Abstract
Nanofiltration, as innovative membrane technology, has been applied for various interesting separation applications including wastewater treatment and molecular separation including salts, dyes, and other small organic molecules. In recent years, several molecularly designed framework systems such as metal–organic framework (MOFs), covalent organic framework (COF), two-dimensional (2D) materials such as MXene, typical natural polyphenols like tannic acid, dopamine, and carbon nanomaterials like graphene oxide (GO) have been employed in the fabrication process of nanofiltration membranes for developing the presentation and overcome the drawbacks of conventional ones. COFs and MOFs as crystalline porous materials with distinct superiorities including high surface areas, structure tolerability, versatile topology architectures, and well-defined nanometer pores, as well as readily customizable functionalities, are promising candidates to prepare next-generation membranes. Also, 2D MXene material has potential in building membranes and molecular separation owing to high conductivity, excellent physicochemical performance, hydrophilic surface, and advanced stability. On the other side, tannic acid has excellent chemical activity due to phenolic hydroxyl groups in the structure, so, it has been extensively used for water-phase monomer in an interfacial polymerization process. Furthermore, GO with 2D construction, outstanding chemical stability, and anti-fouling features have been applied for the functionalization of the NF membrane. Moreover, bioinspired polydopamine with strong adhesion to metal ions, and increased hydrophilicity, is a good candidate for nanofiltration membranes. Since the interest and attention in this field are increasing, here, a review is conducted for summarizing the latest fabrication techniques for membranes based on tannic acid, COF, MOF, MXene, graphene oxide, and dopamine and their uses in terms of water treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Membrane separation, as an environmentally friendly technique with low energy consumption and high separation efficiency, has been rapidly developed in various fields like medicine, food, and wastewater treatment. Nanofiltration (NF) membrane with a high removal rate for mono-/multi-valent salts, as well as diverse size organic molecules, is appropriate to be applied for the elimination of heavy metals, dyes, etc. The chemical construction of NF membranes and their physical features control the water permeability, mechanical/thermal stability, selectivity, anti-fouling features, and separation proficiency, as well as operation cost. NF membrane with features in between reverse osmosis and ultra-filtration has been applied in various interesting uses particularly in water desalination and wastewater treatment. NF process is complex, and it is dependent on interfacial events and microhydrodynamic occurring in the membrane nano-pores and at the surface of the membrane. Steric, dielectric, and transport effects are responsible for rejection from the membrane, and the solutes transportation is done by steric mechanism. Diverse examinations have been shown production and modification methods to fabricate membranes with improved features. Interfacial polymerization and grafting polymerization methods are used in this regard. Also, recently, additives such as nanoparticles like TiO2 have been applied to prepare thin-film nanocomposite. Modifications of the thin-film composite membrane are in three methods: in situ (most commonly used), grafting from, as well as grafting to. For the creation of a selective layer on the substrate, many modifications such as dip-coating, layer-by-layer, grafting, plasma, biocoating, and pressure assistant assembly, and chemical vapor deposition have been employed. Among these methods, interfacial polymerization is a quick and effective technique to prepare commercial composite membranes. By this technique, at the interface between two liquid phases, a selective layer is produced, and temperature, monomer type, and time affect their functional moiety [1,2,3,4,5].
Currently, polymers such as polyamide, polyimide, polysulfone, and polyethersulfone are used for the preparation of available NF membranes. However, for the more complicated utilization, NF membranes containing greater separation performance as well as anti-fouling features are necessary. Membrane scientists have been introduced modifiers for the construction of NF materials with high performance in diverse applications. Recently, a great development was carried out in the progress of NF membranes with a great performance by using a metal–organic framework (MOF), MXene, covalent organic framework (COF), typical natural polyphenols like tannic acid, carbon nanomaterials, and dopamine [6,7,8,9,10,11,12,13,14]. In the present context, the latest strategies based on these valuable materials have been reported to fabricate various NF membranes which are summarized. As can be observed in Fig. 1, numerous studies have been conducted in the NF membranes field, and a variety of materials and methods have been adopted for the preparation of NF membranes for diverse applications.
2 Tannic acid as a promising material for fabrication NF membranes
Between modern NF membranes, thin-film composite-based membranes have been attracted attention for high-performance NF and high perm-selectivity due to separate optimization of selective layer and substrate. In most cases, this kind of membrane is manufactured via interfacial polymerization of a monomer (amine) in aquatic and HCl in an organic media, creating a selective/thin layer of polyamide on the porous substrate. However, this method has many drawbacks like eco-unfriendly (owing to toxic materials and phases), risk of polymer peeling because of poor interaction of selective layer/substrate, and poor resistance to chloride. Coating method managed in water solution has dominated the fabrication of non-polyamide membrane, which has many advantages such as eco-friendly, more cost-efficient, simpler, as well as facile controlling thickness, construction, and wettability of membranes via changing coating materials and cross-linking. But, non-polyamide membranes have disadvantages. Compared to polyamide membranes, they have lower perm-selectivity for desalination that mixes very thin and dense films [15].
Newly, polyphenols like tannic acid with many good features including environmental friendliness and film-forming feasibility, as well as adhesion nature, have attracted attention. Thin films of tannic acid can be fast (in 20 s or even rapidly) created on a substrate via coordination of its organic ligand as well as inorganic cross-linkers in water solution. In this regard, Liu et al. [15] reported rapid (2 min) tannic acid assembly on a polymeric substrate (hydrolyzed polyacrylonitrile) to develop a highly perm-selective non-polyamide membrane through Fe3+ intermediated coordination and regulation. For this aim, at first, by nitrile group hydrolysis, carboxylic groups were created on the porous substrate of polyacrylonitrile. Carboxylic groups were employed to immobilize and regulate Fe3+ ions through coordination interaction. Then, via fast tannic acid assembly on the support, a dense as well as thin layer of tannic acid/Fe3+ network was produced. The coordination of ions and functional groups showed a major influence on the selectivity, stability, and permeability performance of the membrane. The prepared membrane presented high and needed rejection to salts and organic contaminants, respectively, while permeability of pure water was maintained. The rejection for salts and pollutants was as follows: Na2SO4 (90.2%) > MgSO4 (83.4%) > NaCl (50.0%) > MgCl2 (35.2%) and (> 99.0% dyes, 92.2% streptomycin and 81.8% chloramphenicol). Besides, thin-film composite-based membranes displayed outstanding anti-fouling features and structural stability toward pressure as well as alkalinity.
Tannic acid, a natural polyphenol, contains ester groups comprising glucose cores as well as pyrogallol via covalent links. The phenolic hydroxyl groups in it, and other features, provide the chemical activity and coating ability for it [16]. Also, a facile method for the construction of NF membranes is co-deposition among Fe3+ and polyphenols of tannic acid. But in addition to economic problems, this method causes the production of waste materials in the environment. Moreover, a common process to manufacture NF membranes is layer-by-layer self-assembly. Xiao et al. [16] proposed a novel, green, and facile layer-by-layer self-assembly approach by using FeCl3 and tannic acid as safe, low-cost, and available materials to prepare super hydrophilic NF membranes for sustainable molecular separation. With immersing of polyacrylonitrile support into solutions containing FeCl3 and tannic acid, Fe-tannic acid complex layer facilely constructed on the surface of a support. This method did not need toxic chemicals or solvents, pre-treatment on support, and production aggregate waste. After optimization of the membrane, it displayed a high water permeability (40.9 L m−2 h−1 bar−1) and rejections > 93.9% for various dyes. Also, it showed a good ion/organic selective separation behavior.
