Abstract
Biodiesel is an alternative energy sources to petroleum and its quantity is continuously decreasing due to an increase in demand, and produced through a chemical process called transesterification, which refers to a synthesizing the extracted oil, catalyst with methanol to produce biodiesel and a byproduct (glycerin). The production of biodiesel is carried out from non-edible oil extracted from Rumex Crispus leaves and root oils and optimizing the effects of independent process variables. For the optimization study, a Response Surface Methodology centered on Central Composite Design was used to optimize the independent process variables such as methanol/oil molar ratio, reaction time, reaction temperature, and catalyst concentration. A quadratic model was used to predict the performance of biodiesel yield. The optimal conditions were obtained at a molar ratio of methanol to oil of 8:1, a reaction time of 3 h, reaction temperature of 65 ℃, and catalyst concentration of 1.5 wt%. Under these conditions the predicted and experimental biodiesel yields were 93.72% and 94.18% respectively. The R2 value of the model was 0.9855, indicating the accuracy of the model. The properties of produced biodiesel from Rumex Crispus leave and root oil meets the requirements of American Society of Testing Material and European standard for biodiesel, and characterized by Gas Chromatography-Mass Spectroscopy and Fourier transform infrared analysis. The study investigated the potential of Rumex Crispus leaves and roots oil to biodiesel, using a heterogeneous catalysis system to bypass current demand issues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Currently, numerous researchers have conducted many experimental studies on biodiesel to find alternative solutions to energy toward fuel for diesel engines without amendment, and the demand of clean sustainable energy sources [17, 27]. Biodiesel is the most promising alternative diesel fuel, it has received a lot of attention in recent years, as global energy demand has increased (Surya et al. [4, 11]).
During the previous decades, worldwide petroleum consumption has always increased due to the development of the human population and industrialization, which affected the depleting fossil fuel reserves and increased petroleum prices [1]. Energy is basic for development and central to sustainable development, poverty reduction, and progressively improved the economy of any country energy consumption and demand [28].
Energy demand is expected to increase due to rapid population growth, expanding urbanization, rising income levels, improved living standards, and economically feasible options to tackle the depleting fossil fuels and their harmful environmental impact [24, 36]. Biomass is the most alternatives renewable energy to fossil fuels energy, used globally for 15% of the primary energy supply [2]. This indicates that, fossil fuels remain the dominant source of energy used either for domestic or industrial consumption, this situation will pose a growing threat, economic, societal, and environmental to the ongoing development of human activities through it is non-renewable and problems of worldwide climate changes [32]. However, biodiesel has received a lot of attention in recent years due to its advantages over nonrenewable source, since it is biodegradable, renewable, non-toxic, and emits less gaseous and particulate pollutants with higher cetane numbers than regular diesel. In addition, the growing global energy demands that are heavily dependent on oil-based fuel resources will be depleted in the near future, if current energy consumption patterns continue [14].
Rumex Crispus is a wild herbaceous perennial weed plant, which has been used as an abundant source of traditional medicine in Ethiopia to treat and cure a variety of diseases [9], Nigussie [26]). The occurrence of a large number of biologically active compounds in different species of the Rumex Crispus leaves and roots made it significant in medicine as well as the pharmaceutical industry and economic importance [33]. These active compounds are called secondary metabolites like flavonoid glycosides, anthraquinones, steroids, proanthocyanidins, and phenolic compounds [23, 33].
In agricultural land, Rumex Crispus leaves and root are common on waste ground and a very serious weed throughout the world, because of the ability to flower numerous times annually, and production of a huge number of seeds per plant, which remain viable in the soil for many years to survive, and ability to re-grow from vegetative fragments left in the soil after cultivation or cutting. These are undesirable in agriculture because they are found in all types of crop as an early colonizer after disturbance; since it affects crop growth, decreases yields, and reduces forage feeding value by competition [3, 30].
A convenient way to lower the use of fossil fuels is by using biomass as a potential source of energy rather than treating them as waste. A cheaper feedstock of Rumex Crispus leaves and roots oil can be used to improve the economics of biodiesel, which will lower the price of petroleum diesel. Besides, recycles Rumex Crispus leaves and roots rather than discarded as wastes, and resolves environmental problems regarding the usage of fossil fuels. However, extensive use of fossil fuels for energy has resulted in a discouraging range of energy-related challenges, both related to the quantity and quality of fuels.
Catalyst plays an important role in the production of biodiesel. In line, various heterogeneous and homogeneous catalysts have been used for the production of biodiesel. Homogeneous catalysts have drawbacks such as low Free Fatty Acid (FFA) and moisture tolerance and complex purification processes. On the other hand, researchers are considering heterogeneous catalysts instead of homogeneous catalysts to eliminate this cause [35]. Heterogeneous catalysts have many advantages over homogeneous catalysts such as recycling, reuse, easily separated from the product, and can be designed to give higher selectivity and longer life leading to economical production costs. However, heterogeneous catalysts also have many disadvantages such as resistance to mass transfer, time consumption, rapid deactivation, and inefficiency [18].
