Abstract
The growth, biosorption efficiency (%), and exopolysaccharides (EPS) production of Lysinibacillus fusiformis KMNTT-10 strain in nutrient broth containing Pb2+ ions was investigated in this study. Further, the interaction of Pb2+ ions with KMNTT-10 biomass and the state of the adsorbed Pb2+ ions were studied by FTIR, SEM–EDS, and XRD analysis. Experimental results showed that the growth and biosorption efficiency of KMNTT-10 strain for Pb2+ ions were highest at pH 6.0, 96 h incubation time, and 10 mg/L of initial Pb2+ ions concentration. Also, the biosorption efficiency and EPS production of KMNTT-10 strain for Pb2+ ions were dependent on their growth in nutrient broth containing Pb2+ ions. Under nutrient broth containing 10, 50, and 100 mg/L of Pb2+ ions, the biosorption efficiency of KMNTT-10 strain for Pb2+ ions was 98.2, 82.9, and 68.5%, respectively, and their EPS production was 0.42, 0.36, 0.29 mg/100 mL, respectively. From this, it is apparent that bacterial growth, biosorption capacity, and EPS production are interrelated and depend on the concentration of Pb2+ ions in nutrient broth. Characterization studies revealed that the KMNTT-10 strain exhibited a distorted cell surface when grown under Pb2+ ions treated broth, and they adsorbed the Pb2+ ions via an ionic interaction and transformed it into PbS form either intra/extracellularly. The present study’s results suggested that the biosorption and biotransformation potential of KMNTT-10 strain for Pb2+ ions could be used in metal removal applications at the pilot scale.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Lead (Pb), the second most toxic and persistent metal, is naturally found in the Earth’s crust in very limited quantity (0.002%). Although lead is naturally present in the Earth’s crust, its contamination of the environment is extensively caused by anthropogenic activities. As a result of the increased use of lead in anthropogenic activities, the environment becomes progressively more contaminated with lead [1]. Over the last five decades, it is estimated that over 783,000 tons of lead have been released into the environment worldwide [2, 3]. Smelting, Mining, manufacturing, and recycling processes are the principal causes of lead pollution. Recently, worldwide lead production has increased due to their increased use in automobiles and mobile phone batteries. Living beings exposed to lead contamination are mostly from water, batteries vent, soil, lead paint, contaminated diet, and dust particles [3, 4].
Lead has both acute and chronic toxicity as they (including its compounds) are mutagenic, carcinogenic, and highly toxic. Both organic and inorganic form of lead is highly toxic at even low dose and therefore, causes potential health hazards to humans [5]. Both short and long-term exposure can induce hypertension, anemia, cognitive deficiencies, infertility, immunological imbalances, vitamin D deficiency, and gastrointestinal problems in humans [6]. Thus, lead and its compounds should be treated before being discharged into the environment. There are many physical and chemical treatment methods available to treat lead pollution, including, flotation, flocculation, coagulation, ion exchange, adsorption, membrane filtration, chemical precipitation, and electrochemical techniques. Although there are many physical and chemical treatment methods, they all have some limitations such as high cost, high energy requirements, inefficiency at low concentrations, and secondary waste production [7, 8]. As a consequence, researchers have started to focus on bioremediation strategies that impart sustainable solutions to lead pollution in an eco-friendly manner. Biological systems such as bacteria, fungi, algae, plants, and their products are widely used in the bioremediation of metal pollution due to their cost-effective and eco-friendly nature [9]. Amongst, bacteria have drawn great interest in the bioremediation of pollutants due to their highly diverse and adaptable nature, ease of cultivation, and short generation time [10, 11]. In general, bioremediation of metal by bacteria begins with complex formation, ion exchange, chelation, micro-precipitation, and adsorption by anionic functional groups on their surface [12]. Both live and dead bacterial biomass can adsorb metal ions, but their efficiency varies. Bacterial biomass, both live and dead, has advantages and drawbacks in metal ions adsorption. In comparison to live biomass, dead biomass has several advantages including lower operational cost, no growth media or nutrient requirement, no concern about metal ions toxicity, and reusability of biomass. Despite being vulnerable to metal toxicity and requiring nutrients for growth, live biomass nevertheless has certain advantages. Live bacterial biomass is capable of producing extracellular polymeric substances in their surroundings, and the products thus produced contain more negatively charged functional groups at neutral pH [13]. EPS production by bacteria serves as a defense strategy against environmental stress, particularly metal ions stress. In Particular, live biomass under metal ions stress can produce more extracellular polymeric substances than normal and this can be a protective barrier to survive under harsh conditions. Furthermore, as bacteria produce more EPS under metal ions stress conditions, the number of functional groups in them increases, allowing them to adsorb more metal ions [14, 15]. Bacteria can change the composition and concentration of EPS production in response to metal ions concentrations, and these alterations can improve its metal biosorption ability and reduce metal stress [16]. Live biomass not only adsorbs the metal ions but also reduces the toxic effects by transforming the state of metal ions via oxidation, reduction, and precipitation mechanism [17]. Further, the transformation in the state of metal ions by live bacterial biomass is different for each element and bacterial species. It has been reported that E.coli FACU can reduce the highly toxic Cr(VI) into less toxic Cr(III) through the production of chromium reductase [18]. Abbas et al. [19] stated that isolates Enterobacter sp. (MNZ1), Klebsiella pneumoniae 1 (MNZ4) and Klebsiella pneumonia 2 (MNZ6) converted more toxic form of arsenite (As (III)) into less toxic form arsenate (As(V)) through an oxidation process. In addition, the transformation of metal ions into metal sulfide is one resistance mechanism of bacteria that is controlled by their hydrogen sulfide (H2S) production and enzymatic reaction [20].
Understanding the metal ions transformation by live bacterial biomass is an important measure in the biogeochemical cycle as well as environmental remediation of metal ions. A perfect remediation process is not only removing the metal ions from polluted sites but also transforming them into useful substances. So, the bacteria that possess high metal biosorption efficiency and detoxifying potential are important for successful environmental decontamination. The biosorption of lead ions by live bacterial biomass has been extensively studied but their transformation is still unclear in some bacteria. In our previous study, the Pb2+ ions biosorption of Lysinibacillus (L.) fusiformis KMNTT-10 was investigated in the absence of a growth medium and this study showed that biomass of this strain effectively adsorbed Pb2+ ions from aqueous solutions [10]. However, the Pb2+ ions biosorption of this strain in the growth medium is still unknown. By keeping the above bottlenecks, biosorption of Pb2+ ions by L. fusiformis KMNTT-10 in the growth medium under the effect of pH, incubation time, and initial lead ions concentrations was investigated. In addition, the exopolysaccharides (EPS) production, interaction, and chemical nature of biomass with Pb2+ ions were studied.
