Abstract
Englerina woodfordioides is a hemi-parasitic shrub known for its various ethnomedical uses. Nonetheless, these alleged therapeutic benefits are not scientifically validated. In addition, the study of its phytochemical composition remains unexplored. This study aimed at investigating the antimicrobial activity of E. woodfordioides collected from four different host plants. The antibacterial activities and toxicity of crude leaf extract were evaluated using the agar disk diffusion assay and animal model, respectively. The phytochemical composition of active leaf extracts was determined using LC-MS Q-TOF analysis. The crude chloroform, ethyl acetate, and aqueous extracts of E. woodfordioides did not inhibit the growth of Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus at 100 mg/ml. Conversely, the methanolic extracts of E. woodfordioides inhibited antibacterial effects against S. aureus. The acute toxicity test using dry methanol extract revealed no signs of toxicity or death of white mice at doses of 175, 550, and 2000 mg/kg body weight. Thirty-four and eleven metabolites were detected in the methanolic extract of E. woodfordioides collected from Schinus molle and Vachellia abyssinica host plants, respectively. The presence of flavonoids, phenolic acids, and alkaloids may be responsible for the antimicrobial activity of E. woodfordioides. This is the first comprehensive study on the antimicrobial activity, toxicity, and phytochemical constituents of E. woodfordioides collected from different host plants. Further study may reveal the potent bioactive principles with antimicrobial properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Natural products deriving from medicinal plants are exploited in the traditional medicinal systems of many communities around the world. About 80% of the world’s population living in developing countries uses traditional medicine to address their primary healthcare needs [1]. Various secondary metabolites in these plants serve as source of novel molecules for drug discoveries [2, 3]. Meanwhile, the pharmacological activities of several species of medicinal plants in Africa have not been validated scientifically for their alleged health benefits [3]. In recent decades, there has been a growing interest in exploring medicinal plants to overcome the rapid emergence of antibiotic-resistant microorganisms [4, 5].
The genus Englerina belongs to the family Loranthaceae. All 25 species of the genus are grown in tropical Africa and are hosted by 19 families of plant species on the continent [6]. Englerina woodfordioides (syn. Loranthus woodfordioides) is a shrub that grows on a wide range of host plants [7,8,9]. It is distributed in Burundi, Ethiopia, Kenya, Rwanda, Tanzania, Uganda, and Zaire [7, 10]. In the traditional medicine of Ethiopia, E. woodfordioides has been utilized for the treatment of earaches [11], wounds [12], cutaneous leishmaniasis [13, 14], syphilis [15], and malaria [16]. In the southern region of Ethiopia, the therapeutic importance of E. woodfordioides has been observed to vary depending on the host plant on which it grows [9].
The scientific study on the medicinal properties of Loranthaceae species has attracted less attention in Ethiopia. This is mainly associated with the recurrent removal of these plants because of their devastating effect on the survival of host plants, the uneven distribution of species, the difficulty of propagating them, and the lack of enough samples for study. As a result, there is no literature on the biological activity and identity of chemical compounds of Loranthance species, including E. woodfordioides, in Ethiopia. Therefore, the present study was designed to investigate the antimicrobial activity, acute toxicity, and phytochemical constituents of the leaf extracts of E. woodfordioides in Ethiopia.
2 Materials and Methods
2.1 Collection and Authentication of Plant Samples
The leaves of E. woodfordiodes were collected from Shashemene, Gambo, and Menagesha localities in Central Ethiopia between April and June 2018. These samples were harvested from Shinus molle, Discopodium penninervum, Eucalyptus globulus, and Vachellia abyssinica, hereafter designated as EWSM, EWDP, EWEG, and EWVA, respectively. Taxonomic identification of these specimens was done by Melaku Wondafrash Herbarium Specialist at the National Herbarium (ETH) at Addis Ababa University, Ethiopia. These specimens were given the voucher numbers (AY 2, AY 19, AY 4, and AY 34 for EWSM, EWDP, EWEG, and EWVA, respectively) and deposited at ETH.
2.2 Material Processing and Extraction
Healthy leaves were washed under running tap water. These leaves were air-dried for 7 days and pulverized using a Retsch electrical grinding mill (Hulme-Martin Ltd, UK). Twenty (20) grams of each sample specimen’s were macerated in 200 ml of analytical grade chloroform, ethyl acetate, methanol, and sterilized distilled water in a separate glass jar for 72 h. The extracts were filtered using Whatman® no1 filter paper (Maidstone, England). These filtrates were concentrated under reduced pressure in a RE-100D rotary evaporator (Phoenix Instrument) at 40 °C for 45 min for organic solvents, and using a lyophilizer (CHRIST, Germany) for aqueous extracts.