In the water treatment sector, thin-film nanocomposite membranes have attracted considerable attention. So far, various nano-fillers such as molybdenum disulfide (MoS2) have been applied for the construction of these selective membranes. However, due to the poor affinity of pristine MoS2 nano-sheets with polyamide substrate, modification of MoS2 nano-sheets as a hopeful approach is performed for enhancement of the interfacial compatibility between nano-fillers and polymer. According to studies, tannic acid can form a robust thin film on substrates by easily cross-linking it with Fe3+ ions via coordination bonds, and tannic acid-Fe3+ is a good choice for modification MoS2 nano-sheets [17]. In comparison with surface modification approaches which include multiple stages and poisonous chemicals, modification of MoS2 nano-sheets with tannic acid-Fe3+ coordination networks has many advantages such as simplicity, high speed, and being environmentally friendly. Furthermore, tannic acid-Fe3+ films are stable in aqueous media at pH > 5. In a recent study, MoS2 nano-sheets were modified with tannic acid-Fe3+ for embedding in the polyamide substrate and fabrication of thin-film composite NF membranes. Via interfacial polymerization on polysulfone support, NF membranes were prepared with tannic acid-MoS2 nano-sheets embedded in the polyamide layer. Tannic acid-Fe3+ coordination networks containing phenol groups increased the distribution of MoS2 nano-sheets in the polymeric phase and produced cross-linking sites for unreacted HCl in an interfacial polymerization process. Moreover, the presented covalent bonding among unreacted HCl/phenol groups in the interfacial polymerization process improved the degree of cross-linking of the polyamide layer and avoided the creation of non-selective interfacial cavities. The optimized membrane (adding 0.01 wt% modified MoS2 nano-sheets) exhibited improvement in surface roughness and the presence of scattered protuberances, reduced hydrophilicity, and higher polyamide cross-linking degree. Also, it revealed 1.6-fold water permeance and better salt rejection owing to improved permeable surface area and cross-linking degree of the polymeric layer, respectively [17].
Owing to the multiple interactions (electrostatic and coordination interaction) of tannic acid with zwitterionic polymer and metal ion, so, it is good for the preparation of NF membranes. Liu et al. [18] proposed multiple layer-by-layer strategies for fast fabrication of zwitterionic NF membranes based on tannic acid for improvement of the anti-fouling behavior of the membranes. In comparison with conventional layer-by-layer assembly (through single contact among two assembly constituents), the reported strategy was performed through multiple relations among three materials containing tannic acid, metal ions (Fe3+, due to the strongest ability to coordinate with tannic acid), and zwitterionic poly(sulfobetaine methacrylate). The prepared membranes showed multiple features, for example, respectable nanofiltration properties, improved anti-fouling act, developed roughness, and hydrophilicity, as well as zeta potential (compared to non-zwitterionic NF membranes). The effect of tannic acid/Fe3+ bilayer number and amount of poly(sulfobetaine methacrylate) were investigated, and, rejection properties were also examined. In optimal condition, the prepared zwitterionic membrane [(tannic acid/Fe3+)1.5/poly(sulfobetaine methacrylate)] displayed 40.36 LMH/bar water permeability, > 98% and 60% rejections for rose Bengal and Na2SO4, respectively. Compared to the non-zwitterionic membrane (tannic acid /Fe3+)2.5, the resulted zwitterionic membrane exposed superior anti-fouling behavior.
In 2020, a research study reported a mussel-inspired co-deposition of amine/tannic acid for getting NF membrane with countless actions in inorganic salt rejection [19]. In this study, tannic acid with various amines with diverse Mw, comprising PEI (polyethyleneimine), EDA (ethylenediamine), PEPA (polyethylene polyamine), and DETA (diethylenetriamine), was co-deposited on surfaces of polyacrylonitrile ultra-filtration membrane for construction selective NF membranes. The fabrication process of the membrane can be observed in Fig. 2a. Covalent as well as non-covalent connections (like H-bonding and π-π stacking) can be formed between tannic acid and amines. Based on the outcomes, Mw had countless influences on co-deposition behavior and the performance of the membrane. Amines with low Mw (like DETA:) were useful to co-deposition on the membrane surface, so, dens NF membranes with improved rejection for inorganic salt were developed, whereas, amines with high Mw amines like PEPA obtained loose membranes with low rejection. Indeed, small Mw intermediates in the solutions are better co-deposited on the surface of the membrane, as illustrated in Fig. 2b. Limited active sites for binding are insignificant and intermediates can be dissolved in solution. But, owing to abundant binding sites in high Mw amines, aggregate precipitation can be observed. The co-deposited membrane based on tannic acid/DETA with the thickest selective layer showed the highest (83.5%) rejection for MgCl2, and pure water permeance in comparison with other amines. Moreover, the optimum co-deposition membrane presented outstanding long-term stability.
a: Schematic of the NF membrane preparation process via a simple co-deposition between TA and amine. b: Illustration of the effect of the amine nature on the co-deposition behavior (NF: nanofiltration, TA: tannic acid) [19] (copyright 2020, Elsevier)
In a study by Li et al. [20], in order to develop a facile strategy to fabricate modified thin-film nanocomposite NF membranes, a coating solution, as aqueous phase, comprising tannic acid and TiO2 nanoparticles was applied for the interfacial polymerization of tannic acid and trimesoyl chloride in hexane solution. Figure 3 shows the fabrication process of thin-film nanocomposite NF membranes by tannic acid–coated TiO2. Also, the filtration process can be observed in this figure. With the existence of a plentiful phenolic hydroxyl group in the construction of tannic acid, and reaction with a functional group of trimesoyl chloride (acyl chloride), a polyester thin film can be formed. The TiO2 modification, as well as the polymerization process, was done in a one-step (with no pre-modification process). By coating tannic acid on TiO2, the aggregation of nanoparticles was reduced and interfacial compatibility of TiO2-polyester matrix was enhanced. By the addition of 0.020 wt% of TiO2, the fabricated membrane showed 28.8 L m−2 h1 pure water flux (very higher compared to the controlled membrane), and enhancement in salts (NaCl, Na2SO4) rejections.
a: The schematic diagram of preparation process of the TFN membrane with TA-coated TiO2. b: Schematic illustration of the separation performance of TFN membrane containing TA-coated TiO2 nanoparticles (TFN: thin-film nanocomposite, TA: tannic acid) [20] (copyright 2020, Elsevier)
In another study, Guo et al. [21] fabricated thin-film nanocomposite NF membranes using the low-cost and green tannic acid (comprising catechol groups) and a hydrophilic Jeffamine (with flexible long chains comprising amino groups) via mussel-inspired layer-by-layer self-assembly on polyacrylonitrile support membrane without pre-treatment because of the support-independent covering the behavior of tannic acid. Catechol and amino groups in the components were interacted via covalent (through Michael addition or Schiff base) and non-covalent (hydrogen bonding) bonds, and acted as the driving force for a layer-by-layer procedure. Furthermore, these interactions improved surface hydrophilicity to inhibit the adhesion of hydrophobic molecules on a surface. The application of the resulting membranes was studied for wastewater separation. In 15 min, pH = 8, 0.3 g moL−1 monomer, and 2 bilayers, the optimum membrane was attained. It showed > 90% rejection to several dyes and 37 L m−2 h−1 bar−1 water permeance. Besides, it exposed exceptional anti-fouling as well as long-term presentation due to covalent bonds.
In a facile effort, Rahimi et al. [22] fabricated polyethersulfone NF membrane by using a green, simple, and economical technique for the preparation of the nano-filler. In this study, in mild situations, tannic acid was functionalized with citric acid, and after that, it was inserted into the body of the NF membrane. The effect of the modified tannic acid on morphology, porosity, roughness, pore size, and hydrophilic features was examined. Besides, the efficiency of the resultant product was studied as anti-fouling, rejection capacity of dye, and pure water flux. In the modification process, firstly, a mixture of tannic acid/citric acid was prepared and water was added. After agitation and heating, a viscous brownish solution was obtained. Then, it was heated for other three times to complete the modification of tannic acid as well as dendrimer formation. Finally, the resulting solution was dried and the product was achieved. Owing to the numerous carboxyl and hydroxyl groups in tannic acid as well as citric acid, the resultant dendrimer showed an extraordinary content of hydrophilic groups. Via employing the phase inversion precipitation technique, the polyethersulfone NF membrane was fabricated. The findings showed a membrane containing 1 wt% of modified tannic acid nano-filler had the highest dye rejection (95.7%) and flux recovery ratio (93.2%), best anti-fouling features, and increase in pure water flux.