2 Materials and Methods
2.1 Strain maintenance and Pb2+ ions solution preparation
L. fusiformis KMNTT-10, a multi-metal resistant bacterium, was previously isolated from polluted estuarine in Tamil Nadu, India, and maintained in the 20% seawater nutrient (SWN) agar [21]. The KMNTT-10 has grown in 20% SWN broth at 29 ± 2 ºC for a day and is used as an inoculum for the biosorption studies. The analytical grade Pb(NO3)2 (RANKEM, India) was used to prepare the stock solution (1000 mg/L), which was then diluted to prepare the initial working concentrations for biosorption studies.
2.2 Growth and biosorption efficiency of L. fusiformis KMNTT-10 under Pb2+ ions treated conditions
The biosorption of Pb2+ ions by growing L. fusiformis KMNTT-10 in culture media was studied in a batch system using the method described by De et al. [22]. For this study, 1 mL of 24 h grown KMNTT-10 culture was inoculated in 20% SWN broth treated with Pb2+ ions and incubated at room temperature (27 ± 2◦C) on a rotary shaker (150 rpm) for 120 h. The culture without the addition of Pb2+ ions was maintained as a control to compare the growth of KMNTT-10 strain in the Pb2+ ions treated condition. Every 24 h, the growth of KMNTT-10 strain in the Pb2+ ions treated and the untreated broth was checked using a UV/vis spectrophotometer at 600 nm (Model UV 1800 SHIMADZU, Japan). The biosorption of Pb2+ ions by KMNTT-10 strain was determined by the estimation of Pb2+ ions in the supernatant. To study the biosorption of Pb2+ ions by KMNTT-10 strain, 1 ml of culture grown in Pb2+ ions treated broth was collected and centrifuged (12,000 RPM for 10 min at 4 ºC) to collect the supernatant. The collected supernatant was filtered through 0.22-µm membrane filters, digested in 10% HNO3, and used for estimation of Pb2+ ions using a flame-type atomic absorption spectrophotometer (model AA-7000, Shimadzu, Japan). The experiment was performed in triplicate, with the mean value recorded. The biosorption (%) of Pb2+ ions by KMNTT-10 biomass was computed using the following formula:
where Ci and Ce are the concentrations of initial and equilibrium Pb2+ ions in the solution (mg/L), respectively.
To determine the optimal pH favors better growth and biosorption efficiency of KMNTT-10 in 20% SWN broth containing Pb2+ ions, the various pH levels from acidic (3.0) to alkaline (8.0) were studied. The pH of Pb2+ ions treated with 20% SWN broth was adjusted using 1N NaOH and 1N HCl before each experiment. For this study, the initial Pb2+ ions concentration, culture inoculum, and contact time were fixed at 50 mg/L, 1 mL, and 96 h, respectively. The impact of initial Pb2+ ions concentrations (10, 50, 100 mg/L) on the biosorption efficiency was studied at constant parameters; pH 6.0, 1 mL culture inoculum, and contact time (96 h). Similarly, the impact of contact time was also assessed at constant pH 6.0, biomass concentration (1 mL), and initial Pb2+ ions concentration (50 mg/L) on Pb2+ ions biosorption.
2.3 Exopolysaccharides production by L. fusiformis KMNTT-10 under Pb2+ ions treated conditions
To determine the influence of Pb2+ ions on exopolysaccharides production, the L. fusiformis KMNTT-10 strain was cultured in 20% SWN broth with different concentrations of Pb2+ ions (10, 50, and 100 mg/L) at pH 6.0 for 96 h. After 96 h, the supernatant was collected from the culture broth and subjected to the alcohol-precipitation method to obtain EPS, as the method described earlier by Kazy et al. [23]. In brief, the supernatant was mixed with two-fold cold ethanol (96%), then left at 4 ºC overnight for EPS precipitation. The precipitated EPS was collected, lyophilized, and quantified with reference to its total carbohydrate content by phenol sulphuric acid method [24].
2.4 Characterization of KMNTT-10 biomass and its EPS
2.4.1 SEM–EDS analysis of biomass
The morphological and elemental information of KMNTT-10 biomass grown in Pb2+ ions treated and the untreated broth was determined using Scanning Electron Microscope (SEM) Coupled with Energy Dispersive X-ray Spectroscopy (EDS). For this, KMNTT-10 biomass was cultured in 50 mg/L 20% SWN broth containing Pb2+ ions (pH 6.0) for 96 h at room temperature. Then, the biomass was collected, washed, fixed, and dehydrated according to the method described by De et al. [22]. Then, the samples were smeared in coverslip, air dried, mounted on SEM stub, and sputter-coated with gold. The coated samples were analyzed using SEM–EDS analysis (model Vega III, TESCAN, Czech Republic).
2.4.2 FTIR and XRD analysis of biomass
The Pb2+ ions binding sites in the KMNTT-10 biomass and its chemical nature after biosorption was investigated using Fourier transform infrared spectroscopy (FT-IR) and X-ray diffraction spectroscopy (XRD) analysis, respectively. For this, the KMNTT-10 strain was cultured in 20% SWN broth treated with 50 mg/L Pb2+ ions (pH 6.0) for 96 h. To compare, the KMNTT-10 strain was cultured in 20% SWN broth without Pb2+ ions. Then, the KMNTT-10 biomass from the Pb2+ ions treated and the untreated broth was collected, dried, and powdered as the method described by Zhang and Huang [25]. For FT-IR analysis, the powdered biomass samples were mixed, ground with KBr to form a disc, and analyzed in the range of 4000 to 400 cm−1 using FT/IR-6600, JASCO, Japan. For XRD analysis, the powdered samples of Pb2+ ions treated and the untreated broth was analyzed in the range between 10° and 79° (2θ) with a step length of 0.05° (2θ) using X’Pert Pro, PANanalytical, United Kingdom. The obtained XRD results were compared with diffraction data of samples with the powder diffraction data files for metals and alloys, JCPDS, International Centre for Diffraction Data (ICDD, 1997).
2.4.3 FTIR analysis of EPS
To study the contribution of exopolysaccharides in Pb2+ ions biosorption by KMNTT-10 biomass, the EPS was collected from Pb2+ treated and untreated culture broth. The condition and collection of EPS from Pb2+ treated and untreated culture broth were described in Sect. 2.3. The EPS collected from Pb2+ treated and untreated culture broth was mixed and ground with KBr to create a disc, which was then analysed in the range of 4000 to 400 cm−1 using FT/IR-6600, JASCO, Japan.
