Abstract
Purpose
Polycyclic aromatic hydrocarbons (PAHs) are toxic and volatile in nature. They can persist for a long time in the environment. The aim of this work was to investigate the levels of PAHs and their diagnostic ratios in the samples of lake water of Grovnes peninsula, East Antarctica.
Methods
Total fourty five samples were collected from fifteen different lakes of the Northern & Southern Grovnes peninsula. Sixteen compounds of PAHs were estimated in the samples by GC–MS/MS (Gas chromatography-mass spectrometry) technique.
Results
PAHs levels were found from 10.33 to 44.35 pg ml−1 in the lake water samples. Fluorene was detected in all fifteen different lakes. The highest concentration of fluorene was found in the L7BNG lake. PAHs diagnostic ratios were found in the range of 0.76–4.68 for ∑LMW/∑HMW, 0.44–0.80 for FL/(FL + PYR), 1.00 for ANT/(ANT + PHE), 0.37–0.58 for FLA/(FLA + PYR), 0.44–0.47 for BaA/(BaA + CHR) in all fifteen different lakes.
Conclusion
The present study confirms the presence of PAHs in a small amount in Grovnes peninsula, East Antarctica. The presence of additional pollutants should be investigated in order to comprehensively assess the scale of environmental pollution in Antarctica.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
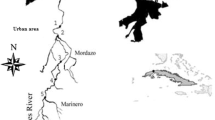
More than 150 lakes are situated on different islands and peninsulas of Larsemann Hills. The Larsemann Hills (69o24′S, 76o13′E) are an approximately 50 km2 oasis which are located halfway between the Vestfold Hills and the Amery Ice Shelf on the southeastern coast of Prydz Bay, Princess Elizabeth Land, East Antarctica (Fig. 1). The lakes are the source of precious water and provide habitats for animals [1]. Most of the Antarctic lakes are freshwater lakes which exhibit a wide range of physical and chemical conditions. These lakes may be perennially or annually ice-covered and usually contain simplified truncated food webs.
Gillieson [2] has explained that the melted polar ice gets accumulated in the depression around the Larsemann Hills and forms newly freshwater lakes. The ice melting increased in the summer season, which then supplies water to the lakes. While in the winter season, water is frozen and makes Antarctica the largest stock of freshwater on the globe [3, 4]. These lakes are being polluted due to anthropogenic activities such as various research activities, tourism, and logistic support [5]. The environment of Antarctica is changing hastily because anthropogenic activities and their impact are increasing incontinent. However, these effects are not evenly spread on the continent because the human footprints are minor with respect to the size of Antarctica.
PAHs are hydrophobic compounds and ubiquitous in nature [6, 7]. These chemical compounds are migrating globally due to the long-range atmospheric transport mechanism as well as through anthropogenic activities [8]. According to Fuoco et al. [9] and Barrie et al. [10] some amount of PAHs is transported to Antarctica through the migrant species, water and after travel an extensive distance, these pollutants are dumped far from their original place. The soils/sediments have been identified as a final sink for PAHs where they can be released into the water [11]. These toxic compounds are semi-volatile and persist in the atmosphere for a long period [12]. They are highly toxic to organisms due to their carcinogenic and mutagenic potential [13, 14]. The toxicity of PAHs depends on their chemical structure and shares some characteristics like teratogenic, mutagenic, carcinogenic [15].
PAHs are the most frequent petroleum byproducts and are used as energy sources by research stations [16]. In the Antarctic environment, PAHs are usually associated with local activities and come from petroleum products [17]. They have lethal properties, especially in the case of chronic exposure, and may be harmful to the environment [18, 19]. Cripps [20] stated that supply ships and a few tourist vessels carry the major PAHs hazard. Dobbins et al. [21] and Liang et al. [22] have reported that the major source of the PAHs in the lakes of East Antarctica is diesel fuel which is commonly used in Antarctica. Local activities linked with transportation, fuel storage, and borehole drilling have inevitably led to the emission of fuel scums, combustion exhausts. Oil spills rather than long-range atmospheric transport appeared to be the more leading source of PAHs in the Antarctic lakes. Fuel combustion may also be a viable source of PAHs to the Antarctic lakes. Incomplete combustion from the agricultural, industrial, natural, and domestic is the major source of PAHs [23, 24].
PAHs resist biological, photolytic, chemical degradation and can be accidentally formed from industrial activities. Several researchers have reported the presence of PAHs in different components of abiotic and biotic [25,26,27]. PAHs diagnostic ratio is a new tool to differentiate PAH sources between the pyrogenic (such as high-temperature combustion of biomass and fossil fuels) and the petrogenic (such as fuels, lubricants, crude oil, and their derivatives). It has been used to distinguish diesel and gasoline combustion emission [28], different crude oil processing products and biomass burning processes including bush, savanna, and grass fires [29]. Mackay et al. [30] stated that the compounds of the PAHs diagnostic ratio have similar physicochemical properties and similar molar mass. Manoli et al. [31] reported that the profile of PAH emission for a source depends on the PAHs producing processes. Low molecular weight PAHs (LMWPAHs) (less than 4 rings) are formed during the low-temperature processes such as wood-burning while high molecular weight PAHs (HMWPAHs) (greater than 3 rings) are formed during the high-temperature processes such as the combustion of fuels in engines [32, 33].
The aim of this study was to estimate the occurrence of PAHs and their diagnostic ratios in the lakes which were near the Bharati research station, on the Grovnes peninsula, East Antarctica. This study is novel itself because this is one of the rare studies which is analyzing PAHs and identify their diagnostic ratios in the Grovnes peninsula. This study exactly falls in the thrust area of the topic water sciences & environment of the journal. In the future, this study can make an important contribution to the checking concentration of PAHs in the Antarctic environment.