In this study, we optimize and investigate the effects of the most important independent process variables affecting the production of biodiesel (methyl ester) yield through transesterification reaction using Calcium Oxide as a solid heterogeneous catalyst to attain optimal conditions by Response Surface Methodology (RSM) via Central Composite Design (CCD). The molar ratio of methanol to oil, reaction temperature, reaction time, and concentration of catalyst is the parameters that varied with considering the constant stirring rate. The composition of Fatty Acid (FAC) and the properties of biodiesel are estimated according to the standards ASTM D6751 and EN14214, and FTIR and GC–MS chromatographs are used to identify the functional groups, and composition of biodiesel respectively.
2 Materials and Methods
2.1 Materials and Reagents
The fresh Rumex Crispus leaves and roots were collected from near Jimma University, Jimma Ethiopia, and the eggshells were collected from Jimma university institute of technology staff launches. The chemical n-hexane (99%), methanol (99.8%), and Sulphuric acid (98%), were purchased from Chem-Supply Kirkos Ltd. in Addis Ababa, Ethiopia, these used chemicals were pure analytical grade.
2.2 Sample Collection
The collected leaves and roots of Rumex Crispus were separated manually for drying purposes and washed with distilled water several times to remove any impurities, and unwanted materials from the leave, and roots. Consequently, dried at 105 ℃ for 3 h in an oven until constant weight, to determine the moisture contents, and milled to a fine powder with a mortar and pestle, and kept in room temperature until extracted oil.
2.3 Extraction of Rumex Crispus Leaves and Roots Oil FAMEs
The prepared Rumex Crispus leaves and roots (Fig. 1) and prepared powder was used for the oil extraction by Soxhlet extractor with n-hexane as a solvent [12, 29]. The extracted crude oil was kept in a water bath at 70 ℃ using a rotary evaporator until the solvent n-hexane was recovered. The oil was placed in an oven at 60 ℃ for 15 min to remove any remaining solvent in the separated oil, and stored at 4 ℃ in plastic-lipped bottles, and used as a raw material for the production of biodiesel [4]. The oil yield extracted by Soxhlet extraction was estimated in percentage using Eq. (1).
2.4 Catalyst Preparation
To prepare a heterogeneous catalyst from the collected waste eggshells were thoroughly washed two or three times with distilled water and placed in an oven at 105 ℃ for a day to dry completely. Then the dried eggshell was heated to 650 ℃ at a rate of 10 ℃/min for 3 h to ensure the calcination process. The calcined eggshell was ground using a mortar and pestle [16]. The powder (CaO) was stored at room temperature. Subsequently, the prepared catalyst was refluxed and stirred in methanol for 1 h, centrifuged, and then placed in a furnace at a temperature of 650 ℃ (temperature rise of 5 ℃/min) for 1 h. The catalyst produced was stored in a desiccator in a polyethylene container to prevent absorption of water and used for transesterification reaction to produce biodiesel with methanol in the presence of a heterogeneous catalyst (CaO).
2.5 Design of Experimental and Statistical Analysis for Optimization
Design-Expert software (version 13.0), used to optimize the process variables and to predict the percentage yield of the response obtained at the design points and determine the optimal operating parameter for producing the maximum yield of biodiesel by the transesterification process without adversely affecting process parameters (Rajesh & Devan, 2021). Response surface methodology (RSM) were used in this study to examine various independent process variables for biodiesel production from Rumex Crispus leaves and roots oil utilizing a heterogeneous (CaO) catalyst based on the analysis of variance (ANOVA) has a significant impact on biodiesel yield through the transesterification process. The statistical analysis saves time, raw materials, and other costs associated with running experiments. The four independent process variables selected for this work are shown in Table 1. This range level of the factors were selected by initial tests carried out on the effects of the individual factors on the biodiesel yield and the operating limits of the production process conditions are shown in Table 1 with the coded symbols, ranges, and levels of the studied factors.
2.6 Transesterification Process Using a Heterogeneous CaO Catalyst
Choosing the right technology to produce biodiesel is critical to addressing the challenges of feedstock processing, yield, quality, and consumption of chemicals and inputs. The transesterification reaction is the most common chemical reaction, which was used to produce biodiesel from oils extracted from Rumex Crispus leaves and roots, and convert oils or triglycerides (TAG) to methyl esters (biodiesel) via methanol (FAME) in the presence of a catalyst calcium oxide (Fig. 2). To carry out the transesterification reaction, a certain amount of methanol was mixed with certain amounts of the produced catalyst, and preheated oil was condensed. Finally, the by-product (glycerol), which needs to be separated from the final product along with drops of methanol, unreacted triglycerides, and the catalyst. The conversion of Rumex Crispus leaves and roots oil into biodiesel through the transesterification process reduces the; molecular weight, a viscosity of oils, flashpoint slightly, and increases the volatility marginally. To prevent the evaporation of methanol in the extract mixture, the temperature value is below the boiling point of methanol (64.7 \(^\circ{\rm C}\)) [7].