3 Results and Discussion
3.1 Growth and biosorption efficiency of KMNTT-10 strain for Pb2+ ions
3.1.1 Effect of pH
It is apparent from Fig. 1a that the growth and Pb2+ ions biosorption efficiency of KMNTT-10 are linearly correlated to pH, i.e., biosorption efficiency was increased with an increase in pH to optimum value, and then efficiency started to decline at a very high pH. The optimum pH condition for maximal growth and Pb2+ biosorption of the KMNTT-10 was found to be pH 6.0 in which biosorption performance and growth of KMNTT-10 in 50 mg/L Pb2+ treated broth were determined to be 2.289 (OD at 600 nm) and 82.68%, respectively. Mwandira et al., [26] stated that Oceanobacillus profundus removed 97% of Pb2+ ions at pH 6.0 from LB medium containing 20 mg/L of PbCl2. Mushtaq et al. [12] reported the strain Levilactobacillus brevis MZ384011 and Levilactobacillus brevis MW362779 biosorbed 70 and 83% of Pb2+ ions, respectively at pH 6.0 from 10 mg/L lead nitrate treated broth. Li et al. [27] showed the Pb2+ ions biosorption capacity (%) of Bacillus cereus SEM-15 in nutrient broth containing Pb2+ ions was good at a pH range from 6.0–7.0. In comparison to the results of others, the Pb2+ ions biosorption capacity (%) of most bacterial strains in nutrient broth containing Pb2+ ions is optimum at pH 6.0–7.0. From this, it can be known that growth and biosorption efficiency are interrelated to each other and the amount of Pb2+ ions biosorption is determined by their excellent growth of KMNTT-10 strain in the nutritional condition. Further, the initial pH value in the broth is vital for biomass growth and metal ions availability in the broth. Jin and Kirk [28] stated that the environmental pH is generally controlling microbial occurrence and distribution by affecting microbial respiration. So, the changes in environmental pH may alter metabolic activity, which limits bacterial growth. Also, pH affects metal solubility and speciation in the solution as well as biomass surface properties [26]. Metal ions availability and biomass surface properties are two important phases in the biosorption process and alterations in either will have an impact on the biosorption process [29]. The first phase, the availability of metal ions in the solution, is determined by their speciation as a function of pH. The different metal forms present in the solution are due to speciation, which also determines the biosorption process. For instance, lead exists as Pb2+ ions predominantly in the range between 3.3 and 5 while, several low soluble hydroxylated species namely Pb(OH)2 and Pb(OH)−3 occur at pH values higher than 5 [30]. The second phase of biosorption is determined by freely available interactive sites of biomass surface for metal ions. The biomass surface charges vary depending on the pH value, for instance, H+ and hydronium (H3O+) ions are abundant at lower pH, and this protonates the biomass surface, limiting the metal ions and biomass interaction. The availability of hydrogen (H+) and hydronium (H3O+) ions is low at higher pH values, which considerably facilitated the contact of metal ions with the surface area of the bacterial biomass [31]. This present study’s data unveiled that the biosorption efficiency of growing bacterial biomass for metal ions in the nutrient broth is determined by their outstanding growth, which is influenced by the initial pH of the nutrient broth.
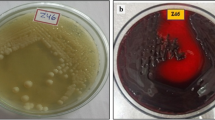
Growth and biosorption efficiency of KMNTT-10 for Pb2+ ions in Pb2+ ions treated 20% SWN broth (a) different pH ranges (3.0–8.0) (b) different incubation times (0–120 h) (c) different initial Pb2+ ions concentrations (10, 50, and 100 mg/L). Error bars represent standard deviations. (d) Growth of KMNTT-10 in 50 mg/L Pb2+ ions treated and untreated 20% SWN broth after 24 and 96 h incubation. The green circle highlights the black colour precipitate with biomass collected from Pb2+ ions treated broth
3.1.2 Effect of incubation time
The incubation time is important for achieving complete growth of the bacterium in the batch culturing system. Figure 1b depicts the growth and biosorption efficiency of KMNTT-10 under 50 mg/L Pb2+ ions treated condition at various incubation times. The growth of KMNTT-10 strain increased as the incubation times were increased up to 96 h, after which it began to decrease significantly. At 96 h, the maximal growth of KMNTT-10 strain was 2.29 at 600 nm. Like growth, biosorption of Pb2+ ions by KMNTT-10 biomass was increased with incubation time and reached a maximum at 96 h incubation and it indicated that biosorption efficiency was dependent on the KMNTT-10 growth in the broth. De et al. [32] found that the biosorption potential (%) of Pseudomonad strain (CH07) was achieved higher in Pb2+ ions treated nutrient broth at 96 h incubation. A study by Naik et al. [33] reported that the growth and biosorption of P. stutzeri strain M-9 and V. harveyi strain M-11 in TMM broth containing lead ions depended on incubation time. Typically, bacteria have four phases of growth in a batch system: lag, log/exponential, stationary, and decline. Due to adaptability, the bacterial growth phases may also be prolonged under stress conditions [34]. Further, the biosorption efficiency of bacteria in the batch system can be greater in the exponential and stationary phases, possibly due to their constant growth and secondary metabolite production. Furthermore, the exopolymeric substances produced by bacteria can enhance their biosorption efficiency for metal ions. EPS production can be higher at stationary phase of bacteria growth as reported by [35, 36]. Results showed that Pb2+ ions biosorption by KMNTT-10 in the batch system was higher at the stationary phase, which could be attributed to their higher EPS production at this phase. Zhou et al., [37] observed similar findings while studying the removal efficiency of Pseudoalteromonas sp. SCSE709-6 under various initial cadmium concentrations, and his study revealed that stationary phase culture had the highest removal efficiency. Whereas, Shirdam et al. [38] stated that maximum metal uptake by Pseudomonas pseudoalkaligenes PTCC 1666, Pseudomonas putida PTCC 1664, Bacillus cereus PTTC 1665, and occurred during the stationary growth phase. Park and Chon [39] reported that the stationary phase biomass of Exiguobacterium sp. had the highest cadmium biosorption efficiency when compared to other growth phases of biomass (early exponential phase, middle exponential phase, late exponential phase). The findings of Chang et al. [40] pointed out that the higher Pb adsorption by Pseudomonas aeruginosa during the stationary phase could be due to its biopolymer production.