2 Experimental Section
2.1 Sampling Sites and Collection
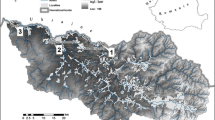
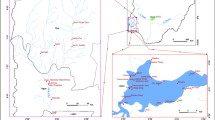
Larsemann Hills are the biggest area of East Antarctica’s coastline and has the two major peninsulas Stornes and Broknes, 130 nearshore islands, and four minor peninsulas [34]. Grovnes peninsula is situated between Broknes and Stornes peninsula. It is divided into Northern and Southern Grovnes peninsula. The sampling sites for the study were on the Grovnes peninsula, which are shown in Figs. 2 and 3.
A total of fourty five surface samples, 1.0 L each from fifteen lakes in triplicate were collected randomly during the 34th Indian Scientific Expedition to Antarctica (ISEA). The sample collection was carried out from 2014 to 2015 by helicopter. Thirty samples were collected from the ten different lakes of the Northern Grovnes peninsula and fifteen samples were collected from the five different lakes of the Southern Grovnes eninsula. The samples were collected in pre-cleaned amber glass bottles. By using the method USEPA-1699 [35], the sampling bottles were pre-treated using deionized water, then rinsed with lake water before use. After that they were stored at − 4 °C. The lake’s identification, coordinates, and depth are given in Table 1.
2.2 Requirements (Instruments/Reagents)
GC–MS/MS (Model-7000, Agilent), electronic weighing balance (Sartorius), rotary evaporator (Buchi), separatory funnel (2000 ml), glass chromatography column (250 mm length × 10 mm width) and round bottom (RB) (250 ml) flask were used for the PAHs analysis. The standard for PAHs (XA11729V) was procured from Supelco. Hexane (SJ75F71067), cyclohexane (29,029,090), pentane (109,660), and methylene dichloride (2,233,940,917) were procured from Merck. Silica gel (S8171) of mesh size (100–200) and anhydrous sodium sulfate (Q14446) were purchased from Fisher Scientific. Before the use, silica gel was activated at 130 °C for 16 h and anhydrous sodium sulfate was kept at 400 °C for 4 h.
2.3 Analytical Methods
2.3.1 Sample Extraction
The lake water samples were extracted through the USEPA 3510B [36] method with slight modification. One liter of each lake water sample was taken into the glass separatory funnel and then 60 ml methylene dichloride was added. The sample was shaken for 1–2 min and released the extra pressure. The funnel was kept for 5–10 min on the stand to allow the organic layer separation from the water layer. The lower layer of methylene chloride was taken into an RB flask. This procedure was repeated two more times. The collected solvent was vaporized at 45 °C by the rotary evaporator. Then the final residue solution (2 ml) in cyclohexane was ready for a cleanup.
2.3.2 Sample Cleanup
The final residue solution was cleaned up through the USEPA 3630C [37] method. The glass chromatography column was organized with sodium sulfate and silica gel. Approx. 5 g silica gel and 2–3 g of sodium sulfate were transferred into the column. The column was prewashed with 50 ml of pentane. The final extract solution was transferred into the column. A 50 ml of solution (pentane: methylene chloride, 1:1) was transferred into the column and then the column elution was collected into the RB flask with the flow rate of 0.5 ml. The whole process was repeated two times and the collected solvent was evaporated at 45 °C by the rotary evaporator. The residue was re-dissolved in 1 ml of hexane and then was transferred into a GC vial for the analysis of PAHs by GC–MS/MS.
2.4 Chromatographic Analysis
GC–MS/MS was used for the estimation of PAHs. The final sample solution (1 µl) was introduced into the system. The column HP-5MS (30 m × 0.25 mm × 0.25 µm, Agilent) was used for the separation of compounds. Helium was the carrier gas with a constant flow of 1 ml/min and nitrogen was the make-up gas. The temperature of the injector and detector was set at 280 °C. The initial temperature of GC oven was set at 70 °C for 2 min and ramped at 25 °C/minute, to 150 °C and then ramped at 3 °C/minute, to 200 °C and then ramped at 8 °C /minute to 280 °C for 10 min [38]. The overall time for the analysis was 41.87 min. The temperature of the transfer line was set at 280 °C and the quadrupole (Q1 & Q2) temperature was 180 °C.
2.5 Quality Assurance/Quality Control (QA/QC)
PAHs were quantified with their standards of different concentrations (10 pg ml−1, 25 pg ml−1, 50 pg ml−1, 100 pg ml−1, and 250 pg ml−1) by using GC–MS/MS technique. Each lake water sample was evaluated in triplicate and then the mean value was taken. With every set of five samples, one blank was examined to check the contamination. For the recovery test, seven injections of the same concentration of a particular compound were injected into the system and the recovery rate was found between 40 and 100%, which fulfilled the acceptance criteria of the recovery limit of the USEPA 1699 [35] method. The limit of detection (LOD) for PAHs was 10.00 pg ml−1 while the RSD was < 2.0%. The multiple reaction monitoring (MRM) of PAHs is listed below in Table 2 and basic detail (abbreviation, number of rings & molar mass) is shown in Table 3.
3 Results
In this study, the concentration of PAHs was varied from 10.33 pg ml−1 to 44.35 pg ml−1 in the fifteen lakes of Grovnes peninsula, East Antarctica. Fluorene was higher in the samples of L7BNG.
3.1 Estimation of PAHs in the Samples of the Northern Grovnes peninsula
The concentration of PAHs was found from 10.45 pg ml−1 to 44.35 pg ml−1 in the ten lakes of the Northern Grovnes peninsula. The concentrations of PAHs compounds are shown in Fig. 4 and Tables 4 and 5.
