PB, Darko G et al (2021) Contamination, exposure and risk assessment of mercury in the soils of an artisanal gold mining community in Ghana. Chemosphere. https://doi.org/10.1016/j.chemosphere.2020.128910
Agyemang J, Gyimah E, Ofori P et al (2021) Pollution and health risk implications of heavy metals in the surface soil of pollution and health risk implications of heavy metals in the surface soil of Asafo Auto‑Mechanic Workshop in Kumasi, Ghana. Chem Africa. https://doi.org/10.1007/s42250-021-00297-x
Asamoah DB, Asare A, Wonders S, Aidoo P (2021) Heavy metal levels and their ecological risks in surface soils at Sunyani magazine in the bono region of Ghana. Sci African 13:e00937. https://doi.org/10.1016/j.sciaf.2021.e00937
Adjei A, Kwame E, Ebo E et al (2019) Potential heavy metal pollution of soils from artisanal automobile workshops: the case of Suame Magazine, Ghana. Environ Earth Sci. https://doi.org/10.1007/s12665-019-8069-7
CCME (2007) Canadian soil quality guidelines for the protection of environmental and human health: Mercury (inorganic) (1999)
VROM (2000) Dutch target and intervention values
Mandeng EPB, Bidjeck LMB, Bessa AZE et al (2019) Contamination and risk assessment of heavy metals, and uranium of sediments in two watersheds in Abiete-Toko gold district, Southern Cameroon. Heliyon. https://doi.org/10.1016/j.heliyon.2019.e02591
Darko G, Boakye KO, Nkansah MA et al (2019) Human health risk and bioaccessibility of toxic metals in topsoils from Gbani mining community in Ghana. J Health Pollut 9:1–11. https://doi.org/10.5696/2156-9614-9.22.190602
Wang H, Lu S (2011) Spatial distribution, source identification and affecting factors of heavy metals contamination in urban-suburban soils of Lishui city, China. Environ Earth Sci 64:1921–1929. https://doi.org/10.1007/s12665-011-1005-0
Egbueri JC, Ukah BU, Ubido OE, Chinanu O (2022) A chemometric approach to source apportionment, ecological and health risk assessment of heavy metals in industrial soils from southwestern Nigeria. Int J Environ Anal Chem 102:3399–3417. https://doi.org/10.1080/03067319.2020.1769615
Demir M, Tunç E, Thiele-bruhn S et al (2023) Status, sources and assessment of potentially toxic element (PTE) contamination in roadside orchard soils of Gaziantep (Türkiye). Int J Environ Res Public Health 20:18. https://doi.org/10.3390/ijerph20032467
Department of Environmental Affairs (DEA) (2010) Framework for the management of contaminated land, South Africa. Republic of South Africa. 79 p. Framework 326
Acknowledgements
This study received no funding but the authors are grateful to Soil Research Institute of Council for Scientific and Industrial Research Ghana for allowing the use of their laboratory for some of the analysis.
Funding
This research received no funding from any external source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report that there is no conflict of interests or competing or financial interest to declare.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dartey, E., Gyamfi, O. Assessing Contamination Levels and Risk of Toxic Elements in Soils from Automobile Workshop Centre in Asante Mampong, Ghana. Chemistry Africa 7, 929–940 (2024). https://doi.org/10.1007/s42250-023-00799-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00799-w