3.1.3 Effect of initial Pb2+ ions concentrations
It is essential to know the initial metal ions concentration that permits outstanding growth and greater biosorption efficiency of bacteria for successful biosorption. Figure 1c presents the growth and biosorption efficiency of KMNTT-10 at various Pb2+ concentrations. The growth of KMNTT-10 strain was good at low Pb2+ ions concentration (10 mg/L), but it was diminished at higher concentrations (50 and 100 mg/L). This result indicates that the KMNTT-10 strain can grow well by alleviating the toxicity of Pb2+ ions in the broth added with low concentration than with higher concentration. From this, Pb2+ ions create a more stressful environment for bacterial growth in the broth added with higher Pb2+ ions. Similar findings were observed by Yu et al., [41] when they examined the growth of Bacillus sp. Pz-1 in various Pb2+ ions concentrations, and reported that low concentrations promote growth while high concentrations inhibit it. Maldonado et al. [42] investigated the growth of Micrococcus luteus DE2008 in LB medium containing varying concentrations of Pb(NO3)2 (0.1, 0.5, 1, 1.5, and 2 mM), and their findings revealed that growth of Micrococcus luteus DE2008 was higher at lower concentrations and lower at higher concentrations. In general, the bacterial generation time may be prolonged under stress conditions due to their physiological adaptations, and it might be greatly reliant on toxicity and concentrations of metal ions [34, 43]. Like growth, the biosorption efficiency of KMNTT-10 was higher at low Pb2+ ions concentrations and it was 98.2%. However, the biosorption efficiency of KMNTT-10 biomass was reduced at increasing concentrations, with values of 82.9% and 68.5% at 50 and 100 mg/L Pb2+ ions, respectively. De et al. [22] stated the isolate Pseudomonad (CH07), removed > 98% of Pb2+ ions from 100 mg/L Pb2+ ions treated culture broth. Saranya et al. [44] reported a similar trend of biosorption potential (%), and their study showed the biosorption potential of Cronobacter muytjensii for cadmium, chromium, copper, and zinc was greater at lower concentrations (100 mg/L) and reduced at higher concentrations (200 mg/L). Comparing the KMNTT-10 growth and biosorption efficiency at various initial Pb2+ ions concentrations, it is clear that the biosorption of Pb2+ ions is mostly determined by the growth. Guo et al., [43] reported that the cell population should have a significant impact on the bioremediation rate of living bacteria. Further, the bacteria exposed to metal ions can increase the production of the exopolymeric substances on their surface, and this production can be dependent on the metal ions concentration in the broth. Also, the differences in EPS production by living bacterial cells in response to metal ions concentration may have caused changes in biosorption efficiency. For instance, bacteria grown at low metal ions added broth could produce maximal EPS than at higher metal ion concentrations [45].
3.2 EPS production by KMNTT-10 biomass
The EPS produced by KMNTT-10 biomass at various initial Pb2+ ions concentrations is shown in Fig. 2. Results showed that the EPS production by KMNTT-10 biomass under control, 10 mg/L, 50 mg/L, and 100 mg/L was 0.32 mg, 0.42, 0.36, and 0.2 mg/100 mL, respectively. Regarding the EPS production under different metal concentrations, the KMNTT-10 strain produced higher EPS at low Pb2+ ions treated broth (10 mg/L) than in control and higher Pb2+ ions treated broth (50 and 100 mg/L). Also, when comparing the EPS production in Pb2+ treated and untreated culture broth, the EPS production was higher in the Pb2+ ions treated culture broth. Naik et al. [46] investigated the EPS production of Enterobacter cloacae P2B under 1.6-Mm Pb2+ ions treated and untreated broth and the results showed that EPS production was higher in Pb2+ ions treated culture broth (108 mg/L) than in untreated broth (28 mg/L). As evident from the literature, bacteria secrete maximal EPS in response to metal stress, which aids bacteria to grow by counteracting the toxicity. In addition, the EPS production by KMNTT-10 strain was greater at low Pb2+ ions concentration and decreased gradually when Pb2+ ions concentration (50, and 100 mg/L) in the culture broth was increased. This suggested that low Pb2+ ions concentration (10 mg/L) influenced the EPS production by KMNTT-10 strain, whereas high concentrations (50, and 100 mg/L) limited EPS production, which could be related to increased toxicity at high concentrations. Further, the biosorption efficiency and EPS production of the KMNTT-10 strain at Pb2+ ions treated broth were related to each other (Fig. 1c, and Fig. 2). For instance, the biosorption efficiency of KMNTT-10 biomass was dependent on their degree of EPS production under Pb2+ ions concentrations, with the biosorption efficiency being higher in the presence of low Pb2+ ions and lower in the presence of high Pb2+ ions. Similar results were reported by Zeng et al. [47], who found that Bacillus sp. S3 produced more EPS at lower Cd(II), Cr(VI), and Cu(II) concentrations and less EPS at higher concentrations. Similarly, when Klebsiella pneumoniae MCC 3091 were grown under Cd2+-treated conditions, they produced more EPS at low Cd2+ ions concentration (1000 μg/mL) and less EPS at higher Cd2+ ions (2000 and 3000 μg/mL) concentration [48]. Based on these results, bacteria can produce EPS up to a certain degree of metal stress and thereby grow under metal stress; however, when metal ions concentration increases, the EPS production of bacteria decreases, and this decrease in EPS production may be a reason to bacterial growth inhibition at high metal ions concentrations.
3.3 Characterization studies
3.3.1 SEM–EDS analysis of KMNTT-10 biomass
The surface morphology and elemental composition of KMNTT-10 strain grown in the Pb2+ treated and untreated broth after 96 h are shown in Fig. 3a-d. The KMNTT-10 cells of control broth (Pb2+ ions untreated) had rod-shaped with clear and smooth surface morphology. But there were some changes in the morphology of the KMNTT-10 cells that grew under 50 mg/L Pb2+ stress. Although 50 mg/L Pb2+ ions concentrations had no adverse effects on KMNTT-10 growth, they disrupted the cell morphology. External distortion such as membrane disruption and uneven surface morphology was found in the KMNTT-10 cells grown under Pb2+ treated broth (Fig. 3b). Further, more distortions were observed in matured cells than in young cells, implying that matured cells were more vulnerable to Pb2+ stress than young cells, which could be related to declined growth, nutrient scarcity, and Pb2+ toxicity after 96 h incubation. Renu et al. [49] observed distorted morphology with the bulging surface in Ochrobactrum intermedium BB12 grown in 25 mg/L cadmium-added broth. The morphological changes that occur under stress conditions may not only allow bacterial cells to survive but may also benefit them to escape from metal toxicity [50]. Figure 3c&d illustrates the elemental composition of KMNTT-10 biomass grown in Pb2+ ions untreated (control) and treated broth. In the spectrum of KMNTT-10 biomass from control, the elements such as P, S, C, K, O, and Si were majorly observed. In contrast, the spectrum of KMNTT-10 biomass grown in Pb2+ treated broth exhibited a Pb element additionally, indicating the biosorption of Pb2+ from the broth. The mechanisms such as surface complexation, electrostatic attraction, ion exchange, or micro-precipitation are generally involved in the biosorption of metal ions. Further, elements present in the biomass could also determine the ion exchange process and surface complexation during metal biosorption [51]. Hegazy et al., [52] observed noticeable changes in the level of elemental composition of biomass grown in metal-amended conditions than untreated biomass and that these changes might be attributed to the ion exchange of elements with metal ions.