3.1.1 L1CNG Lake
Naphthalene was the main pollutant with a high concentration of 26.33 ± 1.53 pg ml−1, followed by fluorene 21.00 ± 1.00 pg ml−1, anthracene 18.00 ± 4.58 pg ml−1, pyrene 18.00 ± 2.65 pg ml−1, acenaphthene 14.68 ± 1.54 pg ml−1, fluoranthene 13.01 ± 1.72 pg ml−1, chrysene 11.47 ± 0.80 pg ml−1 while other compounds were found in BDL.
3.1.2 L1DNG Lake
Naphthalene was the main pollutant with a high concentration of 36.00 ± 2.00 pg ml−1, followed by fluorene 27.77 ± 2.25 pg ml−1, pyrene 26.00 ± 2.00 pg ml−1, fluoranthene 24.00 ± 3.61 pg ml−1, anthracene 23.03 ± 0.99 pg ml−1, acenaphthene 19.00 ± 1.00 pg ml−1, chrysene 14.36 ± 1.52 pg ml−1 while other compounds were found in BDL.
3.1.3 L1ENG Lake
Pyrene was the main pollutant with a high concentration of 37.34 ± 3.05 pg ml−1, followed by naphthalene 33.00 ± 1.00 pg ml−1, fluorene 29.00 ± 3.61 pg ml−1, anthracene 27.00 ± 2.00 pg ml−1, acenaphthene 22.33 ± 1.53 pg ml−1, fluoranthene 22.03 ± 4.38 pg ml−1, chrysene 12.00 ± 2.00 pg ml−1 while other compounds were found in BDL.
3.1.4 L3NG Lake
Pyrene was the main pollutant with a high concentration of 14.00 ± 1.73 pg ml−1, followed by anthracene 12.33 ± 2.08 pg ml−1, fluorene 11.17 ± 0.85 pg ml−1, naphthalene 11.00 ± 1.00 pg ml−1, chrysene 10.45 ± 0.50 pg ml−1 while other compounds were found in BDL.
3.1.5 L5NG Lake
Pyrene was the main pollutant with a high concentration of 15.35 ± 2.51 pg ml−1, followed by fluorene 12.69 ± 1.53 pg ml−1, naphthalene 11.67 ± 0.58 pg ml−1, anthracene 11.13 ± 0.85 pg ml−1 while other compounds were found in BDL.
3.1.6 L6NG Lake
Anthracene was the main pollutant with a high concentration of 16.35 ± 2.50 pg ml−1, followed by chrysene 13.00 ± 2.65 pg ml−1, pyrene 12.00 ± 2.00 pg ml−1, fluoranthene 11.30 ± 0.82 pg ml−1, fluorene 11.20 ± 0.80 pg ml−1 while other compounds were found in BDL.
3.1.7 L7NG Lake
Fluorene was the main pollutant with a high concentration of 41.00 ± 4.00 pg ml−1, followed by naphthalene 35.33 ± 3.06 pg ml−1, anthracene 35.00 ± 4.36 pg ml−1, acenaphthene 17.36 ± 1.54 pg ml−1, fluoranthene 14.35 ± 1.52 pg ml−1, chrysene 14.35 ± 1.51 pg ml−1, benz[a]anthracene 12.53 ± 2.25 pg ml−1, pyrene 10.43 ± 0.51 pg ml−1 while other compounds were found in BDL.
3.1.8 L7ANG Lake
Fluorene was the main pollutant with a high concentration of 42.80 ± 0.72 pg ml−1, followed by naphthalene 38.67 ± 1.53 pg ml−1, pyrene 37.00 ± 2.65 pg ml−1, fluoranthene 35.69 ± 2.50 pg ml−1, anthracene 32.53 ± 2.55 pg ml−1, acenaphthene 26.38 ± 0.56 pg ml−1, chrysene 14.04 ± 0.05 pg ml−1, benz[a]anthracene 11.47 ± 0.50 pg ml−1 while other compounds were found in BDL.
3.1.9 L7BNG Lake
Fluorene was the main pollutant with a high concentration of 44.35 ± 3.07 pg ml−1, followed by naphthalene 39.80 ± 1.59 pg ml−1, pyrene 34.67 ± 2.52 pg ml−1, anthracene 33.00 ± 5.57 pg ml−1, acenaphthene 28.00 ± 2.00 pg ml−1, fluoranthene 25.36 ± 0.60 pg ml−1, chrysene 15.33 ± 2.08 pg ml−1, benz[a]anthracene 11.87 ± 1.20 pg ml−1 while other compounds were found in BDL.
3.1.10 Murk Water Lake
Pyrene was the main pollutant with a high concentration of 15.70 ± 1.50 pg ml−1, followed by fluorene 12.67 ± 1.53 pg ml−1, naphthalene 12.33 ± 2.08 pg ml−1, anthracene 11.40 ± 1.51 pg ml−1, acenaphthene 11.07 ± 0.90 pg ml−1 while other compounds were found in BDL.
3.2 Estimation of PAHs in the Samples of the Southern Grovnes Peninsula
The concentration of PAHs was found from 10.33 pg ml−1 to 41.00 pg ml−1 in the five lakes of the Southern Grovnes peninsula. The concentrations of PAHs compounds are shown in Fig. 5 and Table 6.
3.2.1 L1SG Lake
Anthracene was the main pollutant with a high concentration of 13.69 ± 2.05 pg ml−1, followed by fluorene 11.68 ± 1.53 pg ml−1, pyrene 11.13 ± 1.10 pg ml−1, acenaphthene 10.53 ± 0.42 pg ml−1 and other compounds were found in BDL.
3.2.2 L2SG Lake
Fluorene was the main pollutant with a high concentration of 36.00 ± 3.61 pg ml−1, followed by pyrene 30.53 ± 0.50 pg ml−1, acenaphthene 23.35 ± 2.05 pg ml−1, naphthalene 15.67 ± 2.52 pg ml−1 and other compounds were found in BDL.