Biodiesel production from Rumex Crispus leaves and roots oil in presence of heterogeneous CaO catalyst was conducted in three-neck flasks (50 mL) under a variety of independent operating process parameters was adjusted in Table 1. The right quantity of methanol was combined with the right quantity of CaO catalyst and added to preheated the Rumex Crispus leaves and roots oil at the right temperature to carry out the transesterification reaction. The transesterification reaction was carried out in all tests at a constant mixing rate of 500 rpm [15].
Upon completion of the transesterification reaction, the generated catalyst and glycerol were separated from the biodiesel by centrifuging and a funnel. The solution was transferred to a separator funnel and the mixture was allowed to settle down for 24 h to form two layers. The upper layer is biodiesel and the lower layer is glycerol [25]. Once the glycerol and FAME phases were separated, the last phase was washed with distilled water to remove any residual catalyst, glycerol, and soap (Rajesh and Devan. [31]). The washing process was continued until the pH reached 7 (after 3 successive rinsing with water).
Finally, the produced biodiesel was heated in a rotary evaporator at 90 ℃ for 50 min to entirely separate the methanol from the biodiesel [16]. Once the biodiesel has been produced, the percentage yield of biodiesel through transesterification reaction was calculated at each stage using Eq. (2).
2.7 Characterization of Oil, Catalyst, and Produced Biodiesel
FT-IR analysis (PerkinElmer spectrometer) was performed to investigate the functional groups of the extracted oil, catalysts, and produced biodiesel. FT-IR analyzes were performed in the range of 500–4000 cm−1. To measure the functional groups in the oil and biodiesel, droplets were poured onto the KBr plate, the second plate was placed on the first plate (sandwich mode) and the oil was converted to a thin film. To measure the functional groups of the catalyst, 1 mg of catalyst powder was first mixed with completely dried potassium bromide (KBr). After that, a small amount was poured into a special mold and pressed with a hydraulic press to obtain a transparent tablet, and finally, the FT-IR analysis was performed [20].
The Physicochemical properties of extracted oil from Rumex Crispus leaves and roots oil and biodiesel was characterized by kinematic viscosity, viscosity, density, specific gravity, acid value, calorific value, saponification number, and iodine value, which were analyzed according to international standard of ASTM D 6751 and EN 14214.
3 Results and Discussion
In this study, the relationship between the yield of biodiesel as a response and four operating conditions, methanol-to-oil molar ratio, reaction temperature, reaction time, and catalyst concentration was investigated. Thirty (30) experiments were performed based on the response surface methodology (RSM) matrix, and the results were examined (Table 3), and optimized the transesterification variables using the CCD.
3.1 Characterization of Catalyst
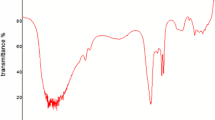
The prepared calcined egg shell was characterized by FTIR analysis to determine their functional groups of various compounds found in calcined egg shell. Figure 3 shows the results of an FT-IR analysis of the Calcium Oxide sample prepared from waste egg shell in the range of 400–4000 cm−1. Thus, observed peaks peak at 3450 cm−1; indicating that the stretching vibration associated with hydroxyl (OH) and carbonyl functional groups in the single bond region of the calcined egg shell respectively, while the faint bands in 2920 cm−1 delivered to C–H asymmetrical stretch. Furthermore, the peaks observed in the range of 1590 and1290 cm−1 were assigned to the pyrone C=C and C=O to the carboxyl group correspondingly, and the presence of C-O stretch at wavenumber 1200 cm−1 indicates the presence of alkoxy functional class. The peak in the region of 1100–900 cm −1 is also associated with the expansion and contraction of Si–O or C-O in alcohol and ether.
3.2 Characterization of Oil, and Produced Biodiesel
The density of the oil is 900 kg/m3, which is consistent with the density according to ASTM D6751 and EN 14214. The acid and saponification values of the oil were determined using standard titration methods, and by using the procedure described by [8]. The density at 15 ℃ was measured with a densimeter [5, 8]. The kinematic viscosity was measured using a viscometer, and the kinematic viscosity was estimated using the density and kinematic viscosity at 40 ℃. The acid value of Rumex Crispus leaves and roots oil is 1.5 mg KOH/g, and free Fatty Acid are half of the acid value is 0.75.