2.3 Antimicrobial Activity
The antibacterial activities of E. woodfordiodes extracts were evaluated against Escherichia coli ATCC 25,922, Pseudomonas aeruginosa ATTC 27,853, and Staphylococcus aureus ATCC 25,923 provided by the Ethiopian Public Health Institute (EPHI), Addis Ababa, Ethiopia. Disk diffusion method was used for the screening of antimicrobial activity of each specimens extracts. The broth cultures of these bacteria were diluted with 0.9% (w/v) sterilized normal saline solution from an overnight grown colonies. The bacterial suspensions were adjusted to 0.5 McFarland turbidity [DEN-1 (BioSan, Latvia)] and spread on Muller Hinton Agar (MHA) growth medium using a sterile cotton swab. The extracts were diluted in dimethyl sulfoxide (DMSO). Sterile filter paper disks (Ø = 6 mm) loaded with 20 µL of the test extracts (100 mg extract dissolved in 1mL DMSO) were placed over the MHA medium. Similarly, disks impregnated with DMSO and the four extraction solvents were prepared to serve as negative controls and placed over the MHA medium. The standard disks of Amoxycillin/Clavulanic acid (30 g for E. coli and S. aureus), Ampicillin (10 g for E. coli and P. aeruginosa), Erythromycin (10 g for S. aureus), Gentamycin (10 g for P. aeruginosa and E. coli), and Tetracycline (30 g, for E. coli) were used as positive controls. The inoculated plates were incubated for 16 to 18 h at 35 ± 2°C. The diameter of the inhibition zone was measured using a transparent ruler and reported in mm, including the 6 mm paper disk. Plant extracts having an apparently higher zone of inhibition were selected for further study.
2.4 Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
The MIC and MBC of plant extracts were determined using a rapid p-Indonitrotetrazolium chloride (INT; ROTH, Carl Roth GmbH Co., Germany) colorimetric assay by the broth macrodilution method as described by [17, 18] with slight modifications. Briefly, 0.5 mL of the stock solution (100 mg of crude plant extract dissolved in 1 mL of 99.9% DMSO) was drawn and diluted with 0.5 mL of 2% DMSO to minimize the toxicity effect of DMSO [19, 20]. The resulting solution was diluted in a two-fold dilution to a range of 50–0.098 mg/mL in 10 separate test tubes. One milliliter of plant extract solution was discarded from the last test tube. Each of these solutions was transferred into 10 test tubes that were filled with 2 ml Muller-Hinton broth (MHB, sterilized broth at 121°C, 15 Pa for 15 min). Test tube 11 (no extract added, but with 1 mL of 2% DMSO) and test tube 12 (no extract but with 1 mL of 2% DMSO and 60 µL of 30 mg/ml erythromycin) served as a negative or growth control and a positive control, respectively. Bacterial suspension (100 µL) was added to each of these test tubes. Then, 60 µL of INT (2 mg/ml of INT in sterile distilled water) was added to each of these test tubes to assess the viability of the bacterium after 18 h of incubation [21]. These test tubes were further incubated for 2 h at 35 ± 2°C to determine the MIC of extracts, which is the lowest concentration of plant extract that inhibited bacterial growth [22]. This is evidenced by the absence of pink coloration of the dye [21]. Finally, the MBC of plant extracts was determined after determination of the MIC of the test plant extracts. Aliquots of 100 µl of each gradient concentrated solution were transferred into new test tube filled with fresh MHB, and incubated at 35 ± 2°C for 48 h. The MBC endpoint was considered as the lowest concentration of plant extract that showed no color change after the addition of INT [23].
2.5 Acute Toxicity Test
Adult healthy female white mice (21.15 ± 2.11 g, 8–12 weeks old) were purchased from the EPHI. These mice were acclimatized for five days in a polypropylene cage (5 mice each) before dosing. Mice were supplied with animal food and water for free access. They were kept at 22 ± 3 °C and on a 12 h light/dark cycle in the Animal House of the College of Natural and Computational Sciences of Addis Ababa University.