Zhao et al. [23] performed a fast in situ coupling reaction of dual diazonium salt (DDS) and tannic acid for the construction of a new stable interlayer based on azo on the polysulfone NF membrane. In this study, an interlayer with a porous organic network was obtained from the quick covalent coupling of DDS and tannic acid which was uniform and stable. Thus, on the modified polysulfone substrate, a quite thin layer was fabricated via interfacial polymerization. After pouring piperazine solution, the substrate color with interlayer was changed which showed significant interaction among the functional groups (phenolic hydroxyl) in the interlayer and the piperazine in aquatic media. By decreasing the amount of piperazine, an active layer with 35 nm thickness was achieved. Also, water permeance in the made-up membrane was meaningfully increased by decreasing the concentration of piperazine. In comparison with the conventional one, the optimized NF membrane showed an increase in water permeance (2.71-fold).
In an investigation by Meng et al. [24], for preparation of ultra-filtration membranes, by employing tannic acid and toluene 2,4-diisocyanate as aqueous and organic, polyurethane NF functional layer was constructed on the polysulfone surface. Semiconductor photocatalytic materials including Ag3PO4 and AgBr–Ag3PO4 were placed on the membrane’s surface via functional layers through a simple alternate impregnation technique for obtaining NF membranes with visible photocatalytic performance. The fabrication situations of NF membranes were optimized along with the interception performance of pure water flux and Na2SO4. Photoelectric chemical features of the manufactured materials were characterized, and the photocatalytic degradation for ciprofloxacin, Congo red, and sulfamethoxazole was studied. Moreover, the anti-pollution action and the stability of composite NF membranes were examined. The NF membranes showed good features such as respectable photochemical characteristics, sensitive photocurrent response, strong visible light absorption, anti-pollution self-cleaning performances, and good stability, as well as low electrochemical impedance.
Guo et al. [25] proposed a new strategy (“selective-etching-induced reinforcing”) for the preparation of anti-swelling NF membranes for bioseparation via alkaline post-etching after the interfacial polymerization procedure (Fig. 4). In this study, water-phase monomers and organic monomers (n-hexane) were selected as tannic acid/piperazine and trimesoyl chloride/Fe(acac)3, respectively. At first, tannic acid-Fe3+/polyamide NF membrane was prepared via interfacial polymerization procedure employing water- and organic-phase monomers. After that, for attaining loose NF membranes, a facial alkaline post-treatment was done for etching the polyester construction. The influence of etching, as well as interfacial polymerization conditions on the anti-swelling feature in alkaline, was methodically examined. Also, the long-term stability and separation performance of the membranes for sucrose and pigments were investigated. The gained NF membrane had an outstanding anti-swelling feature and long-term continuous filtration and good selectivity.
a: Schematic diagram of loose NF membrane preparation; b: chemical reaction during IP; and c: operation steps for membrane performance evaluation [25] (copyright 2021, American Chemical Society)
3 Covalent organic framework as a promising material for fabrication NF membranes
Today, a global concern is growing due to the environmental hazards of pollutants. These pollutants, some of which are highly toxic and non-degradable, enter water sources from various industries such as pharmaceuticals and dyes. Traditional membranes are less efficient against the effective removal of these contaminants. Recently, conjugated microporous polymer membranes have received a lot of attention due to their good benefits such as amazing stability in organic solvent NF. However, providing a fine-defined pore construction in the amorphous polymeric membrane is a challenge. COFs as innovative crystalline porous materials with well-organized nano-pore, easy functionalization [26], and stability of pore structure in polar and non-polar organic solvents have appealed to extensive attention and they are talented candidates for membrane construction. Newly, COF-based membranes for high-flux aqueous NF have been reported. Also, with the integration of ionic modules in the membrane, fantastic functions like charge-controlled molecular sieving can be provided for the separation process. Indeed, by using the charged microporous channel in the membrane, molecule transportation can be dual-controlled via charge effect and pore size. Likewise, for the removal of organic pollutants, the introduction of charged sites in membranes is more important. In this regard, Chen et al. [27] successfully fabricated a self-standing 2D -SO3Na anionic membrane based on COF including uniform nano-channels through interfacial crystallization method for removing organic contaminants (Fig. 5). The integration of ionic module in nano-pore rendered the fabricated membrane including particular electronegativity in comparison with neutral COF-based membranes. Molecular size sieving influence, as well as electrostatic interaction in the COF-based membrane (TpPa-SO3Na membrane), provided outstanding NF separation ability to organic pollutants (with diverse charges). Wonderfully, it could efficiently recycle > 99% of cationic molecules, although maintained brilliant solvent permeability because of the high porosity and rigid construction. For evaluation of selective molecular separation, a dye recycle experiment was done. A mixed solution of pollutants (methylene blue, p-Nitroaniline) was used for filtration by the prepared membrane which can be observed in Fig. 5.
A: (a) Schematic diagram of prepared TpPa-SO3Na membrane by condensation reaction of sodium 2,5-diaminobenzenesulfonate (Pa-SO3Na) and 1,3,5-triformylphloroglucinol (Tp). (b) Schematic diagram of interfacial growth method for fabricating TpPa-SO3Na membrane. (c) The model of mass transport across 2D COF membrane along the vertically aligned nano-channels. B: (a) Schematic for molecular sieving mechanism through ordered-vertical arrayed 1D nano-channels with negative charge of TpPa-SO3Na membrane [27] (copyright 2021, Elsevier)
Hao et al. [28] reported a facile fabrication of COF composite NF membrane (COF–LZU1) via stoichiometric spraying layer-by-layer self-assembly technique. In this study, stoichiometric ratios of aryl amine PDA (p-Phenylenediamine) and aldehyde TFB (1,3,5-benzene tricarboxaldehyde) were alternately sprayed on the functionalized polyacrylonitrile using NaOH solution and reacted. In the presence of catalysis of Brønsted acid, the building blocks formed imine-based COFs. This method showed many advantages like a simple operation, thickness control, mild condition, and no rinsing needed. The fabricated membranes showed > 90% rejection and > 400 L m−2 h−1 MPa−1 permeance for dyes.
As crystalline nano-porous materials, COFs comprised of strong covalent connection of organic building units are used as membranes nano-fillers. These organic materials with designable functionality have an outstanding affinity to the polyamide layer and adhesion to the polymeric supports. Due to the nano-sized of pores (1–4 nm), they are suitable for NF separation processes. Also, with facial functionalization through the decoration of organic linkers, the desired membrane with good catalytic, perm-selectivity, or anti-fouling performances can be achieved. Furthermore, by controlling the position of COF nanoparticles, the surface morphology of the membrane can be flexibly tailored. Although the introduction of COFs into the membrane improves the performance of the NF membrane, there are still some problems in the use of COFs in the preparation of the membrane, for example, inhomogeneous distribution in polyamide films, or rejection capacities. Gu et al. [29] facilely prepared novel nanoporous COF in mild condition, and then, used and deposited it as nano-filler on a surface of the substrate along with piperazine through pressure-aided filtration shadowed via the simplistic interfacial polymerization reaction on polyethersulfone membrane for manufacturing COF-based thin-film membranes (Fig. 6). The influence of the incorporated COF on the chemical properties and morphology of membranes was methodically examined. Also, the NF performances such as long-term separation stability, water permeance, anti-fouling feature, and solute rejections were estimated and compared with control samples. The insertion of COFs showed important effects on NF performance and both physiochemical features of membranes. COF nanospheres with uniform dispersion and hydrophilic functional groups provided additional water transport channels, so, they enhanced water permeance of the membrane. The fabricated membrane showed twice higher water permeance, 98.9% rejections of Na2SO4, and 94.2% of MgCl2. Besides, it displayed improved anti-fouling ability and, also, presented sufficient constancy in the saline solution filtration.