Table 2 summarizes the physicochemical properties (i.e. density, viscosity, acid value, iodine value, and saponification value) of Rumex Crispus leaves and roots oil and produced biodiesel based on the standard methods of ASTM D6751 and EN 14214. These have been significantly improved and meet the required specifications. This result shows that the Rumex Crispus leaves and roots oil can be used as substitute feedstock for the production of biodiesel.
3.3 Analysis of Rumex Crispus Leaves and Root Oil, and Biodiesel by FT-IR
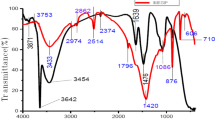
The infrared spectrum (FT-IR) analysis of the Fourier transform of produced biodiesel shown in Fig. 4 was used for quantitative analysis. The peak in the spectrum represented the functional groups contained in the oil. The wave number, functional group, band assignment, and absorption intensity of the absorption peaks were detected in the infrared spectrum of the Fourier transform of oil, and biodiesel. Figure 4 shows the FTIR spectrum of the extracted oil and biodiesel in the range of 400–4000 cm−1. The broad bond in the range of 1813–2678 cm−1 was attributed to the stretching mode of the O–H groups, as well as weak bond of the OH- bending vibration at 1600 cm−1, which occurred due the adsorption of water molecules from the air on the surface of the catalyst. The carbon-hydrogen bonds formed at 625–3330 cm−1, the functionality of the ester at 1715–1750 cm−1, the terminal carbons CH3 at 1354–1589 cm−1, and the carbon–oxygen bonds at 762–1120 cm−1. The results demonstrated that the produced biodiesel is composed of long-chain Fatty Acid esters. The transesterification process are chemically similar to the refined oil, the CaO stretch peak is 1663 cm−1 located in the range 2966–611 cm−1 is typical of esters, the spectrum is often found in FAME and refined oil.
3.4 GC- MS Analysis of Biodiesel
GC–MS (Gas chromatography-mass spectrometry) was performed to determine the composition of the methyl ester. While using GC–MS, we should always consider two important pieces of information: retention time (RT) and peak area (PA). However, due to the unique physical properties of the sample, the RT must remain constant as long as the analytical method is the same. It is also important to understand the elution order of the compounds in the mixture before the GC—MS analysis of the sample. Figure 5, shows the chromatogram of biodiesel with the presence of derivatives of (a) C16:0 (palmitic acid methyl ester), (b) C17:0 (Methyl heptadecanoate), (C) C18:1 (oleic acid methyl ester), (d) C18:2 (linoleic acid methyl ester), (e) C18:3 (linolenic acid methyl ester), (f) diglycerides, (g) unreacted triglycerides present in the biodiesel. The chromatogram of biodiesel obtained from Rumex Crispus leaves and roots oil confirmed the formation of methyl esters.
3.5 Optimization of Operating Variables by Statistical Analysis via Response-Surface Methodology (RSM)
The quadratic regression model is used to investigate the effects of numerous independent operating variables on the produced biodiesel. The commonly statistical schemes used for response surface methodology (RSM) are central composite designs (CCD), which can be used under certain parametric conditions. RSM was used to optimize the interaction of four variables: the methanol to oil ratio, reaction temperature, reaction time, and catalyst loading. Analysis of variance (ANOVA) based on the analysis of parameters that have a significant impact on biodiesel yield through the transesterification process from Rumex Crispus leaves and roots oil biodiesel yields ranged from 74.14 to 94.18%. Table 3 shows the results of the Box-Behnken design model for optimizing the process parameters. These results indicate that biodiesel yields vary by the production process. All running orders have been randomized to avoid systematic errors.
The Predicted biodiesel yield values were generated from a quadratic regression model taken from the statistical analysis of experimental data using response surface methodology (RSM). The response surface methodology was used to calculate the effect of each parameter and its interaction with other parameters. The response (biodiesel yield %) was correlated with other parameters using a full quadratic regression model shown in Eq. (3). This model represents the predicted yield (Y) of Rumex Crispus leaves and roots oil biodiesel as a function of methanol: oil molar ratio (A), reaction temperature (B), reaction time (C), and catalyst concentration (wt%) (D).
This model equation in terms of coded factors shows that the positive coefficients are A, AB, AC, AD, BD, A2, C2, and D2, which are the high levels of the factors and the negative coefficients are B, C, D, \({\mathrm{A}}^{2}\),\({\mathrm{B}}^{2}\) and the interaction of BC, and CD are the low levels of the factors. The final coded equation is useful for identifying the relative impact of factors by comparing the coefficients of the factors. The estimated coefficient represents the expected response changes per unit change in the factor by assuming that all remaining factors are constant. Table 4 shows the importance of these parameters related to the probability value (p-value).