3.2.3 L3SG Lake
Fluorene was the main pollutant with a high concentration of 12.00 ± 1.00 pg ml−1, followed by pyrene 11.73 ± 2.05 pg ml−1, naphthalene 11.00 ± 1.00 pg ml−1 and other compounds were found in BDL.
3.2.4 L4SG Lake
Fluorene was the main pollutant with a high concentration of 41.00 ± 1.00 pg ml−1, followed by naphthalene 29.73 ± 1.55 pg ml−1, anthracene 20.00 ± 4.58 pg ml−1, acenaphthene 13.69 ± 2.06 pg ml−1, chrysene 11.97 ± 1.05 pg ml−1, pyrene 10.33 ± 0.58 pg ml−1 and other compounds were found in BDL.
3.2.5 L5SG Lake
Fluorene was the main pollutant with a high concentration of 40.07 ± 2.00 pg ml−1, followed by anthracene 25.00 ± 2.65 pg ml−1, naphthalene 20.00 ± 1.00 pg ml−1, chrysene 11.00 ± 1.00 pg ml−1, pyrene 10.43 ± 0.12 pg ml−1 and other compounds were found in BDL.
3.3 3.3. PAHs Diagnostic Ratio in the Samples of the Northern Grovnes Peninsula
PAHs diagnostic ratio is based on the parent PAHs and is applicable to the determination of PAHs in water, air, soil, and sediment. Different PAHs diagnostic ratios were calculated in the samples on the basis of the concentration of PAHs. They were found in the range of 0.76 to 3.02 for ∑LMW/∑HMW, 0.44–0.80 for FL/(FL + PYR), 1.00 for ANT/(ANT + PHE), 0.37–0.58 for FLA/(FLA + PYR), 0.44–0.47 for BaA/(BaA + CHR) in the ten different lakes of the Northern Grovnes peninsula. PAHs diagnostic ratios are shown in Table 7.
3.4 PAHs Diagnostic Ratio in the Samples of the Southern Grovnes Peninsula
Different PAHs diagnostic ratios were calculated in the samples on the basis of the concentration of PAHs. They were found in the range of 1.96–4.68 for ∑LMW/∑HMW, 0.51–0.80 for FL/(FL + PYR), 1.00 for ANT/(ANT + PHE) in the five different lakes of the Southern Grovnes peninsula. PAHs diagnostic ratios are shown in Table 8.
4 Discussion
The result of the present study indicates that lakes in the vicinity of the Bharati research station on Grovnes peninsula have been polluted with traces of PAHs. We found that the lakes close to the research station, such as L1CNG, L1DNG, L1ENG, L7NG, L7ANG & L7BNG had relatively high PAHs concentration while lakes far from the research stations such as L3NG, L5NG, L6NG, L1SG, L2SG, L3SG, L4SG & L5SG had low PAHs concentration. This contamination may be due to the discharge from the research station and the influence of anthropogenic activities such as the use of carbon-based fuels for household activities, power generation, and transportation. Lenihan et al. [39] & Cripps [20] also stated in their study that the presence of PAHs levels was due to anthropogenic activities, which came from the research station and tourism while Wania and Mackay [40] supported the theory of migration of PAHs from world to pole. The discharge from the McMurdo research station was affecting the lakes near the research station [41].
Human activities such as oil spills from transportation or even oil spills from vehicles or the use of oil or gasoline, which burns at high temperatures in light-duty gasoline vehicles, as well as fossil-fuel combustion, were responsible for the low PAHs in the lakes far from the research station [42,43,44]. Most of the locations in Antarctica at which PAHs have been reported by researchers for example- Western Antarctica Dickhut [45], Gerlache Inlet, Ross Sea & Terranova Bay [46]. These locations have a relatively long history of human activities such as improper disposal of old electrical equipment and incineration of wastes generated by the research stations. The presence of PAH concentration near to the different research stations (McMurdo station 621–5024 ng g-1; Jubany station 28–1908 ng g-1; James Ross Island 1–2005 ng g-1) [47,48,49] was due to oil spills or fuel combustion.
The highest total PAH concentrations (Σ16PAHs) were observed in L7ANG followed by L7BNG, L1ENG, L7NG, L1DNG, L4SG, L1CNG, L5SG, L2SG, L6NG, Murk Water Lake NG, L3NG, L5NG, L1SG & L3SG. Phenanthrene, pyrene, and fluoranthene were reported as the most abundant PAHs on King George Island, Antarctica [50]. Alekseev and Abakumov [51] reported the PAHs (fluorene and acenaphthylene) in the soil of Larsemann Hills, Fulmar Island, Eastern Antarctica. Our study also reported a high concentration of fluorene and naphthalene. Apart from these two, pyrene, fluoranthene, anthracene, acenaphthene, chrysene, benz-ananthracene, etc. reported in trace quantities. While acenaphthylene, phenanthrene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene, indeno[1,2,3-cd]pyrene, dibenz[a,h]anthracene, benzo[g,h,i]perylene were found below the detection limits.
Several researchers have reported the presence of PAHs from the different parts of Antarctica such as, in penguins and seabirds [52], naphthalene, and acenaphthalene in the lake water samples of Sarah Tarn near the Law Base station of East Antarctica [53], in Inland Lake of Larsemann Hills from 14 to 360 ng l−1 [54], in the wreck (up to 600 µg kg−1) of the Bahia Paraiso near the USA Palmer research station, Anvers Island [16], in sewage effluents and small oil spillages [55], in the air (7134.49 pg m−3) and in soil (61.09 ng g−1) from the Filds peninsula, King George Island [56], in oil exposed soil samples (0.9–30.8 mg kg−1) of King George Island [57], naphthalene, phenanthrene, and fluorene from the Fildes peninsula, King George Island [58]. Szopińska et al. [59] have studied the PAHs in Antarctic diesel-exposed soils or sediments (10–40 mg kg−1) and in non-exposed samples (10–100 µg kg−1).