The acute oral toxicity test was conducted as described by the Economic Cooperation and Development guideline [24]. The dry crude extract of E. woodfordioides with apparent antimicrobial activity was simultaneously administered to three (3) groups of five (5) mice each, namely 175 mg/kg, 550, and 2000 mg/kg body weight doses. The positive and negative control groups of mice (5 mice each) were administered with normal saline and tap water, respectively. Each mouse was observed for signs of toxicity during the first half an hour, after 4 and 24 h, and subsequently daily for 14 days. The body weight of an individual mouse was determined shortly before the commencement of dosing and afterwards in the first and second week of the dosing period. The relative organ weight (ROW) of the lungs, heart, kidneys, and liver of an individual mouse was weighed after the end of the acute oral toxicity test. The percentage weight gain [25] and relative organ [26] weight of mice were calculated using the following formulae.
2.6 Silica Gel Fractions Activity
The crude extracts of EWSM and EWVA with apparent antimicrobial activity were further fractionated using a silica gel column (60 g) eluted with n-hexane, n-hexane/chloroform, chloroform, chloroform/ethyl acetate, ethyl acetate, ethyl acetate/methanol, and methanol. All sub-fractions were tested for their antimicrobial activity as mentioned above.
2.7 Phytochemical Analysis of Metabolites Using LC-MS Q-TOF Analysis
Metabolomic analyses of the crude extracts were done using liquid chromatography-mass spectrometry (LC-MS) analysis on Agilent HP 1260 Infinity Series Liquid Chromatograph equipped with a DAD system (Agilent Technologies system, Santa Clara, CA, USA) coupled to MS Q-TOF model G6540B (Agilent Technologies). The C-18 column (Adamas® 4.6 × 50 mm, 3.5 μm, SepaChrom Srl, Rho, Mi, Italy) was maintained at a constant temperature of 25°C, and used as the chromatography separations column. The mobile phase consisted of 0.1% (v/v) formic acid in water (phase A) and 0.1% (v/v) formic acid in acetonitrile (phase B). The analyses were performed at a flow-rate of 0.5 mL min− 1, 95% A graduating to 70% B in 4 min, 100% B 4–5 min, graduating from 70 to 80% B in 3 min, graduating from 80% B to 100% B in 2 min and equilibrating at 95% A 10–14 min. The UV spectra were collected by DAD every 0.4 s from 190 to 750 nm with a resolution of 2 nm. The mass spectrometer system was equipped with a DUAL ESI ionization source that was set in both positive and negative mode. Mass spectra were recorded from m/z 100 to 1700, with 3 scans per second. The optimized conditions were: nebulizer pressure 11psig; desolvation gas temperature, 350°C; desolvation gas flow, 11 L/min and capillary voltage, 2 kV, fragmentor at 180 V, cone 1 (skimmer 1) at 45 V, Oct RFV at 750 V. A 10 µL of sample was injected into the system. Raw data were evaluated based on retention time and characteristic behavior of MS, including the exact mass, quasimolecular ions and in-source fragmentation, using Mass Hunter Qualitative Analysis Software version B.06.00 (Agilent Technologies). These data were compared with known compounds in an in-house plant database and existing literature [27]. Positive identifications of plant metabolites were considered for analysis if the compound was detected with a mass error below 10 ppm and with a sufficient score.
2.8 Statistical Analysis
All results on the antimicrobial assay and acute toxicity test were carried out by one-way analysis of variance (ANOVA) using the SAS version 9.4 software package. The mean ± standard deviation was used to express these data. Tukey’s test was used to calculate a statistically significant difference among treatments. All results were considered statistically significant at p ≤ 0.05.
3 Results
3.1 Antimicrobial Activity
The crude methanolic extracts of EWSM, EWEG, EWDP, and EWVA inhibited the growth of S. aureus at 100 mg/ml of crude extracts. At this concentration, the crude methanol extracts of EWSM and EWVA showed a higher zone of inhibition compared with EWEG and EWDP at 100 and 50 mg/ml (Table 1, Supplementary file Figs. 1, 2, 3 and 4). In contrast, the crude extracts of ethyl acetate, chloroform, and aqueous solvents did not inhibit the growth of S. aureus. In addition, the crude ethyl acetate, chloroform, aqueous, and methanol extracts of these four specimens have shown no activity against E. coli and P. aeruginosa. The standard antibiotic disks have shown a higher zone of inhibition than all crude extracts. Consequently, further investigation into the antimicrobial activities, MIC, MBC, acute toxicity and phytochemical composition of E. woodfordioides was conducted using the crude methanol extracts of EWSM and EWVA.