3.3.2 FT-IR analysis of KMNTT-10 biomass
The identification of functional groups is crucial to understand the interactions of bacteria with metal ions during biosorption. The FT-IR spectra of KMNTT-10 biomass grown in Pb2+ untreated and treated broth are shown in Fig. 4. The FTIR spectrum of KMNTT-10 biomass (control) exhibits absorption peaks at wavelengths of 3278.93 (O–H/N–H stretch); 2923.93 (C–H stretch); 1640.41 (amide I; mainly C = O stretch); 1539.81 (amide II; N–H bend); 1397.73 (symmetric stretching of COO–; CH2/CH3 bending), 1233.86 (COO–, P = O, and P–O stretching), and 1065.20 (C–O, C–N stretching) [53, 54]. However, the IR spectrum of KMNTT-10 biomass grown in Pb2+ treated broth showed variation in the intensity and peak position. The peak position detected at the IR spectrum of KMNTT-10 biomass were 3289.70, 2919.01, 1637.60, 1534.72, 1389.14, 1233.09, and 1062.50 cm−1. As shown in Fig. 3, significant changes including decreased intensity and peak position shift were noted in the functional group region of the IR spectrum of KMNTT-10 biomass. Furthermore, the intensity and peak position shifts at 3278.93 to 3289.70 cm−1 and 2923.93 to 2919.01 cm−1 may be owing to interactions between these peaks corresponding functional groups primarily involved in Pb2+ ions biosorption. An increase and decrease in the peak intensity, as well as the shift in peak position, could be caused by the interaction of these peak corresponding functional groups involved in the Pb2+ biosorption [31, 55]. Rajaram et al. [56] reported that hydroxyl groups of EPS were mainly involved with Cu2+ interaction, and this interaction altered the position of other functional groups. Zhang et al. [57] stated that the functional groups of EPS such as –OH and C–O–C of EPS mainly interact with cadmium ions during biosorption. Results showed that there were no differences in the functional groups of biomass when compared to both IR spectra. The findings of Yue et al. [15] revealed that functional groups did not get affected by heavy metals, but it increased the concentrations of surface functional groups in the EPS samples. Overall, IR spectral analysis of biomass from Pb2+ treated and untreated broth revealed that the main functional groups present on the biomass were hydroxyl, carbonyl, carboxyl, amide, imidazole, phosphate, and phosphodiester groups which are primarily present in the cell components such as polysaccharides, proteins, and lipids.
3.3.3 XRD analysis of KMNTT-10 biomass
X-ray diffraction (XRD) was used to understand the nature, and crystallinity of biomass as well as their changes due to interaction with Pb2+ ions. Figure 5 shows the XRD spectra of KMNTT-10 biomass grown in Pb2+ untreated (control) and treated broth. The XRD spectrum of KMNTT-10 strain grown in Pb2+ untreated broth showed very low-intensity peaks indicating that the biomass was amorphous. Whereas, the XRD spectrum of KMNTT-10 biomass grown in Pb2 + treated broth displayed distinct peaks at 2θ values of 30.93°, 43.62°, 62.39°, and 71.87° which corresponded to the planes of (200), (220), (400), and (420), respectively. The XRD spectrum obtained for KMNTT-10 biomass grown in Pb2+ treated broth was compared to powder diffraction files of known compounds (ICDD/JCPDS). Data analysis with a JCPDS card (PDF Nos. 78–1901) showed the presence of PbS in the KMNTT-10 biomass, indicating the KMNTT-10 strain transformed Pb2+ ions into PbS form. A similar finding was reported by Zhang and Huang, [25] who found that the Pb2+ ions were transformed into PbS when Shinella Zoogloeoids PQ7 were cultured in Pb ions containing Luria–Bertani broth. Wei et al. [58] found that the Lysinibacillus sphaericus SH72 could form the PbS nanocrystallites from Pb ions in the presence of L-cysteine. Our previous studies reported that KMNTT-10 strain can transform the Pb2+ ions into PbS when exposed to Pb(NO3)2 containing aqueous (nutrient-limited) solution [10], but the transformation ability differs when compared to nutrient-supplemented conditions. De et al. [22] found that Brevibacterium iodinium (GP13) and Bacillus pumilus (S3) precipitated the adsorbed Pb ions into their sulfide form. It has been reported that bacteria use the biotransformation process to stay alive in a metal-polluted environment [59, 60, 61]. Bacteria can transform the toxic form of metal ions into a less toxic form either intracellularly or extracellularly to counteract the metal toxicity. The processes such as oxidation, reduction, and other metabolic reactions can be involved to reduce the toxicity of metal ions into less toxic forms. Brink et al. [62] hypothesized that the precipitation of Pb by microbes under aerobic conditions occurs due to oxidation–reduction mechanisms, whereas, the precipitation of Pb under anaerobic conditions occurs due to a combination of oxidation–reduction mechanisms and sulfide-liberation mechanisms through the catabolism of sulfur-containing amino acids such as cysteine and methionine.
3.3.4 FTIR analysis of EPS
Figure 6 shows the FTIR spectra of EPS produced by KMNTT-10 strain in 50 mg/L Pb2+ ions treated and untreated broth. Comparing the two spectra, the functional groups were similar, but their positions and intensities changed in the spectrum of EPS obtained from Pb2+ ions stress. In the spectrum of the EPS obtained from Pb2+ ions untreated broth, absorption peaks showed at 3293.07, 2942.81, 1644.09, 1552.39, 1409.57, 1232.28, and 1030.48 cm−1 positions which are O–H/N–H stretch, C–H stretch, amide I; mainly C = O stretch, and amide II; N–H bend, respectively [53, 54]. Also, these functional groups are mostly present in carbohydrates and proteins of EPS. In the spectrum of EPS collected from Pb2+ ions treated broth, the changes in peak position and intensity of these functional groups were observed. In particular, it could be seen that some peak intensity was strong in some positions and weak in some positions in the spectrum of EPS collected from Pb2+ ions treated broth. In the spectrum of the EPS obtained from Pb2+ ions stress, major absorption peaks were observed at 3277.52, 2980.54, 1642.93, 1409.50, 1251.28, and 1041.37 cm−1 positions. There were shifts in the position of most of the peaks when compared to the spectrum of EPS obtained Pb2+ ions treated broth. It has been reported that shifts in peak position imply that functional groups corresponding with these peaks may be involved in metal biosorption [63, 64]. Comparing the spectra of biomass and EPS obtained under Pb2+ ions treated and untreated broth (Figs. 4 and 6), it can be seen that the peak intensity at the regions of 2919 to 2980 cm−1 (C-H stretch) and 1030 to 1065 cm-1 (C–O, C–N stretching) was weak in the spectrum of biomass obtained under Pb2+ ions treated broth. In contrast, the peak intensity at those same regions was strong in the spectrum of EPS obtained under Pb2+ ions treated broth. These peak intensity changes may be functional groups associated with this peak involved in Pb2+ ions biosorption. Furthermore, the position shift and intensity changes of peaks in the spectrum of EPS collected from Pb2+ treated condition indicated that the functional groups associated with these peaks present in the biomass might be primarily involved in Pb2+ ions interaction and biosorption.