The occurrences of PAHs in the Antarctic lake water samples explain the transportation of contamination. Atmospheric transportation may be a possible source of PAHs. The presence of PAHs in the Antarctic residents is due to biomagnification [60] while in the lake water, soil and sediment is due to the activities of the Antarctic residents [61]. The presence of PAHs in the Antarctic lakes may constitute a potentially negative effect on the environment of Antarctica [62]. The accumulation of PAHs in the Antarctic organisms and its environment may cause mutagenicity, genotoxicity, reproductive disorders, and interference in the immune system.
4.1 Identification of PAHs Sources by Diagnostic Ratio
Several scientists reported different PAHs emission sources based on the PAHs diagnostic ratio. Wang et al. [63] reported combustion and petroleum-related sources of PAH emission on the basis of ANT/(ANT + PHE) and FLA/(PYR + FLA) in the water samples of the Yellow River Delta while Wang et al. [64] studied the mixed petrogenic and pyrogenic sources of PAHs in the samples of the Pearl River Delta. The combustion is a source of waterborne PAHs on the basis of BaA/(BaA + CHR), ANT/(ANT + PHE) and IcdP/(IcdP + BghiP), while petroleum combustion and coal/biomass burning are the sources of PAHs in Elelenwo Creek water of Nigeria on the basis of FLA/(PYR + FLA) [65].
The ratio FLA/(PYR + FLA) indicated sampling points affected by fuel combustion processes, unburnt petroleum products and biomass burning [66]. Riccardi et al. [67] reported the PAHs in the groundwater originated from fuel leaks on the basis of ANT/(ANT + PHE) and FLA/(PYR + FLA). Wang et al. [68] studied that coal combustion is the source of PAHs in the precipitation samples of Taishan Mountain, China on the basis of PHE/(PHE + ANT), FLA/(FLA + PYR), and BaA/(BaA + CHR) ratios. The LMWPAHs/HMWPAHs ratio may be much higher for water samples than PM2.5 and particulate matter samples [69]. Hwang and Foster [70] described that coal-burning as PAHs emission sources on the basis of high BbF/BkF and BaA/CHR. Jacobs et al. [71] stated that PAHs undergo photolysis in the aquatic environment which may alter the values of diagnostic ratios. The ratios FLA/(FLA + PYR), IcdP/(IcdP + BghiP) are more conservative than ANT/(ANT + PHE), BaA/(BaA + CHR), and are sensitive to photodegradation. The ratio ANT/(ANT + PHE) is sensitive to environmental changes. Tobiszewski and Namieśnik [72] have well presented the PAHs emission sources with the value of PAHs diagnostic ratios in Table 9.
Our study reported the PAHs ratio ∑LMW/∑HMW > 1 for all lakes of the Grovnes peninsula except L6NG. The ratio FL/(FL + PYR) was found > 0.5 for all lakes except L1ENG, L3NG, L5NG, L6NG & Murk Water Lake NG. The ratio ANT/(ANT + PHE) was found > 0.1 for all lakes except L2SG & L3SG. The ratio FLA/(FLA + PYR) was found < 0.4 for L1ENG, 0.4–0.5 for L1CNG, L1DNG, L6NG, L7ANG & L7BNG, > 0.5 for L7NG. The ratio BaA/(BaA + CHR) was found > 0.35 for L7NG, L7ANG & L7BNG.
In future research, recently emerging contaminants must be studied with the estimation of PAHs. Through a long time study, future research will diagnose different diseases and it will relate to the concentration of PAHs over a period [78]. The present study provides an idea about the concentration of PAHs and their harmful effect on the Antarctic environment. We can decrease or replace the usage of PAHs with the outcome of other alternates. But we should ensure that these alternatives may be other chemicals and cannot replace one toxic chemical with others.
5 Conclusions
In the present study, fifteen different lake water samples were examined for the estimation of PAHs and the result indicates that the Grovnes peninsula has been contaminated with trace amounts of PAHs. Fluorene was the most dominant pollutant and the highest concentration was found in L7BNG lake while other lakes were less polluted. This study confirms the presence of PAHs in another region of Antarctica as reported earlier. Several factors such as oil spills, fossil fuels, different transport resources, human activities, etc. may be the major cause of PAHs in the pristine environment of Antarctica. Long-range atmospheric transport is another cause which helps to carry the PAHs into the poles. PAHs diagnostic ratios have been used for the identification of PAHs sources. It should be used with caution, as PAHs ratio values may change during the environmental fate of these compounds. More than one diagnostic ratio should be used to confirm the results. LMWPAHs are emitted from different sources than HMWPAHs.
We can regulate and measure the emission of PAHs through an effective monitoring system. In the future, we can make stronger guidelines for the minimization of PAHs from the continent. Scientific support and tourist activities have increased over the last few decades. These activities are affecting the ecosystem of Antarctica. Due to the large size of the continent, PAHs do not evenly distribute in Antarctica but if not checked properly, they will leave the residue in the future. Monitoring of the PAHs plays a key role to control its emission and continental migration at the global level by which we can reduce PAHs contamination globally and protect the pristine ecosystem of Antarctica.
Data availability
Not applicable.
Code availability
Not applicable.
References
Bharti PK, Niyogi UK (2015) Assessment of Pollution in a freshwater lake at Fisher Island, Larsemann Hills over east Antarctica. Science International 3(1):25–30
Gillieson D (1990) An atlas of the lakes of the Larsemann Hills, Princess Elizabeth Land, Antarctica (Vol. 74). Australian National Antarctic Research Expeditions, Antarctic Division, Dept. of the Arts, Sport, the Environment, Tourism, and Territories.