The MIC for EWSM and EWVA against S. aureus was observed at 25 and 12.5 mg/mL, respectively. The negative controls showed full growth of S. aureus at 2% DMSO. Conversely, there was no bacterial growth in erythromycin-treated test tubes. On the other hand, the MBC of EWSM and EWVA were more than 50 mg/mL against S. aureus (Supplementary file Figs. 5, 6, 7 and 8). The ethyl acetate and ethyl acetate: methanol fractionates of EWSM and the ethyl acetate fractions of EWVA exhibited antimicrobial activity against S. aureus. None of the remaining fractions showed inhibition activity against S. aureus.
3.2 Acute Toxicity Test
The administration of crude methanol extracts of EWSM and EWVA exhibited hair erection, hiding in the shavings of the softwood, and being sleepy during the first 4 h of observation. Then after, all mice were active and showed no sign of diarrhea, death, or toxicity throughout the 14 days of observation at all test doses. There was an overall weight gain of mice in the second week of dosing. However, these weight gains were not significantly different among the treatment and control groups of mice (Table 2).
There was a significant difference (p < 0.05) in the mean weight of the kidney with respect to the body weight of the mice. The mean weight of the heart, lungs, and liver did not differ significantly. An exceptional significant difference (p > 0.05) was observed between the body weight of the mice treated with 2000 mg/kg of plant extract and the tap water treated mice (Table 3).
3.3 Phytochemical Analysis of Metabolites Using LC-MS Q-TOF Analysis
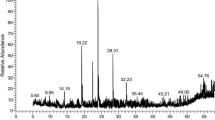
In total, 34 and 11 metabolites were identified in the crude methanol extracts of EWSM (Table 4, Supplementary file Figs. 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42 and 43) and EWVA (Table 5, Supplementary file Figs. 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71 and 72), respectively. In these crude extracts, a greater number of metabolites was detected in the negative ionization mode than in the positive ionization mode. Eight metabolites were shared between the crude methanol extracts of EWSM and EWVA specimens. Flavonoids and phenolics were the most detected classes of compounds in these specimens.
In the EWSM, four compounds were identified in both positive and negative ionization modes. Six out of the nine compounds were found in both ethyl acetate-methanol fractions of EWSM (Table 6, Supplementary file Figs. 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59 and 60). Most of these compounds were detected in the positive ionization mode. In contrast, 23 compounds were identified in ethyl acetate I and II fractions of EWVA (Table 7, Supplementary file Figs. 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103 and 104). Most of the compounds in ethyl acetate-I were identified in the negative ionization mode. An equivalent number of compounds were identified in both ionization modes of ethyl acetate-II. In addition, eight metabolites were commonly shared between the ethyl acetate I and II fractionates of EWVA (Table 7). Five compounds, namely albine, hyperin, melanoxetin, reynoutrin, and trifolin were shared between the two fractions of EWSM and EWVA.
4 Discussion
The present study demonstrated that the crude methanol leaf extracts of E. woodfordioides inhibit the growth of S. aureus. The antimicrobial activity of methanol extracts over other solvent extracts may be associated with the polarity of methanol solvent and the presence of polar bioactive secondary metabolites in these specimens [28]. This is also supported by the antimicrobial activity of polar fractions (ethyl acetate-methanol and the ethyl acetate fractionates) than the low to medium polar (n-hexane, n-hexane/chloroform, chloroform, chloroform/ethyl acetate) fractions of EWSM and EWVA, respectively, against S. aureus. Conversely, the relatively lower inhibition zones exhibited by the methanol extracts of EWEG and EWDP may be related to the difference in the quality and quantity of bioactive compounds available in these specimens, which may partly depend on the host plant species [29]. The sensitivity of S. aureus (Gram-positive) and the resistance of E. coli and P. aeruginosa (Gram-negative) bacteria with respect to the different solvent extracts of E. woodfordioides may be explained by the variation in the cell wall morphology and composition of the two groups of bacteria [30]. In addition, the lower inhibition zone by EWSM and EWVA compared with the standard drugs may be the result of the quality and quantity of bioactive compounds in the crude extracts of E. woodfordioides. Despite the fact that no previous study report is available on the acute toxicology of E. woodfordioides extracts, the absence of physical and behavioral changes, and the death of mice after the administration of 2000 mg/kg bw may indicate the low toxicity of E. woodfordiodes as described in the Globally Harmonized System of Classification and Labeling of Chemicals [31]. Meanwhile, the mean weight of the kidney may be due to other intrinsic factors of the extract.