Analysis of variance (ANOVA) is performed to determine the significance and validity of the quadratic model, as well as the effects of individual significant terms and interactions on the selected responses (S. [10]. As the ANOVA results are shown in Table 4, the quadratic regression model has an F-value (73.01) higher and implies the model is significant and a p-value or probability error value is used to check the significance of each regression coefficient which is (0.0001) lower than the significance level \(\left(\mathrm{P}<0.05\right)\), indicating that the model terms are significant. In this case, A, B, C, D, AB, AC, AD, BD, and CD are significant model terms, and values greater than 0.1000 indicate that the model terms are not significant. If there are many insignificant models, this implies reduction may improve the model.
The lack of fit is also determined by the quadratic regression model, which shows a lack of fit, this indicates that the model does not sufficiently describe the relationship between the independent variables (i.e. the methanol to oil molar ratio, the reaction temperature, the reaction time and the concentration of the catalyst) and the dependent variable (i.e. biodiesel yield). In this study, the F and p-value of the fit parameter were found to be 1.42 and 0.3670, respectively. The p-value of the missing adjustment parameter is greater than 0.0500, indicating that there is a good fit between the quadratic regression model and the experimental data.
The coefficient of determination R-squared (R2) reflects the variability of the dependent variable explained by its relationship with the independent variables (predictive variables). In general, a high R2 value indicates that the model is responsible for more data variability and therefore the data points will be closer to the regression line. In other words, a high R2 value indicates a good fit between the model and the experimental data. Based on the results of Table 4, it can be seen that the R2 value is 0.9955, indicating that 99.55% of the variability of biodiesel yield is explained by the quadratic regression model.
However, it should be noted that the value of R2 can increase as the number of predictive variables in the model increases and therefore care must be taken in interpreting the value. The corrected coefficient of determination (adjusted R2) is used to compensate for this undesirable effect, as it not only indicates how well the model fits the experimental data but also takes into account the number of predictive variables. Adjusted R2 will increase if useful predictive variables are added to the model, and likewise, adjusted R2 will decrease as unnecessary predictor variables are added. The adjusted R2 is 0.9720 as shown in Table 4, indicating that the model accounts for 97.20% of the variation in biodiesel yield.
The predicted and experimental values of the biodiesel yield are shown in Fig. 6, as the line of a perfect fit with the point corresponding to the zero error. The differences between the experimental and predicted values appear to be less than 0.2, indicating good agreement between the model and the experimental data. A point close to a straight line indicates that the experimental and predicted values are in good agreement. This result is consistent with R2 obtained earlier and R2 modified to a value close to unity. Therefore, the regression model gives a good estimate of system response (i.e. biodiesel yield) with changes in process variables (Fig. 7).
3.6 Effect of Process Variables on Biodiesel Yield
This study examined the effect of the molar ratio of methanol to oil on the biodiesel yield by varying the methanol to oil ratios within the ranges of \(6:1-8:1.\) The most effective variable affecting the conversion efficiency of the produced methyl ester yield during the transesterification reaction is the molar ratio of alcohol (methanol) to extracted oil. Since transesterification is an equilibrium reaction, a large excess of alcohol is required for the reaction to proceed and avoid the reversible reaction (Elkady et al. [13]).
Figure 8a shows the three-dimensional surface plot of the combined effects of methanol to oil molar ratio and catalyst concentration on biodiesel yield. Finding the appropriate methanol-to-oil ratio is critical in determining the methyl ester yield. If the methanol to oil ratio is insufficient for the transesterification reaction, glycerides will not be converted to Fatty Acid methyl esters, resulting in lower biodiesel yields. In general, it can be observed that at a fixed catalyst concentration increasing the methanol to oil molar ratio has an insignificant effect on the yield of biodiesel. Increasing the amount of methanol increases the time required to extract excess methanol from biodiesel after the transesterification process since methanol has a polar hydroxyl group that acts as an emulsifier and thus improving the solubility of glycerol in the biodiesel phase and ultimately lowering the yield. In general, adding excessive amounts of methanol to oil are not recommended, because it decreases the percent methyl ester yield, this behavior indicates that the separation of glycerol and methyl esters becomes more difficult due to the emulsification and reversibility behavior of the transesterification reaction [20]. The maximum biodiesel yield was achieved at methanol to oil molar ratio of 8:1, keeping the catalytic concentration and reaction temperature at their optimal values. This fact indicates that excess alcohol in the transesterification process will tip to rises in the product, while the opposite side of excess methanol increases glycerol solubility, resulting in lower yields.