Roberts D, McMinn A (1996) Relationships between surface sediment diatom assemblages and water chemistry grdients in saline lakes of the Vestfold Hills. Antarctica Antarctic Science 8(4):331–341
Hodgson DA, Vyverman W, Sabbe K (2001) Limnology and biology of saline lakes in the Rauer Islands, eastrn Antarctica. Antarct Sci 13(3):255
Legrand M, Mayewski P (1997) Glaciochemistry of polar ice cores: a review. Rev Geophys 5(3):219–243
Avino P, Notardonato I, Perugini L, Russo MV (2017) New protocol based on high-volume sampling followed by DLLME-GC-IT/MS for determining PAHs at ultra-trace levels in surface water samples. Mirochemical Journal 133:251–257
Wild S, McLagan D, Schlabach M, Bossi R, Hawker D, Cropp R, King CK, Stark JS, Mondon J, Nash SB (2015) An Antarctic research station as a source of brominated and perfluorinated persistent organic pollutants to the local environment. Environ Sci Technol 49(1):103–112
Hund AJ (2014) Antarctica and the Arctic Circle: A Geographic Encyclopedia of the Earth's Polar Regions [2 volumes]. ABC-CLIO.
Fuoco R, Giannarelli S, Wei Y, Ceccarini A, Abete C, Francesconi S, Termine M (2009) Persistent organic pollutants (POPs) at Ross sea (Antarctica). Microchem J 92(1):44–48
Barrie LA, Gregor D, Hargrave B, Lake R, Muir D, Shearer R, Tracey B, Bidleman T (1992) Arctic contaminant: sources, occurrence and pathways. Sci Total Environ 122(1–2):1–74
Negoita TG, Covaci A, Gheorghe A, Schepens P (2003) Distribution of polychlorinated biphenyls (PCBs) an organochlorine pesticides in soils from the East Antarctic coast. J Environ Monit 5(2):281–286
Jacob J (2013) A review of the accumulation and distribution of persistent organic pollutants in the environment. Int J Biosci Biochem Bioinform 3(6):657
UNEP (United Nations Environmental Programme) (1992) Determination of petroleum hydrocarbons in sediments, reference methods for marine pollution studies. UNEP, Nairobi, Kenya 20:97
Yang Z, Feng J, Niu J, Shen Z (2008) Release of polycyclic aromatic hydrocarbons from Yangtze River sedient cores during periods of simulated resuspension. Environ Pollut 155(2):366–374
Woolfenden EN, Hince G, Powell SM, Stark SC, Snape I, Stark JS, George SC (2011) The rate of removal ad the compositional changes of diesel in Antarctic marine sediment. Sci Total Environ 410:205–216
Kennicutt MC, Sweet ST, Fraser WR, Stockton WL, Culver M (1991) Grounding of the Bahia Paraiso a Arthur Harbor, Antarctica. 1. Distribution and fate of oil spill related hydrocarbons. Environ Sci Technol 25(3):509–518.
Priddle J (2002) Regionally based assessment of persistent toxic substances-Antarctica. United Nations Environment Program Global Environment Facility. Chatelaine, Switzerland: UNEP Chemicals
Dauner ALL, Hernández EA, MacCormack WP, Martins CC (2015) Molecular characterisation of anthropogenic sources of sedimentary organic matter from Potter Cove, King George Island, Antarctica. Sci Total Environ 502:408–416
Moodley L, Arnberg M, Dunaevskaya E, Seear P, Bærheim L, Nilsen M, Ramanand SS, Øysæd KB, Westerlund S, Jonsson G, Tarling GA (2018) Effects of low crude oil chronic exposure on the northern krill (Meganyctiphanes norvegica). J Exp Mar Biol Ecol 500:120–131
Cripps GC (1992) Natural and anthropogenic hydrocarbons in the Antarctic marine environment. Mar Pollut Bull 25(9–12):266–273
Dobbins RA, Fletcher RA, Benner JBA, Hoeft S (2006) Polycyclic aromatic hydrocarbons in flames, in diesel fuels, and in diesel emissions. Combust Flame 144(4):773–781
Liang F, Lu M, Keener TC, Liu Z, Khang SJ (2005) The organic composition of diesel particulate matter, diesel fuel and engine oil of a non-road diesel generator. J Environ Monit 7(10):983–988
Yang R, Xie T, Li A, Yang H, Turner S, Wu G, Jing C (2016) Sedimentary records of polycyclic aromatic hydrocarbons (PAHs) in remote lakes across the Tibetan Plateau. Environ Pollut 214:1–7
Liu B, Xue Z, Zhu X, Jia C (2017) Long-term trends (1990–2014), health risks, and sources of atmospheric polycyclic aromatic hydrocarbons (PAHs) in the US. Environ Pollut 220:1171–1179
Cabrerizo A, Dachs J, Barceló D, Jones KC (2012) Influence of organic matter content and human activities on the occurrence of organic pollutants in Antarctic soils, lichens, grass, and mosses. Environ Sci Technol 46(3):1396–1405
Piazza R, Gambaro A, Argiriadis E, Vecchiato M, Zambon S, Cescon P, Barbante C (2013) Development of a method for simultaneous analysis of PCDDs, PCDFs, PCBs, PBDEs, PCNs and PAHs in Antarctic air. Anal Bioanal Chem 405(2–3):917–932
Bhardwaj LK, Chauhan A, Ranjan A, Jindal T (2018) Persistent organic pollutants in biotic and abiotic components of Antarctic pristine environment. Earth Systems and Environment 2(1):35–54
Ravindra K, Sokhi R, Van Grieken R (2008) Atmospheric polycyclic aromatic hydrocarbons: source attribution, emission factors and regulation. Atmos Environ 42(13):2895–2921
Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S (2002) PAHs in the Fraser River basin: a critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33(4):489–515
Mackay D, Shiu WY, Lee SC (2006) Handbook of physical-chemical properties and environmental fate for organic chemicals. CRC Press
Manoli E, Kouras A, Samara C (2004) Profile analysis of ambient and source emitted particle-bound polycyclic aromatic hydrocarbons from three sites in northern Greece. Chemosphere 56(9):867–878
Mostert MM, Ayoko GA, Kokot S (2010) Application of chemometrics to analysis of soil pollutants. TrAC, Trends Anal Chem 29(5):430–445
Sun F, Wen D, Kuang Y, Li J, Li J, Zuo W (2010). Concentrations of heavy metals and polycyclic aromatic hydrocarbons in needles of Masson pine (Pinus massoniana L.) growing nearby different industrial sources. J Environ Sci 22(7):1006–1013.