Previous studies on the phytochemical composition of E. woodfordioides reported the presence of flavonoids, quinones, terpenoids, steroids [32], and condensed tannins [33]. There were no alkaloids and tannins [32]. Nonetheless, these studies did not report details of these phytochemicals. On the other hand, the presence of 4-hydroxybenzoic acid [34], erythritol [35], isorhamnetin [36], fisetin and hyperin [37], and succinic acid [38] were reported for the antimicrobial activity of these metabolites against S. aureus. In agreement with these studies, the detection of various secondary metabolites such as flavonoids and phenolic acids in the leaves of EWSM and EWVA may be responsible for the antimicrobial activity of these plant extract against S. aureus. The detection of different metabolites between the crude extracts and fractionates of EWSM and EWVA might be associated with the variation in the extraction process and polarities of chemical compounds used during the extraction of the plant [28, 39].
5 Conclusion
The crude methanol leaf extract of E. woodfordioides has antimicrobial activity against S. aureus with no prolonged signs of toxicity and mortality in mice. Accordingly, this plant is safe and non-toxic as stated in the Globally Harmonized System of Classification and Labeling of Chemicals. This is the first study to identify metabolites from the leaves of E. woodfordioides. Therefore, it is necessary to further isolate pure compounds responsible for the antimicrobial activity of the plant.
Abbreviations
- ANOVA:
-
Analysis of variance
- DMSO:
-
Dimethyl sulfoxide
- E. coli :
-
Escherichia coli
- EPHI:
-
Ethiopian Public Health Institute
- ETH:
-
National Herbarium at Addis Ababa University, Ethiopia
- EWDP:
-
Englerina woodfordioides collected from Discopedium penninervum
- EWEG:
-
Englerina woodfordioides collected from Eucalyptus globulus host plant
- EWSM:
-
Englerina woodfordioides collected from Schinus molle host plant
- EWVA:
-
Englerina woodfordioides collected from and Vachellia abyssinica
- INT:
-
p-Iodonitrotetrazolium dye
- MBC:
-
Minimum bactericidal concentration
- MHA:
-
Muller Hinton Agar
- MIC:
-
Minimum inhibitory concentration
- OECD:
-
Organization for Economic Cooperation and Development guideline
- P. aeruginosa :
-
Pseudomonas aeruginosa
- LC-MS Q-TOF/MS:
-
Liquid chromatograph mass spectroscopy quadrupole time-of-flight mass spectrometry
- ESI:
-
Electrospray ionization
References
World Health Organization (2019) WHO global report on traditional and complementary medicine 2019.World Health organization
Jouini M, Horchani M, Zardi-Bergaoui A, Znati M, Romdhane A, Krisa S, Waffo-Teguo P, Jannet HB(2021) Phytochemical analysis, neuroprotective, anticholinesterase, cytotoxic and catalase potentials of Opuntia microdasys var. rufida and Opuntia leptocaulis. Chemistry Africa 4:285–298
Amponsah IK, Ramos GF, Harley BK, Sarkoie JA, Ekuadzi E, Ampofo EK, Ben IO (2022) Anti-inflammatory, Antioxidant and Cytotoxic Activities of Guibourtia ehie on Human Prostate (PC-3) and Breast Cancer (MC-7) Cell Lines and in silico Studies on its metabolite 7,4’-Dihydroxyflavone. Chem Afr 5:627–639
Amole KL, Bello IA, Oyewale AO (2019) Synthesis, characterization and antibacterial activities of new. Chem Afr 3:47–55
Znati M, Zardi-Bergaoui A, Daami-Remadi M, Ben Jannet H (2020) Semi-synthesis, antibacterial, anticholinesterase activities, and drug likeness properties of new analogues of coumarins isolated from Ferula lutea (Poir.) Maire. Chem Afr 3:635–646
Grimsson F, Xafis A, Neumann FH, Scott L, Bamford MK, Zetter R (2018) The first Loranthaceae fossils from Africa. Grana 57:249–259
Gilbert MG(1989) Loranthaceae. Eds. Tewolde BerhanGebre Egziabher, OlovHedberg, MesfinTadesse, IbFriis, InngaHedberg and Sue Edwards, Flora of Ethiopia volume 3 361–374. Addis Ababa, Ethiopia