Preparation of CTN membranes via pressure-assisted deposition and interfacial polymerization (CTN: COF-based thin-film nanocomposite) [29] (copyright 2021, Elsevier)
In a study by Zhang et al. [30], a thin-film nanofibrous composite polyamide membrane was prepared by facile surface modification approach via the introduction of COFs (TpPa: by 1,3,5-triformylphloroglucinol and p-Phenylenediamine monomers) interlayer with nanorods on the polyacrylonitrile support for the NF. Deposition of COF interlayer was performed by interfacial polymerization technology. For the preparation of a TpPa/polyacrylonitrile membrane, a similar approach to interfacial polymerization was applied as presented in Fig. 7. Also, the NF process with polyamide/polyacrylonitrile and polyamide/TpPa-12/ polyacrylonitrile membranes can be observed in the figure. Through changing the concentration of p-Phenylenediamine, the morphology of the COF interlayer could be adjusted. With increasing monomer concentration (12.0 wt%,), a thin layer was formed which was on the surface of the substrate along with nanorods. The size of the surface pores of polyacrylonitrile was decreased and was beneficial for adsorption as well as storing aqueous monomer solution, formation of a thinner layer of polyamide, and improving the water permeability. The frequent micro- and nanopores from COFs helped the distribution of the amine solution to form a selective layer of polyamide. The achieved membrane (polyamide/TpPa-12/polyacrylonitrile thin-film nanofibrous composite) with a thin skin layer of polyamide showed exceptional NF behavior. Compared to the original polyamide membrane without COF interlayer, the prepared membrane had high permeation water flux (104.8 L m−2 h−1), brilliant selectivity to mixed NaCl/dye, outstanding mechanical features, and high rejection of 98.4% for Na2SO4, as well as long-term test stability.
Schematic diagram illustration for the preparation of PA/TpPa/PAN TFNC membrane (PA/PAN TFNC: polyamide/polyacrylonitrile thin-film nanofibrous composite) [30] (copyright 2021, Elsevier)
Su et al. [31] reported a facile production of composite membrane COF-LZU1/poly(ether sulfone) through in situ interfacial polymerization on filtration support for separation dye/salt. COF-LZU1 with 2D eclipsed layered construction (0.37 nm layer spacing and 1.8 nm pore size), as a thin and defectless active layer, was effectively developed on a substrate through polycondensation of 1,3,5-triformylbenzene and p-phenylenediamine. The prepared composite membrane with great stability displayed high (> 99.0%) rejection and low (< 12.0%) rejection rates for dyes and inorganic salts respectively. Compared to other membranes with similar rejections, the resulting membrane showed high water permeance (80.0 L m−2 h−1 bar−1).
To eliminate intercrystalline defects in selective layers of COFs in NF, Yin et al. reported a pressure-modulated synthesis approach for the preparation of crystalline, high-quality, and defect-free COF NF membranes [32]. Solutions of amine and aldehyde were placed with a level interval for the creation of vertical pressure for regulation of monomer’s mobility, and so, intercrystalline gaps and self-repairing of defects were remedied. Indeed, the external pressure in this study provided a dual function for the growth of selective layers of COF on spongy support. Pressure allowed the self-pairing procedure of intercrystalline defects and improved the sieving capability of molecules and ions. The prepared COF-based membranes showed 90.4% and 63.6% rejections for methyl orange and Na2SO4, respectively. Also, it revealed up to ~ 44.2 L m−2 h−1 bar−1 water permeance (2–10 times greater compared to other ones) and was useful for fast separation of ions as well as molecules.
Graphene oxide (GO)–based membrane deficiencies include poor flux and instability. The insertion of nanopores is an efficient method for improving solvent transportation and effective molecular sieving. Chen and co-authors designed and fabricated hierarchically GO/COF membranes through intercalation of imine-based COFs [33]. In GO matrix, these nanoparticles with superhydrophilic performance, inside hollow structure, 1.8 nm nanopores, and plentiful functional groups provided nano-channels for fast transportation of water and organic solvents (Fig. 8). The fabricated membrane displayed outstanding water, as well as organic solvents, permeate flux (59 L m−2 h–1 bar−1) (60–51 L m−2 h−1 bar−1). The rejection rate for methylene blue and Congo red dyes was 99% and 99.82% respectively. Likewise, outstanding construction stability (7 days stability in strong acidic/basic solutions) and reasonable swelling feature were observed for the prepared membrane.
Hierarchically nanostructured GO/COF hybrid membranes: the cross-section images of (a) pure GO membrane and (b, c) GO/COF hybrid membrane. (d) The schematic view of hierarchically nanostructured GO/COF hybrid membranes with the sub-nanometer channels (0.82–0.91 nm) between adjacent GO flakes, in-plane nanopores (1.8 nm), hollow pores on COF nanoparticles and mesopores between COF nanoparticles (GO: graphene oxide) [33] (copyright 2020, Elsevier)
Also, Sui et al. [34] successfully intercalated rigid two-dimensional (2D) COF into partially reduced GO laminates for fabrication robust membrane by the hydrothermal assembly as well as pressure-assisted filtration technique. In this study, the interlayer spacing between partially reduced GO nano-sheets was increased by mesopores 2D COF, as nano-spacers. COF provided direct transfer channels, reduced transfer resistance of water. Also, COF improved the capacity of self-supporting for GO nets on support. With using the COF strategy, membrane surface area showed a 53.4% increase, and water permeance was enhanced 27 times rise for the optimized GO-COF laminate membrane, so, NF performance was promoted.
In a study by Mao et al. [35], a novel, effective, and facile approach for manufacturing COF membrane with limited channels and pores, as well as having both high permeance/dye rejection filling by in situ polyaniline layer growth, was developed for efficient dye NF. At first, the COF membrane was organized via interfacial polymerization with a TpPa layer on hydrolyzed polyacrylonitrile support. Then, a layer of polyaniline was in situ manufactured on the TpPa layer as a cover for obtaining polyaniline/TpPa hybrid NF membrane (Fig. 9). With covering TpPa with polyaniline, polymeric chains inserted in the pore channel of COFs in the in situ growth process. So, the pore size of COF was reduced and dye retention capacity for the membrane was improved. Also, high water permeance was maintained by loose and hydrophilic construction of polyaniline. The achieved polyaniline/TpPa hybrid membrane showed excellent stability, outstanding performance with 85.2 Lm−2 h−1 bar−1, water permeance, 99.4% rejection of Congo red, and 81.0% rejection for acid fuchsin. Overall, the fabrication process was performed at room temperature at 3 h.
a: Fabrication process of TpPa/HPAN, PANI/HPAN, and PANI-TpPa/HPAN membranes. b: Formation process of PANI-TpPa/HPAN membrane (PANI/HPAN: polyaniline/hydrolyzed polyacrylonitrile) [35] (copyright 2021, Elsevier)
Recently, in a facile in situ growth technique, an excellent COF-300 membrane which was held on the NH2-modified porous ceramic α-Al2O3 was developed by Wang et al. [36] COF-300 with high porosity and surface area, and 3D framework construction was fabricated in a one-step simple solvothermal procedure. Characterization methods verified that covalent linkers among the ceramic support and COF-300 via terephthalaldehyde and 3-aminopropyltriethoxysilane, as a surface anchor. COF-300 layer prepared high-efficacy transportation pathways to water and rejection for dye solutions. Also, the tube-like COF-300 membrane showed exceptional stability (90 h continuous test) that confirmed the feasibility of applying for dyeing wastewater treatment. The outcomes revealed an amazing flux of 395.3 Lm−2 h−1 (at 5 bar), and 97.4% rejection of Chrome black T.