Hodgson DA, Verleyen E, Sabbe K, Squier AH, Keely BJ, Leng MJ, Saunders KM, Vyverman W (2005) Late Quaternary climate-driven environmental change in the Larsemann Hills, East Antarctica, multi-proxy evidence from a lake sediment core. Quatern Res 64(1):83–99
USEPA (2007) Method 1699: Pesticides in Water, Soil, Sediment, Biosolids and Tissue by HRGC/HRMS.
USEPA 3510B (1994) Separatory Funnel Liquid Liquid Extraction. Revision 2, September 1994.
USEPA 3630C (1996) Silica Gel Cleanup. Revision 3, December 1996.
Bhardwaj LK, Sharma S, Ranjan A, Jindal T (2019) Persistent organic pollutants in lakes of Broknes peninsula at Larsemann Hills area. East Antarctica Ecotoxicology 28(5):589–596
Lenihan HS, Oliver JS, Oakden JM, Stephenson MD (1990) Intense and localized benthic marine pollution around McMurdo Station. Antarctica Marine Pollution Bulletin 21(9):422–430
Wania F, Mackay D (1996) Peer reviewed: tracking the distribution of persistent organic pollutants. Environ Sci Technol 30(9):390A-396A
Hale RC, Kim LS, Harvey E, Guadia MJL, Mainor TM, Bush E, Jacobs EM (2008) Antarctic research bases: local sources of polybrominated diphenyl ether (PBDE) flame retardants. Environ Sci Technol 42:1452–1457
Ravindra K, Wauters E, Van Grieken R (2008) Variation in particulate PAHs levels and their relation with the transboundary movement of the air masses. Sci Total Environ 396(2–3):100–110
Miguel AH, Kirchstetter TW, Harley RB, Hering RA (1998) On-road emissions of particulate polycyclic aromatichydrocarbons and black carbon from gasoline and dieselvehicles. Environ Sci Technol 32:450–455
Tao S, Li X, Yang Y, Coveney RM, Lu X, Chen H, Shen W (2006) Dispersion modeling of polycyclic aromatic hydrocarbons from combustion of biomass and fossil fuels and production of coke in Tianjin. China Environmental science & technology 40(15):4586–4591
Dickhut RM, Cincinelli A, Cochran M, Ducklow HW (2005) Atmospheric concentrations and air-water flux of organochlorine pesticides along the western Antarctic Peninsula. Environ Sci Technol 39:465–470
Fuoco R, Giannarelli S, Wei Y, Abete C, Francesconi S, Termine M (2005) Polychlorobiphenyls and polycyclic aromatic hydrocarbons in the sea-surface micro-layer and the water column at Gerlache Inlet, Antarctica. J Environ Monitoring 7:1313–1319
Kim M, Kennicutt MC, Qian Y (2006) Molecular and stable carbon isotopic characterization of PAH contaminants at McMurdo Station, Antarctica. Mar Pollut Bull 52:1585–1590
Curtosi A, Pelletier E, Vodopivez CL, Mac Cormack WP (2007) Polycyclic aromatic hydrocarbons in soil and surface marine sediment near Jubany Station (Antarctica). Role of permafrost as a low-permeability barrier. Sci Total Environ 383:193–204
Klanova J, Matykiewiczova N, Macka Z, Prosek P, Laska K, Klan P (2008) Persistent organic pollutants in soils and sediments from James ROSS Island, Antarctica. Environ Pollut 152:416–423
Deelaman W, Pongpiachan S, Tipmanee D, Suttinun O, Choochuay C, Iadtem N, Charoenkalunyuta T, Promdee K (2020) Source apportionment of polycyclic aromatic hydrocarbons in the terrestrial soils of King George Island, Antarctica. J South Am Earth Sci 104:102832.
Alekseev I, Abakumov E (2021) Polycyclic aromatic hydrocarbons, mercury and arsenic content in soils of larsemann hills, pravda coast and fulmar island, eastern antarctica. Bull Environ Contam Toxicol 106(2):278–288
Paruk JD, Long D IV, Perkins C, East A, Sigel BJ, Evers DC (2014) Polycyclic aromatic hydrocarbons detected in Common Loons (Gavia immer) wintering off coastal Louisiana. Waterbirds 37:85–93
Goldsworthy PM, Canning EA, Riddle MJ (2003) Soil and water contamination in the Larsemann Hills. East Antarctica Polar Record 39(4):319–337
Yao Y, Meng XZ, Wu CC, Bao LJ, Wang F, Wu FC, Zeng EY (2016) Tracking human footprints in Antarctica through passive sampling of polycyclic aromatic hydrocarbons in inland lakes. Environ Pollut 213:412–419
Young LY, Cerniglia CE (1995) Microbial transformation and degradation of toxic organic chemicals, vol 15. Wiley-Liss, New York
Na G, Gao Y, Li R, Gao H, Hou C, Ye J, Jin S, Zhang Z (2020) Occurrence and sources of polycyclic aromatic hydrocarbons in atmosphere and soil from 2013 to 2019 in the Fildes Peninsula, Antarctica. Marine Pollution Bulletin 156:111173.