Yirgu A (2014) New host range for parasitic plants in Bonga and Yayu natural forests in Ethiopia. Pest Mgt J Eth 17:37–42
Gebresilase GW(2020) Ethnobotanical study of medicinal plants in two cultures: Hadiya and Yem Peoples, SNNPR, Ethiopia. PhD Dissertation. Addis Ababa University, Addis Ababa, Ethiopia
Polhill R, Wiens D (1998) Mistletoes of Africa. The Royal Botanic Gardens, Kew
Yineger H, Yewhalaw D, Teketay D (2008) Ethnomedicinal plant knowledge and practice of the Oromo ethnic group in southwestern Ethiopia. J Ethnobiol Ethnomed 4(1):1–10
Chekole G (2017) Ethnobotanical study of medicinal plants used against human ailments in Gubalafto District Northern Ethiopia. J Ethnobiol Ethnomed. https://doi.org/10.1186/s13002-017-0182-7
Wubetu M, Abula T, Dejenu G (2017) Ethnopharmacologic survey of medicinal plants used to treat human diseases by traditional medical practitioners in Dega Damot district, Amhara, Northwestern Ethiopia. BMC Res Notes 10:157
Aschale Y, Wubetu M, Reta H (2018) Ethnobotany of medicinal plants used to treat Leishmaniasis in Ethiopia: a systemic review. J Tradit Med Clin Nat 7:2
Zerabruk S, Yirga G (2012) Traditional knowledge of medicinal plants in Gindeberet district, Western Ethiopia. South Afr J Bot 78:165–169
Adelanwa EB, Tijjani AA (2013) An ethno-medical survey of the flora of Kumbotso local Government area of Kano State. Nig J Pharm Sci 12:1–8
Chukwujekwu JC, van Staden J (2016) In vitro antibacterial activity of Combretum edwardsii, Combretum krausii, and Maytenus nemorosa and their synergistic effects in combination with antibiotics. Front Pharmacol 7:208
Mfengwana PMAH, Mashele SS (2016) Antimicrobial activity screening of Philenoptera violacea (Klotzsch) schrire and Xanthocercis zambesiaca (Baker) Dumaz-Le-Grand. J Pharm Sci Res 8(10):1132–1135
Bergman P, Linde C, Putsep K, Pohanka A, Normark S, Henriques-Normark B, Andersson J, Bjorkhem-Bergman L (2011) Studies on the antibacterial effects of statins-in vitro and vivo. PLoS One 6(8):e24394
Graziano TS, Cuzzullin MC, Franco GC, Schwartz-Filho HO, de Andrade ED, Groppo FC, Cogo-Muller K (2015) Statins and antimicrobial effects: simvastatin as a potential drug against Staphylococcus aureus biofilm. PLoS One 10(5):e0128098
Eloff JN (1998) A sensitive and quick microplate method to determine the minimum inhibitory concentration of plant extracts for bacteria. Planta Med 64:711–713
Meot-Duros L, Cerantola S, Talarmin H, Meur CL, Floch GL, Magne C (2010) New antibacterial and cytotoxic activities of falcarindiol isolated in Crithmum maritimum L. leaf extract. Food Chem Toxicol 48:553–557
Kuete V, Teponno RB, Mbaveng AT, Tapondjou LA, Meyer JJM, Barboni L, Lall N (2012) Antibacterial activity of the extracts, fractions and compounds from Dioscorea bulbifera. BMC Complement Altern Med 12:228
Organization for Economic Cooperation and Development Guideline for testing of chemicals (OECD) (2008) Acute oral toxicity-Up-and-Down-Procedure (UDP), Guideline no. 425. Section 4, OECD Publishing. Rome, Italy
Oghobase GE, Aladesanmi OT, Akomolafe RO, Olukiran OS, Akano PO, Eimunjeze MH (2020) Assessment of the toxicity and biochemical effects of detergent processed cassava on renal function of Wistar rats. Toxicol Rep 7:1103–1111
Kifayatullah M, Mustafa MS, Sengupta P, Sarker Md MR, Das A, Das SK (2015) Evaluation of the acute and sub-acute toxicity of the ethanolic extract of Pericampylus glaucus (Lam.) Merr. in BALB/c mice. J Acute Dis 4(4):309–315
Tafuri S, Cocchia S, Carotenuto D, Vassetti A, Staropoli A, Mastellone V, Peretti V, Ciotola F, Albarella S, Del Prete C, Palumbo V, Esposito L, Vinale F, Ciani F (2019) Chemical analysis of Lepidium meyenii (Maca) and its effects on redox status and on reproductive biology in stallions, Molecules 24(10): 1981