Due to the potential uses of COFs in separation, adsorption, and heterogeneous catalysis, Hao et al. [37] prepared COF-TpPa1-based membranes via chemical vapor deposition method for dye separation. For this aim, by using NaOH aqueous solution, the poly(1,1-difluoroethylene) membrane was modified and neutralized with the help of water. Then, the COF-TpPa1 membrane was prepared on it by evaporation of p-phenylenediamine and 2,4,6-trihydroxy-benzene-1,3,5-tricarbaldehyde powders in the chemical vapor deposition reactor. The reduced iron powder was added to p-phenylenediamine for preventing oxidation. Finally, the resulted membrane (COF-TpPa1/poly(1,1-difluoroethylene) was washed and dried. The fabricated membranes showed many advantages such as excellent water permeance, long-term operation stability, and high (> 90%) and low (< 8.2%) rejections for dyes and salts, respectively.
4 Metal–organic framework as a promising material for fabrication NF membranes
MOFs, as organic–inorganic hybrid compounds constructed through the interconnection of metal ions and organic polydentate ligands, comprising fantastic features such as flexible construction (in comparison with the traditional inorganic constituents containing rigid frameworks), high surface area and porosity, and regular micropores, as well as the tunable chemical composition, are being examined widely for preparation of composite NF membranes for molecular separation uses [38].
In a successful investigation, Chen and co-workers [38] reported the production of thin-film composite NF membrane containing great organic solvent flux using MOF materials and interfacial polymerization process. Via in situ growing of an HKUST-1 MOF interlayer onto a cross-linked polyimide substrate, an NF membrane was obtained. MOF interlayer containing outstanding surface hydrophilicity as well as high porosity improved porosity and hydrophilicity of membranes, and also, controlled the piperazine diffusion in the interfacial polymerization, and made a highly cross-linked polyamide layer with outstanding separating performance. The as-organized thin-film composite NF membrane with MOF interlayer showed 9.59 L·m−2 h−1 bar−1 permeation flux for methanol, while maintained 98.8% rejection for brilliant blue G250 dye. Furthermore, the resultant membrane exhibited suitable stability in polar/aprotic solvents (N, N-dimethylformamide), proved the excessive ability for organic solvent NF uses in chemical industries.
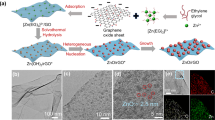
One of the best broadly examined MOFs in the fabrication of membranes is ZIF-8 (zeolitic imidazolate framework-8) containing Zn2+ and Hmim (2-methylimidazole) in a sodalite topology, owing to the mild production condition (compared to other MOFs), excellent chemical stability, and thermal performance, as well as suitable pore size, to apply in molecular sieving. Zn2+ ion is the most and usual metal precursor to manufacture ZIF-8 membrane. In this regard, Xiao et al. [39] fabricated a MOF membrane by using a constant and extreme-thin metal/phenolic linkage layer on the surface of polyethersulfone support. In this study, tannic acid/Zn2+ as a phenolic/metal network was applied as a promising metal precursor in the fabrication process of the ZIF-8 membrane. At first, through layer-by-layer self-assembly of tannic acid and Zn2+ ion, a very thin layer of tannic acid/Zn2+was created on a surface of polyethersulfone support membrane. After that, the prepared layer was immersed into Hmim solution, and with partial self-conversion of tannic acid/Zn2+ to ZIF-8, an ultra-thin top layer of ZIF-8 was facilely made. The process of membrane fabrication and probable mechanism of construction of ZIF-8 with a tannic acid/Zn2+ are demonstrated in Fig. 10. The layer-by-layer self-assembly enabled satisfactory controllability in the formation of a thin and continuous layer on the surface. Through the layer-by-layer self-assembly bilayer number, separation performance and morphology of ZIF-8 membrane could be facilely tailed. The optimized ZIF-8/(tannic acid/Zn2+)2/polyethersulfone membrane showed 5.1 Lm−2 h−1 bar−1 pure water permeance, and 55.2% and 93.6% rejection for NaCl and Na2SO4.
a: Illustration of the synthesis of ZIF-8/(TA-Zn2+)n/PES membrane with TA-Zn2+ network as zinc precursor. b: Possible formation mechanism of ZIF-8 with a TA-Zn2+ network as metal resource [39] (copyright 2021, Elsevier)
Recently, Echaide-Górriz et al. [40] manufactured a bilayered polyamide/MOF membrane inside the surface of hollow fiber support to NF an aqueous solution. For this aim, through liquid-phase crystallization, a continuous MOF layer was fabricated, and then, in the interfacial polymerization process, a layer of polyamide was made on the MOF layer. The effect of time for MOF growth was examined, and selectivity and gas permeance, as well as layer thickness measurements, were tested. Compared to a conventional thin-film composite membrane with no MOF layer and bare support, the resultant membrane showed improved water permeance (from 0.06 to 0.24 L·m−2 h−1 bar−1) and dye rejection (from 88 to 98%).
In another study, UiO-66-NH2 MOF nanomaterial was prepared, and then, in a facile way using palmitoyl chloride, the outside surface was modified [41]. After that, the modified nanomaterial is distributed in an organic phase of benzene-1,3,5-tricarbonyl trichloride/cyclohexane, and after the interfacial polymerization process, a thin-film nanocomposite NF membrane was prepared. In this process, the accumulation affinity between nanoparticles was decreased by increasing the compatibility among organic solvent and UiO-66-NH2 nanoparticles due to employing the long alkyl chain of palmitoyl chloride and similar polarity. Also, by employing cyclohexane as an organic solvent, the nanomaterial distribution in the organic phase was improved. “Ridge-valley” formed Turing construction surface morphology with 380 nm thickness was observed for the prepared NF membrane. Compared to the other thin-film nanocomposite membrane, it demonstrated improved surface roughness and hydrophobicity, as well as zeta potential. Furthermore, the resultant membrane exposed developed dispersibility in the organic phase, long-term stability, and better water permeability, and lower rejection features because of the incorporation of MOF and fabrication interfacial passageway of MOF/polyamide.
In order to facilely fabricate self-cleaning NF membrane, in a recent interesting study, Zhou et al. [42] used CuTz-1 (copper-triazolate MOF) for preparation of a novel nanocomposite membrane (CuTz-1/GO) due to its great features such as robust thermal/chemical stability, and performance in photodegradation organic molecules by visible light. The membrane was made up of a filtration-assisted assembly of MOF and nano-GO sheets on a substrate (hydrolyzed polyacrylonitrile) (Fig. 11). In this process, the cross-linking reaction of poly(vinyl alcohol) (with glutaraldehyde) was used for improving the stability of CuTz-1/GO on the substrate. The utilization of GO in this study had many advantages: having oxygen-containing groups to improve the surface hydrophilicity as well as anti-fouling features, improve anti-bacterial performance by producing ROS, and develop photocatalytic performance via synergism of MOF and GO nano-sheets. With the addition of MOF, long-term NF stability, high (40.2 L m−2 h−1 bar−1) water permeance (due to the existence of CuTz-1 nano-rods between the GO nano-sheets and increased interlayer distance), an outstanding rejection for dyes (99.4% Congo red, 98.2% direct red, and 94.9% for methyl blue), and low rejection for salts (0.3% NaCl: and 19.6% Na2SO4) were observed. Also, by employing MOF, and GO and synergetic effect, photodegradation capacity and anti-bacterial performance (ultra-high (100%) bacteriostasis rate) were increased.
The preparation route of CuTz-1/GO/HPAN composite membranes (CuTz-1/GO/HPAN: CuTz-1/graphene oxide/ hydrolyzed polyacrylonitrile) [42] (copyright 2021, Elsevier)
5 MXene as a promising material for fabrication of NF membranes
Transition metal carbide, also known as MXenes, as attractive 2D materials with M n+1XnTx (n = 1–4) formula and unique features and diverse applications, is comprised of transition metals and carbides/nitrides. Several recent examinations have confirmed the environmental utilization of diverse MXenes for catalysis, sensors, adsorption, molecular separation, and membrane due to outstanding physicochemical and mechanical as well as thermal features [43]. The following are some of the newest applications of these materials in the preparation of membranes.