4 Conclusion
The efficiency of Lysinibacillus fusiformis KMNTT-10 strain for Pb2+ ions removal was evaluated in this study. Pb2+ ions biosorption by KMNTT-10 strain was positively influenced by their excellent growth. The maximal biosorption efficiency of KMNTT-10 biomass was found to be 98.2% at 10 mg/L. The biosorption efficiency of KMNTT-10 biomass for Pb2+ ions is particularly determined by their EPS production in the Pb2+ ions treated broth. Further, the EPS present in the KMNTT-10 strain can primarily interact with Pb2+ ions during biosorption. This present research also revealed that KMNTT-10 strain not only adsorbs Pb2+ ions from the solution but also converts them into a less-toxic sulfide form. Overall, the results showed that the KMNTT-10 strain in Pb2+ ions treated nutrient broth was found to grow and produce exopolysaccharides in response to the concentration of Pb2+ ions. Also, the KMNTT-10 strain reduced the concentration of Pb2+ ions in the nutrient broth by binding through surface active functional groups (hydroxyl, carbonyl, carboxyl, amide, imidazole, phosphate, and phosphodiester groups). Also, the KMNTT-10 strain transforms absorbed Pb2+ ions into PbS by intracellular mechanisms to counteract the toxicity of Pb2+ ions. As a result, the KMNTT-10 strain is a promising candidate for treating Pb2+ contamination and synthesis of lead sulfide (PbS) nanoparticles. Synthesis of PbS nanoparticles from this test strain has various applications as it has a small bandgap, high carrier mobility, and dielectric constant, which will be considered in our future studies. Additional research is required to ameliorate the conditions for greater PbS synthesis by this strain. Understanding the mechanisms of PbS precipitation by this strain would help to decontaminate the lead-polluted region for environmental sustainability in the long term.
Data availability
The datasets used and/or analyzed during the study are available upon reasonable request.
References
L. Jaber, I. Ihsanullah, I.W. Almanassra, S.N. Backer, A. Abushawish, A.K.A. Khalil, H. Alawadhi, A. Shanableh, M.A. Atieh, Adsorptive removal of lead and chromate ions from water by using iron-doped granular activated carbon obtained from coconut shells. Sustainability 14(17) (2022). https://doi.org/10.3390/su141710877
O.V. Singh, S. Labana, G. Pandey, R. Budhiraja, R.K. Jain, Phytoremediation: an overview of metallic ion decontamination from soil. Appl. Microbiol. Biotechnol. 61, 405–412 (2003)
K. Raj, A.P. Das, Lead pollution: Impact on environment and human health and approach for a sustainable solution. Enviro.Chem. Ecotoxicol. 5, 79–85 (2023)
C. O’Callaghan-Gordo, J. Rosales, P. Lizárraga, F. Barclay, T. Okamoto, D.M. Papoulias, A. Espinosa, M. Orta-Martinez, M. Kogevinas, J. Astete, Blood lead levels in indigenous peoples living close to oil extraction areas in the Peruvian Amazon. Environment international 154, 106639 (2021)
D.A. Gidlow, Lead toxicity. Occup. Med. 54(2), 76–81 (2004)
P. Mitra, S. Sharma, P. Purohit, P. Sharma, Clinical and molecular aspects of lead toxicity: An update. Crit. Rev. Clin. Lab. Sci. 54(7–8), 506–528 (2017)
R.S. Dongre, Lead: toxicological profile, pollution aspects and remedial solutions. Lead chemistry. IntechOpen (2020). https://doi.org/10.5772/intechopen.93095
V. Kumar, S.K. Dwivedi, S. Oh, A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J. Water Proc. Eng. 45, 102518 (2022)
M. Bilal, I. Ihsanullah, M. Younas, M. Ul Hassan Shah, Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 278, 119510 (2021). https://doi.org/10.1016/j.seppur.2021.119510
K. Mathivanan, R. Rajaram, G. Annadurai, Biosorption potential of Lysinibacillus fusiformis KMNTT-10 biomass in removing lead (II) from aqueous solutions. Sep. Sci. Technol. 53(13), 1991–2003 (2018)
P.I. Sevak, B.K. Pushkar, P.N. Kapadne, Lead pollution and bacterial bioremediation: a review. Environ. Chem. Lett. 19(6), 4463–4488 (2021)
M. Mushtaq, N. Arshad, M. Hameed, A. Munir, G.A. Javed, A. Rehman, Lead biosorption efficiency of Levilactobacillus brevis MZ384011 and Levilactobacillus brevis MW362779: A response surface based approach. Saudi J. Biol. Sci. 30(2), 103547 (2023)
L. Huang, Y. Jin, D. Zhou, L. Liu, S. Huang, Y. Zhao, Y. Chen, A review of the role of extracellular polymeric substances (EPS) in wastewater treatment systems. Int. J. Environ. Res. Public Health 19(19), 12191 (2022)
O.Y.A. Costa, J.M. Raaijmakers, E.E. Kuramae, Microbial extracellular polymeric substances: ecological function and impact on soil aggregation. Front. Microbiol. 9, 1636 (2018)
Z.-B. Yue, Q. Li, C. Li, T. Chen, J. Wang, Component analysis and heavy metal adsorption ability of extracellular polymeric substances (EPS) from sulfate reducing bacteria. Biores. Technol. 194, 399–402 (2015)
P.H. Zadeh, F.G. Fermoso, G. Collins, A. Serrano, S. Mills, F. Abram, Impacts of metal stress on extracellular microbial products, and potential for selective metal recovery. Ecotoxicol. Environ. Saf. 252, 114604 (2023)
X. Hu, J. Cao, H. Yang, D. Li, Y. Qiao, J. Zhao, Z. Zhang, L. Huang, Pb2+ biosorption from aqueous solutions by live and dead biosorbents of the hydrocarbon-degrading strain Rhodococcus sp. HX-2. PLoS One 15(1), e0226557 (2020)
M.S.M. Mohamed, N.I. El-Arabi, A. El-Hussein, S.A. El-Maaty, A.A. Abdelhadi, Reduction of chromium-VI by chromium-resistant Escherichia coli FACU: a prospective bacterium for bioremediation. Folia. Microbiol. 65, 687–696 (2020)