Truong D-H, Nguyen DH, Ta NTA, Bui AV, Do TH, Nguyen HC (2019) Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J Food Qual 2019: 1–9
Deeni YY, Sadiq NM (2002) Antimicrobial properties and phytochemical constituents of the leaves of African mistletoe (Tapinanthus dodoneifolius (DC) Danser) (Loranthaceae): an ethnomedicinal plant of Hausaland, Northern Nigeria. J Ethnopharmacol 83:235–240
Breijyeh Z, Jubeh B, Karaman R (2020) Resistance of Gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules 25:340
United Nations (UN) (2011) Globally Harmonized System of Classification and Labeling of Chemicals (GHS). UN Economic Commission for Europe, Geneva. http://www.unece.org/trans/danger/publi/ghs/ghs_rev04/04files_e.html
Ngbolua K-N, Dalley-Divin KS-S, Jean MM, Jean-Claude KK, Odilon KK, Ulrich M, Desire MM, Mpiana PT, Mudogo V (2014) Phytochemical investigation and TLC screening for antioxidant activity of 24 plant species consumed by the Eastern Lowland gorillas (Gorilla beringei ssp. graueri: Hominidae, Primates) endemic to Democratic Republic of the Congo. J Adv Med Life Sci 1(3):1–6
Waterman PG, Choo GM, Vedder AL, Watts D (1983) Digestibility, digestion-inhibitors and nutrients of herbaceous foliage and green stems from and African montane flora and comparison with other tropical flora. Oecologia 60:244–249
Cho J-Y, Moon J-H, Seong K-Y, Park K-H (1998) Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci Biotechnol Biochem 62(11):2273–2276
de Cock P, Makinen K, Honkala E, Saag M, Kennepohl E, Eapen A (2016) Erythritol is more effective than xylitol and sorbitol in managing oral health endpoints. Int J Dent 2016
Gong G, Guan Y-Y, Zhang Z-L, Rahman K, Wang S-J, Zhou S, Luan X, Zhang H (2020) Isorhamnetin: a review of pharmacological effects, review. Biomed Pharmacother 128:110301
Mogana R, Adhikari A, Tzar MN, Ramliza R, Wiart C (2020) Antibacterial activities of the extracts, fractions and isolated compounds from Canarium patentinervium Miq, against bacterial clinical isolates. BMC Complement Med Ther 20:55
Silva E, Monteiro R, Grainha T, Alves D, Pereira MO, Sousa AM (2020) Fostering innovation in the treatment of chronic polymicrobial cystic fibrosis-associated infections exploring aspartic acid and succinic acid as ciprofloxacin adjuvants. Front Cell Infect Microbiol 10:441
Abarca-Vargas R, Malacara CFP, Petricevich VL (2016) Characterization of chemical compounds with antioxidant and cytotoxic activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) extracts. Antioxidants 5:45
Acknowledgements
We thank Addis Ababa University (AAU) and the EPHI for providing technical support and bacterial strains. AY is grateful to Marlies Spohn, Helmut Spohn and his colleagues at Ethiopian Forest Development, Central Ethiopia Center and AAU for their unreserved assistance and encouragement. We thank Dr Adey Feleke for her motivation during laboratory works and financial assistance for laboratory consumables. Finally, our thanks go to the editor of the Journal and the anonymous reviewer for their critical comments and suggestions to improve the manuscript.
Funding
Partial financial support was received from Addis Ababa University that covers fieldwork expenses and purchase of some laboratory consumables through thematic projects.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval and consent to participate
The study was carried out following all relevant guidelines and regulations.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yirgu, A., Mekonnen, Y., Eyado, A. et al. Antimicrobial Activity and Phytochemical Constituents of Leaf Extracts of Englerina woodfordioides (Schweinf.) M. Gilbert. Chemistry Africa 6, 845–853 (2023). https://doi.org/10.1007/s42250-022-00535-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00535-w