Yang et al. [44] added diverse percentages of Fe3O4 nanoparticles into 2D MXene nano-sheets on the membrane surface of cellulose acetate for fabrication of Fe3O4/MXene composite NF membranes to remove contaminants (heavy metal ions) from the wastewater. The nanoparticles were uniformly distributed on the nano-sheet construction (with no clear accumulation) and expanded the MXene nano-channels. In comparison with the virgin MXene membrane, the resultant membrane in this study showed significant improvement in water flux as well as the elimination ratio of heavy metal ions. Due to the synergistic influence of adsorption capability and layer sieving of MXene nano-sheets and Fe3O4 nanoparticles, the prepared NF membrane displayed removal of 63.2% Cu2+ and 64.1% Cd2+ as well as 70.2% Cr6+ from wastewater. Additionally, after washing them with HCl, they revealed good recycling capability.
In another study, Kim et al. [45] prepared of large-area MXene-based NF membrane by slot-die coating technique. The applied method as an effective way for manufacturing a high-quality Ti3C2Tx layer allowed the rapid (6 mm s-1speed) manufacture of continuous and scalable coatings, and controlling the thickness (from the nm scale to the μm), and enhancement of nano-sheet alignment via the shear force of the slot-die head. The fabrication process along with the fabricated NF membrane can be observed in Fig. 12. Exceptional NF performance (190 LMH/bar water permeance and 269 Da molecular weight cutoff) was observed for slot-die-coated Ti3C2Tx membrane owing to the well-ordered interlayer construction. Moreover, the prepared membrane exposed improved stability (30 days) in a harsh oxidizing situation.
a: Schematic illustration of Ti3C2Tx coating procedure using a slot-die coater. b: Photographic image of the slot-die coating process with a Ti3C2Tx solution at a concentration of 5 mg/mL. c: Photographic image of an as-prepared Ti3C2Tx membrane. d: Bending test of a Ti3C2Tx membrane at several bending [45] (copyright 2021, American Chemical Society)
For elimination of organic molecules from wastewater, Xu et al. [46] fabricated a novel flexible, fouling-resistant, and self-cleaning MXene-deviated NF membrane with continuous water transport channels through the vacuum filtration approach on a cellulose acetate matrix (Fig. 13). In this work, TiO2 with superior photocatalytic performance was applied to optimize water flux and produce self-cleaning ability. Ti3C2Tx nano-sheets and TiO2 showed a key role in the production of continuous water transmission channels. Also, the surface roughness of the resulted membranes was decreased by the insertion of TiO2 nanoparticles. The fabricated Ti3C2Tx/TiO2 composite NF membrane displayed outstanding (75.4 L/m2 h bar) water flux, and 99% rhodamine B removal. Besides, after washing membranes under UV light, the flux recovery ratio of the fouling membrane was > 80%, which showed recycling efficacy. Moreover, good anti-fouling performance was observed, due to decreased adsorption affinity of contaminants on the membrane surface or the blockage of the pores [46].
Flow chart of membrane preparation and digital photos of the prepared membranes with metallic luster on the surface and flexibility (TTM represents Ti3C2Tx/TiO2 membrane) [46] (copyright 2020, Elsevier)
Zhu et al. [47] prepared a highly effective NF membrane with simultaneously improved salt rejection and water permeance through brush-painting MXene-assisted interfacial polymerization procedure. In this work, on an MXene-painted polyethersulfone substrate, ultra-thin polyamide film (with a thickness of 15 nm) was fabricated. The influence of the MXene painting cycle was examined. Considering the outcomes, MXene improved the hydrophilicity and piperazine adsorptions, and the prepared NF membranes had a narrower pore size, a highly negatively charged surface, and improved integrity. Membranes showed 99.9% rejection of Na2SO4, 27.8 L m−2 h−1 bar−1 of water permeance, and high separation for mono/divalent salts (NaCl/Na2SO4) which revealed the great performance. The fabricated MXene-based NF membrane showed great salt selectivity and water permeance compared to the control NF membrane. Figure 14 shows enhancement mechanisms, the enhanced water permanence by MXene-based membranes could be assigned in four aspects: (1) crumple-organized polyamide layer was formed by MXene interlayer, which increased the filtration area; (2) a thick polyamide layer was in the control NF membrane (ten-fold higher compared to MXene-based membranes), which thinner layer is helpful for reduction of resistance of water transport and creation higher water flux; (3) MXene layer improved hydrophilicity and affinity of the surface of the membrane with water molecules; (4) negatively and hydrophilic MXene nano-sheets possess more nano-channels which enhanced the permeance of water molecules [47].
Schematic illustration of the performance enhancement mechanisms for the resultant NF membranes: (a) the nodular-like polyamide film fabricated via traditional IP and (b) crumple-structured polyamide film formed by the MXene-assisted IP (IP: interfacial polymerization) [47] (copyright 2021, Elsevier)
Shao et al. [48] enhanced the interfacial adhesion and anti-swelling as well as anti-compaction features of MXene NF membranes by employing pillaring CNTs (carbon nanotubes) and promoting the post-drying approach. It is noted that the excessive drying in the air and conventional heat treatment cause zero-permeance of the membranes. This research team suggested a fan-aided partial-dehydration post-modification technique for solving the swelling and improving the performance of the membrane. For this aim, CNTs were intercalated among MXene nano-sheets via intrinsic connecting categories (ionic, covalent, and metal). The fabricated NF membrane with enlarged d-spacing by CNTs showed great molecular separation to dyes in ethanol, enhanced water permeance (from 20.09 Lm−2 h−1 bar−1 to 100.89 Lm−2 h−1 bar−1) stability (60 days in DMF), and 95% rejection of dyes.
6 Fabrication NF membranes based on graphene oxide
GO with unique features like 2D nano-construction, outstanding polymer-like flexibility, and chemical and thermal stability is a promising candidate for fabricating new-generation NF membranes. The formation of 2D nano-channels among GO sheets allows rapid permeate of water, and due to 2D construction, stacked and highly ordered continuous membranes can be formed. For improved performance of membranes, Wang et al. [49] reported NF membranes using GO cross-linked with zwitterion-modified polydopamine. Indeed, in the manufacture of membranes based on GO, there are challenges, for example, the instability of membranes in hydrated conditions due to abundant hydrophilic groups on GO nano-sheets, the possibility of swelling of membranes in organic solutions, and membrane fouling. So, in this work, by immersion of GO in the dopamine solution, polydopamine cross-linked GO was prepared, and then, the zwitterionic polymer (anti-fouling layer) was grafted on it through Michael addition reaction (Fig. 15). The fabricated NF membrane with physical stability, anti-fouling features showed 49.5 Lm−2 h−1 bar−1 flux, high rejection of the pollutants (Congo red 100%, orange G 82%, and methyl orange 67%) [49].
The fabrication process of Z-PEI-GO@PDA/PES membranes (Z-PEI-GO@PDA/PES: polyethyleneimine/graphene oxide@ polydopamine/polyethersulfone) [49] (copyright 2020, Elsevier)
Huang et al. [50] prepared composite NF membranes using GO/silver nanoparticles and investigated the size-effect of nanoparticles on filtration performance. Three types of membranes were prepared by Ag with 8, 20, and 33 nm sizes. Between three membranes, the NF membrane with Ag particles with 20 nm showed the highest (106.1 L m−2 h−1 bar−1) water flux and 97.73% rejection of Rhodamine B [50]. In another study by Amiri et al. [51], GO was synthesized, poly(vinyl alcohol) and alginate were intercalated onto its nano-sheets, and a nanocomposite hydrogel with carboxyl, hydroxyl, carboxylate ions, and epoxy groups was prepared as hydrophilic nano-filler. Next, by embedding this nanocomposite into a microporous polyethersulfone matrix, novel NF membranes were fabricated via phase inversion induced by the immersion precipitation method for enhanced purification performance. Based on the outcomes, nanocomposite hydrogel (1 wt%) enhanced the permeability and anti-fouling ability. The rejection of Lanasol blue 3R dye was obtained more than 83% [51].