S.Z. Abbas, M. Riaz, N. Ramzan, M.T. Zahid, F.R. Shakoori, M. Rafatullah, Isolation and characterization of arsenic resistant bacteria from wastewater. Braz. J. Microbiol. 45, 1309–1315 (2014)
J.D. Holmes, D.J. Richardson, S. Saed, R. Evans-Gowing, D.A. Russell, J.R. Sodeau, Cadmium-specific formation of metal sulfide ‘Q-particles’ by Klebsiella pneumoniae. Microbiology 143(8), 2521–2530 (1997)
K. Mathivanan, R. Rajaram, Isolation and characterisation of cadmium-resistant bacteria from an industrially polluted coastal ecosystem on the southeast coast of India. Chem. Ecol. 30(7), 622–635 (2014)
J. De, N. Ramaiah, L. Vardanyan, Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar. Biotechnol. 10(4), 471–477 (2008)
S.K. Kazy, P. Sar, S.P. Singh, A.K. Sen, S.F.D. Souza, Extracellular polysaccharides of a copper-sensitive and a copper-resistant Pseudomonas aeruginosa strain : synthesis, chemical nature and copper binding. World J. Microbiol. Biotechnol. 18, 583–588 (2002)
M. Dubois, K.A. Gilles, J.K. Hamilton, P.A. Rebers, F. Smith, Colorimetric method for determination of sugars and related substances. Anal. Chem. 28(3), 350–356 (1956)
W. Zhang, Y. Huang, The synthesis of PbS NPs and biosorption of Pb (II) by Shinella zoogloeoides PQ7 in aqueous conditions. Water 12(7), 2065 (2020)
W. Mwandira, K. Nakashima, S. Kawasaki, A. Arabelo, K. Banda, I. Nyambe, M. Chirwa, M. Ito, T. Sato, T. Igarashi, H. Nakata, S. Nakayama, M. Ishizuka, Biosorption of Pb (II) and Zn (II) from aqueous solution by Oceanobacillus profundus isolated from an abandoned mine. Sci. Rep. 10(1), 1–9 (2020)
Q. Li, W. Zhang, S. Liao, D. Xing, Y. Xiao, D. Zhou, Q. Yang, Mechanism of lead adsorption by a Bacillus cereus strain with indole-3-acetic acid secretion and inorganic phosphorus dissolution functions. BMC Microbiol. 23(1), 1–14 (2023)
Q. Jin, M.F., Kirk, pH as a primary control in environmental microbiology: 1. thermodynamic perspective. Front. Environ. Sci. 6, 21 (2018). https://doi.org/10.3389/fenvs.2018.00021
G. Naja, B. Volesky, in Microbial biosorption of metals, ed. by P. Kotrba, M. Mackova, T. Macek. The mechanism of metal cation and anion biosorption (Springer, Dordrecht, 2011). https://doi.org/10.1007/978-94-007-0443-5_3
P. Lodeiro, J.L. Barriada, R. Herrero, M.E. Sastre de Vicente, The marine macroalga Cystoseira baccata as biosorbent for cadmium (II) and lead (II) removal: kinetic and equilibrium studies. Environ. Pollut. 142(2), 264–273 (2006)
M. Oves, M.S. Khan, A. Zaidi, Biosorption of heavy metals by Bacillus thuringiensis strain OSM29 originating from industrial effluent contaminated north Indian soil. Saudi J. Biol. Sci. 20(2), 121–129 (2013)
D. Jaysankar, N. Ramaiah, N.B. Bhosle, A. Garg, L. Vardanyan, V.L. Nagle, K. Fukami, Potential of mercury-resistant marine bacteria for detoxification of chemicals of environmental concern. Microbes Environ. 22(4), 336–345 (2007)
M.M. Naik, S.K. Dubey, D. Khanolkar, B. D’Costa, P-type ATPase and MdrL efflux pump-mediated lead and multi-drug resistance in estuarine bacterial isolates. Curr. Sci. 105(10), 1366–1372 (2013)
R. Shah, S. Jha, Alishewanella sp. strain GIDC-5, Arsenite hyper-tolerant bacteria isolated from industrial effluent of South Gujarat India. Chem. Ecol. 29(5), 427–436 (2013)
S. Petry, S. Furlan, M.-J. Crepeau, J. Cerning, M. Desmazeaud, Factors affecting exocellular polysaccharide production by Lactobacillus delbrueckii subsp. bulgaricus grown in a chemically defined medium. Appl. Environ. Microbiol. 66(8), 3427–3431 (2000)
S.A.M. Moghannem, M. Farag, A.M. Shehab, M.S. Azab, Exopolysaccharide production from Bacillus velezensis KY471306 using statistical experimental design. Brazilian J. Microbiol. 49, 452–462 (2018)
W. Zhou, D. Liu, W. Kong, Y. Zhang, Bioremoval and recovery of Cd (II) by Pseudoalteromonas sp. SCSE709-6: comparative study on growing and grown cells. Bioresour. Technol. 165, 145–151 (2014)
R. Shirdam, A. Khanafari, A. Tabatabaei, Cadmium, nickel and vanadium accumulation by three strains of marine bacteria. Iran J. Biotechnol. 4(3), 180–187 (2006)
J.H. Park, H.-T. Chon, Characterization of cadmium biosorption by Exiguobacterium sp. isolated from farmland soil near Cu-Pb-Zn mine. Environ. Sci. Pollut. Res. 23, 11814–11822 (2016)
J.-S. Chang, R. Law, C.-C. Chang, Biosorption of lead, copper and cadmium by biomass of Pseudomonas aeruginosa PU21. Water Res. 31(7), 1651–1658 (1997)
S. Yu, C. Teng, X. Bai, J. Liang, T. Song, L. Dong, Y. Jin, J. Qu, Optimization of siderophore production by Bacillus sp. PZ-1 and its potential enhancement of phytoextration of Pb from soil. J. Microbiol. Biotechnol. 27(8), 1500–1512 (2017)
J. Maldonado, E. Diestra, L. Huang, A.M. Domènech, E. Villagrasa, Z.M. Puyen, R. Duran, I. Esteve, A. Solé, Isolation and identification of a bacterium with high tolerance to lead and copper from a marine microbial mat in Spain. Ann Microbiol. 60(1), 113–120 (2010). https://doi.org/10.1007/s13213-010-0019-2
H. Guo, S. Luo, L. Chen, X. Xiao, Q. Xi, W. Wei, G. Zheng, C. Liu, Y. Wan, J. Chen, Y. He, Bioremediation of heavy metals by growing hyperaccumulaor endophytic bacterium Bacillus sp. L14. Bioresour. Technol. 101(22), 8599–8605 (2010)
K. Saranya, A. Sundaramanickam, S. Shekhar, M. Meena, R.S. Sathishkumar, T. Balasubramanian, Biosorption of multi-heavy metals by coral associated phosphate solubilising bacteria Cronobacter muytjensii KSCAS2. J. Environ. Manage. 222, 396–401 (2018)