Figure 8b shows the effect of reaction temperature and reaction time on biodiesel yield when the molar ratio of methanol to oil and catalyst load are held constant at 8:1 and 1.5 wt% respectively. The figures show that the maximum biodiesel yield could be achieved with a reaction time of 3 h. A further increase in the reaction time will not affect the biodiesel yield. This could be recognized in the reverse reaction loading to reduce FAME formation. The optimal value of the reaction temperature is observed at 65 ℃. The reaction temperature has a significant influence on the reaction rate and the conversion to methyl ester. The biodiesel yield increases with increasing reaction temperature, but as it approaches the boiling point of methanol, the biodiesel yield decreases. Concerning the reaction time, the effect of the reaction temperature influences the yield to the maximum. However, compared to reaction time, the effect of reaction temperature has the greatest impact on yield [21].
Figure 8c, shows the three-dimensional surface plot of the combined effects of temperature and catalyst concentration on FFA conversion with other variables (i.e. methanol to oil molar ratio and reaction time) kept constant at their mean values \(\left(8:1\mathrm{and }3\mathrm{ hours},\mathrm{ respectively}\right)\) with constant stirring speed. In fact from Fig. 8c, it can be observed that increasing the catalyst concentration from the range of 0.50–1.5wt% decreases the biodiesel yield (Hojjat et al. [19]). This indicates that the optimal operating parameters for the transesterification parameters of methanol to oil ratio at 8:1, reaction time at 3 h, catalyst concentration of 1.5wt%, and reaction temperature of 65 ℃ with maximum biodiesel yield of 94.18%.
Figure 8d, shows the three-dimensional surface plot of the combined effects of methanol concentration, and reaction time on biodiesel yield conversion, with other variables (i.e. reaction temperature and catalyst concentration) kept constant at their mean values \(\left(8:1\mathrm{and }3\mathrm{ hours},\mathrm{ respectively}\right)\). The contour diagram indicated that there were two optimal ranges of the methanol to oil ratio, one in the lower half of the contour diagrams and the other in the upper half of the contour diagrams (Karmakar and Mukherjee. [22]). In the lower half of the contour diagram, at a low ratio of methanol to oil, there was a moderate decrease in biodiesel yield with an increase in reaction time due to the negative time effect of Eq. (3).
From Fig. 9, the ramp diagram, it can be seen that the optimum ester yield is 94.18% when the molar ratio of methanol to oil, catalyst concentration, reaction temperature, and reaction time are 8:1, 1.5wt%, 65 ℃, and 3 h respectively.
4 Conclusion
This study focused on optimizing and characterizing the biodiesel production process independent parameters for biodiesel derived from non-edible feedstocks (Rumex Crispus leaves and roots oil) via transesterification reaction using response surface methodology. The response surface methodology based on the central composite design was applied to investigate the interactive effects of process parameters on the ester yield, to obtain the optimum yield of biodiesel. The optimal operating parameters for transesterification of oil at a molar ratio of methanol to oil of 8:1, a reaction temperature of 65 ℃, a reaction time of 3 h, and a catalyst concentration of 1.5wt%. These optimal operating parameters were validated with the actual biodiesel yield of 94.18%, and the predicted ester yields were 93.72% under optimized conditions. The coefficient of variance (CV %) was 0.7681 with a 98.55% confidence limit of R2. The physicochemical properties of biodiesel are produced to meet the requirements of ASTM D6751 and EN14214 standards. The values are close to the physicochemical properties of diesel leading to the conclusion that optimized biodiesel are a potential replacement for diesel fuel. The results indicated that Rumex Crispus leaves and roots oil could be a possible feedstock for biodiesel production, although more studies are needed to produce a quality fuel and to evaluate the engine performance and emissions of this biodiesel.
Availability of Data and Material
The availability of data, i.e. experimental design, data analysis (RSM), FTIR, Gas chromatography-mass spectrometry (GC–MS) used to support the results of this study are incorporated in the article.