Yu et al. successfully manufactured a photocatalytic self-cleaning membrane based on GO and mineral additive by a vacuum-assisted filtration self-assembly procedure. TiO2 nano-rods were uniformly inserted into GO nano-sheets on a cellulose acetate mat for effective nanofiltration (Fig. 16). In this study, interlamellar spacing between nano-sheets was extended by intercalated nanoTiO2, and separation efficiency was advanced. NF membranes containing TiO2 showed increased waster flux (400%) and high rejection of dyes (99.3, 99.4, 99.3, and 99.6% for methylene blue, rhodamine B, methyl orange, and Congo red, respectively. Indeed, TiO2 among nano-sheets acted as space holders and avoided shrinkage of nano-channels. Furthermore, compared to GO membranes, the resulted NF membranes exhibited anti-fouling ability because of the developed hydrophilicity. Also, these membranes showed excessive photocatalytic self-cleaning capability [52].
Schematic depiction of the preparation of GNR membrane (NR: Nanorods) [52] (copyright 2020, Elsevier)
Eum et al. [53] reported a facile technique for coating GO layers on ethylenediamine-modified polyvinylidene fluoride fibers and preparation NF membranes. Via hydrothermal reaction, ethylenediamine-modified polyvinylidene fluoride fibers were prepared. The performance of NF of the resulted membranes was tested for dye rejection and compared with GO-based membranes fabricated via vacuum filtration. Figure 17 shows a facile assembly of GO layers on ethylenediamine-modified polyvinylidene fluoride fibers. At first, via a dry–wet spinning technique, polyvinylidene fluoride was fabricated; then, this fiber was hydrothermally treated with ethylenediamine solution, in which carbon backbones of polymer were cross-linked with amine groups. After that, ethylenediamine-modified polyvinylidene fluoride fiber was immersed into a GO solution. GO flakes were attached to fiber surfaces due to high reactivity among amine groups and oxygen-comprising groups in fiber and GO. Filtration showed high rejection (100%) for brilliant blue molecules [53].
Schematic for the facile assembly of GO flakes on diamine-cross-linked PVDF fiber. GO layer is spontaneously formed during the immersion in GO aqueous solution (PVDF: polyvinylidene fluoride) [53] (copyright 2020, American Chemical Society)
7 The role of dopamine for the fabrication of NF membranes
Dopamine, as a mussel-inspired bioglue, forms hydrophilic and compact polydopamine layers for adhesion to surfaces. Also, the presence of amine, as well as catechol groups in structure, provides the reaction with functional groups for modification. It is an efficient material for membranes and proposes several promises for the preparation of membranes. In this regard, in a recent study, a new cellulose-based NF membrane with outstanding anti-biofouling and anti-bacterial performances employing chitosan, dopamine, and TiO2 was fabricated for water purification. At first, the regenerated cellulose membrane was functionalized with dopamine, and then, it was reacted with polyethyleneimine via Michael addition. After that, dopamine was used for the modification of TiO2 nanoparticles to improve TiO2 dispersion and immobilization onto membranes. A mixture of chitosan and the modified TiO2 was covered on the membrane by covalent bonds (Fig. 18). After the introduction of dopamine-modified TiO2 and chitosan, the resulted membrane effectively killed bacteria. Besides, dopamine-modified TiO2 improved the hydrophilicity, and enhanced the anti-biofouling feature [54].
Schematic illustration of fabricating CS/DA-TiO2-NFM: a stepwise details of preparing CS/DA-TiO2-NFM (CS/DA-TiO2-NFM: chitosan/dopamine-TiO2 composite nanofiltration membrane) (CS: chitosan, DA: dopamine) [54] (copyright 2021, Springer)
In another study, to prepare new NF membranes, sulfonated dopamine was fabricated and employed as an aqueous monomer for cross-linking by trimesoyl chloride through interfacial polymerization. Sulfonated dopamine with sulfonic acid groups influenced the charged property of the membranes. The performance of NF membranes was simply controlled by altering the immersing time in sulfonated dopamine. The optimized NF membrane showed a 62.2 L∙m−2 h−1 water flux and 90.0% rejection of Na2SO4, and > 99.9% rejection for dyes (brilliant green, congo red, methyl blue). Therefore, the sulfonated dopamine was a hopeful material to prepare a high-performance NF membrane [55].
Li et al. [56] improved the performance of loose NF membranes using polydopamine and zwitterionic polymer. In this work, a facile mussel-inspired technique was used. Hydroxyl radical activation produced through CuSO4/H2O2 was applied for realizing rapid deposition of polydopamine. For obtaining anti-fouling as well as anti-bacterial surface, SBMA (a zwitterionic polymer) was applied for co-deposition polydopamine/zwitterionic polymer and polymerization. Also, the separation performance of the NF membrane showed high rejection and low rejection of dyes and salts, respectively. The resulted membrane showed great anti-fouling behavior [56].
8 Summary, challenges, and perspective
NF membrane which was first known in the late 1980s is appropriate to be applied for the elimination of pollutants from water with a high removal rate. Approaches to fabricate NF membranes have been reasonably effective in production membranes with improved flux, selectivity, and fouling features. Still, interfacial polymerization is the main technique for manufacturing thin-film hybrid membranes. However, the insertion of versatile materials such as MOFs, COFs, MXene, tannic acid, GO, and dopamine in the fabrication process of NF membranes had an important influence in improving their performance and overcome the drawbacks of conventional membranes. Tables 1, 2, 3, and 4 summarize NF separation performance with different kinds of membranes based on these materials.
Recently, studies have verified certainly that tannic acid is useful in membrane-based technologies. But there are several challenges in this regard. The deposition is a common method for constructing tannic acid–based covering on the surface of the membrane, but, deposition mechanisms are very few, in-depth understanding made it challenging to control chemical features and the homogeneity, so, understanding the surface adsorption procedure precisely must be the first sign. Also, it is essential to understand the mechanism of tannic acid–based interlayer on the selectivity and layer and transportation.
On the other side, COF-/MOF-based membranes possess great potential to apply in separation methods. Till now, diverse strategies and surface modifications have been used for the development of MOF-based membranes; however, lack of control of MOFs loading and aggregation reduce the membrane performance, so, the balance between loading amount, MOFs dispersibility, and separation performance is the main issue. Also, the biggest difficulty with COFs is the creation of ultra-microporous (ca. 0.6 nm) COF-based materials to improve selectivity and ultra-fast molecular sieving. For the separation of liquids and gases, the development of continuous COF-based membranes is another challenge. Besides, enhancement of mechanical stability must be more considered. Moreover, preparation of self-supporting polyCOF membranes with superior separation performance and exceptional mechanical features, or employing 3D COF membranes, will be considered.
As stated in this review, MXenes show high potential in the fabrication of membranes for water purification and filtration. In comparison with pristine membranes, MXene-based membranes have exceptional performance due to the permeance and elimination qualities for solutions containing pollutants. They provide more water transport pathways compared to pristine ones. Besides, hydrophilic and negatively charged MXenes improve selectivity and fouling resistance, due to adsorption or electrostatic interactions. In addition, the functionalization of MXene-based membranes develops the transport the several solvents. However, for the fabrication of effective MXene-based membranes, efficient development of more hydrophilic, dispersible, and stable MXenes (in water) is necessary. For understanding the separation mechanisms of membranes based on MXene, the detailed molecular structure of the membrane must be evaluated. The influence of various emerging MXenes on features of membranes like surface smoothness, hydrophilicity, and charge as well as interlayer spacing is essential to examine. Evaluation of the interactions of MXene with contaminants and homogeneity dispersion is challenging.