W. Zeng, S. Zhang, M. Xia, X. Wu, G. Qiu, L. Shen, Insights into the production of extracellular polymeric substances of Cupriavidus pauculus 1490 under the stimulation of heavy metal ions. RSC Adv. 10(34), 20385–20394 (2020)
M.M. Naik, A. Pandey, S.K. Dubey, Biological characterization of lead-enhanced exopolysaccharide produced by a lead resistant Enterobacter cloacae strain P2B. Biodegradation 23(5), 775–783 (2012)
W. Zeng, F. Li, C. Wu, R. Yu, X. Wu, L. Shen, Y. Liu, G. Qiu, J. Li, Role of extracellular polymeric substance (EPS) in toxicity response of soil bacteria Bacillus sp. S3 to multiple heavy metals. Bioprocess Biosyst. Eng. 43(1), 153–167 (2020)
K. Pramanik, S. Mitra, A. Sarkar, T. Soren, T.K. Maiti, Characterization of cadmium-resistant Klebsiella pneumoniae MCC 3091 promoted rice seedling growth by alleviating phytotoxicity of cadmium. Environ. Sci. Pollut. Res. Int. 24(31), 24419–24437 (2017)
S. Renu, K.M. Sarim, D.P. Singh, U. Sahu, M.S. Bhoyar, A. Sahu, B. Kaur, A. Gupta, A. Mandal, J.K. Thakur, M.C. Manna, A.K. Saxena, Deciphering cadmium (Cd) tolerance in newly isolated bacterial strain, Ochrobactrum intermedium BB12, and its role in alleviation of Cd stress in spinach plant (Spinacia oleracea L.). Front. Microbiol. 12, 758144 (2022)
E. Ultee, K. Ramijan, R.T. Dame, A. Briegel, D. Claessen, Stress-induced adaptive morphogenesis in bacteria. Adv. Microb. Physiol. 74, 97–141 (2019)
T.A. Kurniawan, W.H. Lo, X. Liang, H.H. Goh, M.H.D. Othman, K.-K. Chong, A. Mohyuddin, A.O. Kern, K.W. Chew, Heavy metal removal from aqueous solutions using biomaterials and/or functional composites: recent advances and the way forward in wastewater treatment using digitalization. J. Compos. Sci. 7(2), 84 (2023). https://doi.org/10.3390/jcs7020084
G.E. Hegazy, N.A. Soliman, M.E. Ossman, Y.R. Abdel-Fattah, M.N. Moawad, Isotherm and kinetic studies of cadmium biosorption and its adsorption behaviour in multi-metals solution using dead and immobilized archaeal cells. Sci. Rep. 13(1), 2550 (2023)
W. Jiang, A. Saxena, B. Song, B.B. Ward, T.J. Beveridge, S.C.B. Myneni, Elucidation of functional groups on gram-positive and gram-negative bacterial surfaces using infrared spectroscopy. Langmuir 20(26), 11433–11442 (2004)
J. Pan, R. Liu, H. Tang, Surface reaction of Bacillus cereus biomass and its biosorption for lead and copper ions. J. Environ. Sci. 19(4), 403–408 (2007)
S.K. Kazy, P. Sar, S.F. D’Souza, Studies on uranium removal by the extracellular polysaccharide of a Pseudomonas aeruginosa strain. Bioremediat. J. 12(2), 47–57 (2008)
R. Rajaram, J.S. Banu, K. Mathivanan, Biosorption of Cu (II) ions by indigenous copper-resistant bacteria isolated from polluted coastal environment. Toxicol. Environ. Chem. 95(4), 590–604 (2013). https://doi.org/10.1080/02772248.2013.801979
D. Zhang, J. Wang, X. Pan, Cadmium sorption by EPSs produced by anaerobic sludge under sulfate-reducing conditions. J. Hazard. Mater. 138(3), 589–593 (2006)
S. Wei, C. Guo, L. Wang, J. Xu, H. Dong, Bacterial synthesis of PbS nanocrystallites in one-step with l-cysteine serving as both sulfur source and capping ligand. Sci. Rep. 11(1), 1216 (2021)
G.S. Nagvenkar, N. Ramaiah, Arsenite tolerance and biotransformation potential in estuarine bacteria. Ecotoxicol. 19(4), 604–613 (2010)
A.D. Chaturvedi, D. Pal, S. Penta, A. Kumar, Ecotoxic heavy metals transformation by bacteria and fungi in aquatic ecosystem. World J. Microbiol. Biotechnol. 31(10), 1595–1603 (2015)
V. Pande, S.C. Pandey, D. Sati, P. Bhatt, M. Samant, Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front. Microbiol. 13 (2022)
H.G. Brink, C. Hörstmann, J. Peens, Microbial Pb (II)-precipitation: the influence of oxygen on Pb (II)-removal from aqueous environment and the resulting precipitate identity. Int. J. Environ. Sci. Technol. 17(1), 409–420 (2020)
Y. Li, M. Xin, D. Xie, S. Fan, J. Ma, K. Liu, F. Yu, Variation in extracellular polymeric substances from Enterobacter sp. and their Pb2+ adsorption behaviors. ACS Omega. 6(14), 9617–9628 (2021)
Y. Shuhong, Z. Meiping, Y. Hong, W. Han, X. Shan, L. Yan, W. Jihui, Biosorption of Cu2+, Pb2+ and Cr6+ by a novel exopolysaccharide from Arthrobacter ps-5. Carbohyd. Polym. 101, 50–56 (2014)
Acknowledgements
The authors acknowledge support from Shandong Province Central Guidance Science and Technology Development Fund (YDZX2023700002831). The Post-doctoral research plan at the Institute of Oceanology, Chinese Academy of Sciences, P.R. China for K. Mathivanan (Post-doctoral number: 329010) is gratefully acknowledged.
Funding
This work was supported by Shandong Province Central Guidance Science and Technology Development Fund (YDZX2023700002831).
Author information
Authors and Affiliations
Contributions
Krishnamurthy Mathivanan: conceptualization, Investigation, Methodology, Data curation, Writing-original draft, and Writing—review & editing. Jayaraman Uthaya Chandirika: formal analysis. Thangavel Mathimani: Formal analysis, Writing—review & editing. Rajendran Rajaram: formal analysis. Ruiyong Zhang: conceptualization, funding acquisition, resources, and supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mathivanan, K., Uthaya Chandirika, J., Mathimani, T. et al. A study on exopolysaccharides production, biosorption, and detoxification properties of Lysinibacillus fusiformis KMNTT-10 in growth media treated with Pb2+ ions. emergent mater. 6, 1491–1502 (2023). https://doi.org/10.1007/s42247-023-00549-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-023-00549-1