Abbreviations
- ANOVA:
-
Analysis of variance
- CaO:
-
Calcium oxide
- CCD:
-
Central composite design
- FTIR:
-
Fourier transforms infrared
- GC–MS:
-
Gas chromatographs mass spectroscopy
- FAC:
-
Fatty acid
- RSM:
-
Response surface methodology centered
References
Ahmia AC, Danane F, Bessah R, Boumesbah I (2014) Raw material for biodiesel production. Valorization of used edible oil. J Renew Energies 17(2):335–343
Ahmia AC, Danane F, Bessah R, Boumesbah I (2014) Raw material for biodiesel production. Valorization of used edible oil. Revue Des Energies Renouvelables 17:335–343
Alshallash S (2015) Quantifying the efficiency of the beetle (gostrophysavirdula quantifying the efficiency) as biological control agent on weedy plant sorrel dock (control agent on weedy plant sorrel dock (rumex crispus) at different seedling growth stages. Int J Curr Res 7(11):23049–23056
Anwar M, Rasul MG, Ashwath N, Rahman MM (2018) Optimisation of second-generation biodiesel production from Australian native stone fruit oil using response surface method. Energies 11(10):2566. https://doi.org/10.3390/en11102566
Aworanti OA, Agarry SE, Ajani AO (2013) Statistical optimization of process variables for biodiesel production from waste cooking oil using heterogeneous base catalyst. Br Biotechnol J 3(2):116–132
Bai L, Tajikfar A, Tamjidi S, Foroutan R, Esmaeili H (2021) Synthesis of MnFe2O4@graphene oxide catalyst for biodiesel production from waste edible oil. Renewable Energy 170:426–437. https://doi.org/10.1016/j.renene.2021.01.139
Boulifi NE, Bouaid A, Martinez M, Aracil J (2010) Process optimization for biodiesel production from corn oil and its oxidative stability. Int J Chem Eng. https://doi.org/10.1155/2010/518070
Bullo TA, Fana FB (2021) Production and characterization of biodiesel from avocado peel oils using experimental analysis (ANOVA). J Eng Adv. 02(02):104–111. https://doi.org/10.38032/jea.2021.02.006
Ćebović T, Jakovljević D, Maksimović Z, Djordjević S, Jakovljević S, Četojević-Simin D (2020) Antioxidant and cytotoxic activities of curly dock (Rumex crispus L., Polygonaceae) fruit extract. Vojnosanit Pregl 77(3):308–316. https://doi.org/10.2298/VSP170713084C
Dharma S, Masjuki HH, Ong HC, Sebayang AH, Silitonga AS, Kusumo F, Mahlia TMI (2016) Optimization of biodiesel production process for mixed Jatropha curcas-Ceiba pentandra biodiesel using response surface methodology. Energy Convers Manage 115:178–190. https://doi.org/10.1016/j.enconman.2016.02.034
Dharma S, Haji M, Chyuan H, Hanra A (2017) Optimization of biodiesel production from mixed jatropha curcas—ceiba pentandra using artificial neural network- genetic algorithm: evaluation of reaction kinetic models. Chem Eng Trans 56:547–552. https://doi.org/10.3303/CET1756092
El-Gendy NS, Deriase SF, Hamdy A, Abdallah RI (2015) Statistical optimization of biodiesel production from sunflower waste cooking oil using basic heterogeneous biocatalyst prepared from eggshells. Egypt J Pet 24(1):37–48. https://doi.org/10.1016/j.ejpe.2015.02.004
Elkady MFAZ (2015) Production of biodiesel from waste vegetable oil via KM micro-mixer. J Pet Environ Biotechnol. https://doi.org/10.4172/2157-7463.1000218
Fan X, Wang X, Chen F (2011) Biodiesel production from crude cottonseed oil: an optimization process using response surface methodology. Open Fuels & Energy Sci J 4:1–8. https://doi.org/10.2174/1876973X01104010001
Foroutan R, Mohammadi R, Esmaeili H, Mirzaee Bektashi F, Tamjidi S (2020) Transesterification of waste edible oils to biodiesel using calcium oxide@magnesium oxide nanocatalyst. Waste Manage 105:373–383. https://doi.org/10.1016/j.wasman.2020.02.032
Foroutan R, Mohammadi R, Ramavandi B (2021) Waste glass catalyst for biodiesel production from waste chicken fat: optimization by RSM and ANNs and toxicity assessment. Fuel 291:120–151. https://doi.org/10.1016/j.fuel.2021.120151
George A, Joshua A, Akorede OI, Adeoye S, Victoria D (2019) Modelling and optimisation of biodiesel production from Euphorbia lathyris using ASPEN Hysys. SN Appl Sci 1(11):1–9. https://doi.org/10.1007/s42452-019-1522-0
Gupta J, Agarwal M, Dalai AK (2016) Optimization of biodiesel production from mixture of edible and nonedible vegetable oils. Biocatal Agric Biotechnol. https://doi.org/10.1016/j.bcab.2016.08.014
Hojjat M, Nayebzadeh H (2016) Optimization of process conditions for biodiesel production over CaO – Al 2 O 3/ZrO 2 catalyst using response surface methodology. Chem Pap. https://doi.org/10.1007/s11696-016-0096-1
Hundie KB, Shumi LD, Bullo TA (2022) Investigation of biodiesel production parameters by transesterification of watermelon waste oil using definitive screening design and produced biodiesel characterization. S Afr J Chem Eng 41:140–149. https://doi.org/10.1016/j.sajce.2022.06.002