Gran-Scheuch A, Ramos-Zuñiga J, Fuentes E, Bravo D, Pérez-Donoso JM (2020) Effect of Co-contamination by PAHs and heavy metals on bacterial communities of diesel contaminated soils of South Shetland Islands. Antarctica Microorganisms 8(11):1749
Préndez M, Barra C, Toledo C, Richter P (2011) Alkanes and polycyclic aromatic hydrocarbons in marine surficial sediment near Antarctic stations at Fildes Peninsula. King George Island Antarctic Sci 23(6):578
Szopińska M, Namieśnik J, Polkowska Ż (2016) How important is research on pollution levels in Antarctica? Historical approach, difficulties and current trends. In Reviews of Environmental Contamination and Toxicology 239:79–156. Springer, Cham.
Corsolini S, Borghesi N, Ademollo N, Focardi S (2011) Chlorinated biphenyls and pesticides in migrating and resident seabirds from East and West Antarctica. Environ Int 37(8):1329–1335
Kumar KS, Kannan K, Corsolini S, Evans T, Giesy JP, Nakanishi J, Masunaga S (2002) Polychlorinated dibenzo-p-dioxins, dibenzofurans and polychlorinated biphenyls in polar bear, penguin and south polar skua. Environ Pollut 119(2):151–161
Szopińska M, Szumińska D, Bialik RJ, Dymerski T, Rosenberg E, Polkowska Ż (2019) Determination of polycyclic aromatic hydrocarbons (PAHs) and other organic pollutants in freshwaters on the western shore of Admiralty Bay (King George Island, Maritime Antarctica). Environ Sci Pollut Res 26(18):18143–18161
Wang L, Yang Z, Niu J, Wang J (2009) Characterization, ecological risk assessment and source diagnostics of polycyclic aromatic hydrocarbons in water column of the Yellow River Delta, one of the most plenty biodiversity zones in the world. J Hazard Mater 169(1–3):460–465
Wang JZ, Nie YF, Luo XL, Zeng EY (2008) Occurrence and phase distribution of polycyclic aromatic hydrocarbons in riverine runoff of the Pearl River Delta. China Marine Pollution Bulletin 57(6–12):767–774
Opuene K, Agbozu IE, Adegboro OO (2009) A critical appraisal of PAH indices as indicators of PAH source and composition in Elelenwo Creek, southern Nigeria. Environmentalist 29(1):47–55
Tobiszewski M, Tsakovski S, Simeonov V, Namieśnik J (2010) Surface water quality assessment by the use of combination of multivariate statistical classification and expert information. Chemosphere 80(7):740–746
Riccardi C, Di Filippo P, Pomata D, Incoronato F, Di Basilio M, Papini MP, Spicaglia S (2008) Characterization and distribution of petroleum hydrocarbons and heavy metals in groundwater from three Italian tank farms. Sci Total Environ 393(1):50–63
Wang Y, Li PH, Li HL, Liu XH, Wang WX (2010) PAHs distribution in precipitation at Mount Taishan: China. Identification of sources and meteorological influences. Atmospheric Res 95(1):1–7.
Li PH, Wang Y, Li YH, Wang ZF, Zhang HY, Xu PJ, Wang WX (2010) Characterization of polycyclic aromatic hydrocarbons deposition in PM2. 5 and cloud/fog water at Mount Taishan (China). Atmospheric Environ 44(16):1996–2003.
Hwang HM, Foster GD (2006) Characterization of polycyclic aromatic hydrocarbons in urban stormwater runoff flowing into the tidal Anacostia River, Washington, DC, USA. Environ Pollut 140(3):416–426
Jacobs LE, Weavers LK, Chin YP (2008) Direct and indirect photolysis of polycyclic aromatic hydrocarbons in nitrate-rich surface waters. Environ Toxicol Chem 27(8):1643–1648
Tobiszewski M, Namieśnik J (2012) PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119
Zhang W, Zhang S, Wan C, Yue D, Ye Y, Wang X (2008) Source diagnostics of polycyclic aromatic hydrocarbons in urban road runoff, dust, rain and canopy throughfall. Environ Pollut 153(3):594–601
Pies C, Hoffmann B, Petrowsky J, Yang Y, Ternes TA, Hofmann T (2008) Characterization and source identification of polycyclic aromatic hydrocarbons (PAHs) in river bank soils. Chemosphere 72(10):1594–1601
Roberto J, Lee WY, Campos-Díaz SI (2009) Soil-borne polycyclic aromatic hydrocarbons in El Paso, Texas: analysis of a potential problem in the United States/Mexico border region. J Hazard Mater 163(2–3):946–958
Akyüz M, Çabuk H (2010) Gas–particle partitioning and seasonal variation of polycyclic aromatic hydrocarbons in the atmosphere of Zonguldak. Turkey Sci Total Environ 408(22):5550–5558
Oliveira C, Martins N, Tavares J, Pio C, Cerqueira M, Matos M, Silva H, Oliveira C, Camões F (2011) Size distribution of polycyclic aromatic hydrocarbons in a roadway tunnel in Lisbon. Portugal Chemosphere 83(11):1588–1596
Boekelheide K, Blumberg B, Chapin RE, Cote I, Graziano JH, Janesick A, Lane R, Lillycrop K, Myatt L, States JC, Thayer KA (2012) Predicting later-life outcomes of early-life exposures. Environ Health Perspect 120(10):1353–1361
Acknowledgements
The authors acknowledge with thanks to the Director of NCPOR (National Centre for Polar and Ocean Research) and Earth Sciences, Ministry for the participation in 34th ISEA.
Funding
This study was not supported by any funding agency.
Author information
Authors and Affiliations
Contributions
All authors have equal contribution.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Bhardwaj, L.K., Sharma, S. & Jindal, T. Occurrence of Polycyclic Aromatic Hydrocarbons (PAHs) in the Lake Water at Grovnes Peninsula Over East Antarctica. Chemistry Africa 4, 965–980 (2021). https://doi.org/10.1007/s42250-021-00278-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-021-00278-0