Jeyakumar N, Narayanasamy B, Venkatraman B (2019) Optimisation of biodiesel production from Jack fruit seed oil using response surface methodology. Int J Ambient Energy. https://doi.org/10.1080/01430750.2019.1621202
Karmakar A, Mukherjee S (2017) Process optimization of biodiesel production from Neem oil. Indian J Agric Res Process 51(6):529–355. https://doi.org/10.18805/IJARe.A-4831
Kaya E, Akbaş P, Ceyhan G, Karabekmez Erdem T, Alkan H (2020) Determination the fatty acid composition of the Rumex patientia L. leaves and in vitro antimicrobial activity of their different extracts. J Süleyman Demirel Üniversitesi Fen Bilimleri Enstitüsü Dergisi 24:362–367. https://doi.org/10.19113/sdufenbed.643154
Keneni YG, Marchetti JM (2017) Oil extraction from plant seeds for biodiesel production. AIMS Energy 5:316–340. https://doi.org/10.3934/energy.2017.2.316
Naik L, Radhika N, Sravani K, Hareesha A, Mohanakumari B, Bhavanasindhu K (2015) Optimized parameters for production of biodiesel from fried oil optimized parameters for production of biodiesel from fried Oil. Int Adv Res J Sci, Eng Technol. 2:62–65. https://doi.org/10.17148/IARJSET.2015.2615
Nigussie G (2021) Isolation and characterization of the roots of Rumex nervosus. J Trop Pharm Chem 5(1):39–50. https://doi.org/10.25026/jtpc.v5i1.241
Olagunju OA, Musonge P, Kiambi SL (2022) Production and optimization of biodiesel in a membrane reactor using a solid base catalyst. Membranes 12(7):1–15. https://doi.org/10.3390/membranes12070674
Palani Y, Devarajan C, Manickam D, Thanikodi S (2020) Performance and emission characteristics of biodiesel-blend in diesel engine: a review. Environ Eng Res 27(1):200338. https://doi.org/10.4491/eer.2020.338
Panichikkal AF, Prakasan P, Kizhakkepowathial Nair U, Kulangara Valappil M (2018) Optimization of parameters for the production of biodiesel from rubber seed oil using onsite lipase by response surface methodology. 3 Biotech. 8(11):1–14. https://doi.org/10.1007/s13205-018-1477-7
Amini R, Bahmani Y, Amjadi E (2015) Effect of salinity and crop residue on seed germination and early seedling growth of curled dock (Rumex Crispus L.). Int J Plant Anim Environ Sci 1:68–73
Rajesh K et al (2021) Parametric optimization and biodiesel production from coconut fatty acid distillate. Iran J Chem Chem Eng 40(1):343–355
Selvakumar MJ, Alexis SJ (2016) Renewable fuel production technologies. Middle-East J Sci Res 24(8):2502–2509. https://doi.org/10.5829/idosi.mejsr.2016.24.08.23783
Shafiq N, Saleem M, Kousar S, Sahar M, Hussain SM, Jabeen F (2017) Investigation of genus Rumex for their biologically active constituents. Pharm Chem Sci 2:148–165. www.rjlbpcs.comwww.rjlbpcs.com
Singh D, Kumar V, Sandhu SS, Sarma AK (2016) Process optimization for biodiesel production from indigenous non-edible Prunus armeniaca oil. Adv Energy Res 4(3):189–202
Talebian-Kiakalaieh A, Amin NAS, Mazaheri H (2013) A review on novel processes of biodiesel production from waste cooking oil. Appl Energy 104:683–710. https://doi.org/10.1016/j.apenergy.2012.11.061
Zulqarnain, Mohd Yusoff MH, Ayoub M, Ramzan N, Nazir MH, Zahid I, Abbas N, Elboughdiri N, Mirza CR, Butt TA (2021) Overview of feedstocks for sustainable biodiesel production and implementation of the biodiesel program in Pakistan. ACS Omega 6(29):19099–19114. https://doi.org/10.1021/acsomega.1c02402
Acknowledgements
The authors would like to express their highest gratitude to the School of chemical Engineering, Jimma Institute of Technology, and Jimma University to support laboratory materials, and internet free services.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
All the authors: TAB wrote the main parts of the manuscript, YMB, MSB, KBH DAA and DGG have made a substantial contribution in conceptualization, data curation, formal analysis, methodology, designed the study and procedure, interpretation of the data, conducting lab testing, visualization, validation, analysis of FT-IR spectroscopy, and GC–MS analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no materials or financial conflicts of interest in this study.
Ethical Approval and Consent to Participate
This article does not include human or animal studies conducted by any of the authors. Consent to participate in the study was also obtained at the individual level.
Consent for Publication
The paper is original, has not yet been published in a journal, and is not currently peer-reviewed by another journal.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bullo, T.A., Bayisa, Y.M., Hundie, K.B. et al. Optimization, Characterization and Production of Biodiesel from Rumex Crispus Leaves and Roots Oil Using Central Composite Design (CCD). Chemistry Africa 7, 749–761 (2024). https://doi.org/10.1007/s42250-023-00784-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-023-00784-3