Even though significant progress has been made in the preparation of NF membranes with the help of carbonaceous materials such as GO, there are challenges for the commercial use of its NF membranes; for example, the long-term stability of GO-based membranes is a concern in this regard. Besides, in aqueous solutions, pure films of GO suffered from low physical stability. NF membranes based on GO must be resistant to chemical cleaning and backwashing which are still rare. Furthermore, another challenge is the stable precise separation of GO membranes in aqueous media. The influences of physical features such as lateral width as well as surface wrinkles of GO nano-sheets on membrane stability are infrequently reported. So, additional researches are required.
Mussel-inspired surface chemistry is a hopeful technology in membrane surface engineering. Till now, dopamine-assisted co-deposition was used for the preparation of membranes due to the surface functionality, but still, the underlying co-deposition mechanism is unclear, which can be considered more in the future.
Abbreviations
- NF:
-
Nanofiltration
- MOF:
-
Metal-organic framework
- COF:
-
Covalent organic framework
- GO:
-
Graphene oxide
- PEI:
-
Polyethyleneimine
- EDA:
-
Ethylenediamine
- PEPA:
-
Polyethylene polyamine
- DETA:
-
Diethylenetriamine
- DDS:
-
Dual diazonium salt
- PDA:
-
p-Phenylenediamine
- TFB:
-
1,3,5-Benzene tricarboxaldehyde
- 2D:
-
Two-dimensional
- CuTz-1:
-
Copper-triazolate MOF
- CNTs:
-
Carbon nanotubes
References
A.W. Mohammad, Y.H. Teow, W.L. Ang et al., Nanofiltration membranes review: recent advances and future prospects. Desalination 356, 226–254 (2015)
Y. Ji, W. Qian, Y. Yu et al., Recent developments in nanofiltration membranes based on nanomaterials. Chin J Chem Eng 25, 1639–1652 (2017)
F. Sheng, L. Hou, X. Wang et al., Electro-nanofiltration membranes with positively charged polyamide layer for cations separation. J Memb Sci 594, 117453 (2020)
H. Li, W. Shi, H. Zhang et al., Preparation of internally pressurized polyamide thin-film composite hollow fiber nanofiltration membrane with high ions selectivity by a facile coating method. Prog Org Coatings 139, 105456 (2020)
J. Zhang, Y. Ge, Z. Li, Y. Wang, Facile fabrication of a low-cost and environmentally friendly inorganic-organic composite membrane for aquatic dye removal. J Environ Manage 256, 109969 (2020)
Z. Zhang, C. Yin, G. Yang et al., Stitching nanosheets of covalent organic frameworks to build aligned nanopores in nanofiltration membranes for precise ion separations. J Memb Sci 618, 118754 (2021)
R. Dai, X. Wang, C.Y. Tang, Wang Z Dually charged MOF-based thin-film nanocomposite nanofiltration membrane for enhanced removal of charged pharmaceutically active compounds. Environ Sci Technol 54, 7619–7628 (2020)
J. Kujawa, S. Al-Gharabli, T.M. Muzioł et al., Crystalline porous frameworks as nano-enhancers for membrane liquid separation – recent developments. Coord Chem Rev 440, 213969 (2021)
Z. Yang, T. Liu, S. Wang et al., Fabrication of ionic covalent triazine framework-linked membranes via a facile sol − gel approach. Chem Mater 33, 3386–3393 (2021)
Y. Chiao, T. Patra, M. Belle et al., Zwitterion co-polymer PEI-SBMA nanofiltration membrane modified by fast second interfacial polymerization Yu-Hsuan. Polymers 12, 269 (2020)
X. Li, J. Yang, W. Yuan et al., Microstructured MXene/polyurethane fibrous membrane for highly sensitive strain sensing with ultra-wide and tunable sensing range. Compos Commun 23, 100586 (2021)
M. Ding, H. Xu, W. Chen et al., 2D laminar maleic acid-crosslinked MXene membrane with tunable nanochannels for efficient and stable pervaporation desalination. J Memb Sci 600, 117871 (2020)
Y. Sun, D. Xu, S. Li et al., Assembly of multidimensional MXene-carbon nanotube ultrathin membranes with an enhanced anti-swelling property for water purification. J Memb Sci 623, 119075 (2021)
X. Tong, S. Liu, D. Qu et al., Tannic acid-metal complex modified MXene membrane for contaminants removal from water. J Memb Sci 622, 119042 (2021)
D. Liu, Y. Chen, T.T. Tran, G. Zhang, Facile and rapid assembly of high-performance tannic acid thin-film nanofiltration membranes via Fe3+ intermediated regulation and coordination. Sep Purif Technol 260, 118228 (2021)
Y. Xiao, D. Guo, T. Li et al., Facile fabrication of superhydrophilic nanofiltration membranes via tannic acid and irons layer-by-layer self-assembly for dye separation. Appl Surf Sci 515, 146063 (2020)
H. Zhang, X.Y. Gong, W.X. Li et al., Thin-film nanocomposite membranes containing tannic acid-Fe3+ modified MoS2 nanosheets with enhanced nanofiltration performance. J Memb Sci 616, 118605 (2020)
H. Liu, G. Liu, M. Zhang et al., Rapid preparation of Tannic acid (TA) based zwitterionic nanofiltration membrane via a multiple layer-by-layer (mLBL) assembly strategy for enhanced antifouling performance. Sep Purif Technol 253, 117519 (2020)
Y. Xu, D. Guo, T. Li et al., Manipulating the mussel-inspired co-deposition of tannic acid and amine for fabrication of nanofiltration membranes with an enhanced separation performance. J Colloid Interface Sci 565, 23–34 (2020)
T. Li, Y. Xiao, D. Guo et al., In-situ coating TiO2 surface by plant-inspired tannic acid for fabrication of thin film nanocomposite nanofiltration membranes toward enhanced separation and antibacterial performance. J Colloid Interface Sci 572, 114–121 (2020)
D. Guo, Y. Xiao, T. Li et al., Fabrication of high-performance composite nanofiltration membranes for dye wastewater treatment: mussel-inspired layer-by-layer self-assembly. J Colloid Interface Sci 560, 273–283 (2020)
Z. Rahimi, A.A. Zinatizadeh, S. Zinadini, M. Van Loosdrecht, A hydrophilic and antifouling nanofiltration membrane modified by citric acid functionalized tannic acid (CA-f-TA) nanocomposite for dye removal from biologically treated baker’s yeast wastewater. J Environ Chem Eng 9, 104963 (2021)
S. Zhao, L. Li, M. Wang et al., Rapid in-situ covalent crosslinking to construct a novel azo-based interlayer for high-performance nanofiltration membrane. Sep Purif Technol 258, 118029 (2021)
X. Meng, J. Ren, J. Chen et al., Ag3PO4 composite nanofiltration membrane and its visible-light photocatalytic properties. J Memb Sci 631, 119334 (2021)
S. Guo, H. Zhang, X. Chen et al., Fabrication of antiswelling loose nanofiltration membranes via a “selective-etching-induced reinforcing” strategy for bioseparation. ACS Appl Mater Interfaces 13, 19312–19–19323 (2021)
S. Mallakpour, E. Azadi, C.M. Hussain, Emerging new-generation hybrids based on covalent organic frameworks for industrial applications. New J Chem 45, 7014–7046 (2021)
T. Chen, B. Li, W. Huang et al., Highly crystalline ionic covalent organic framework membrane for nanofiltration and charge-controlled organic pollutants removal. Sep Purif Technol 256, 117787 (2021)
S. Hao, L. Jiang, Y. Li et al., Facile preparation of COF composite membranes for nanofiltration by stoichiometric spraying layer-by-layer self-assembly. Chem Commun 56, 419–422 (2020)
Z. Gu, P. Li, X. Gao et al., Surface-crumpled thin-film nanocomposite membranes with elevated nanofiltration performance enabled by facilely synthesized covalent organic frameworks. J Memb Sci 625, 119144 (2021)
T. Zhang, P. Li, S. Ding, X. Wang, High permeability composite nanofiltration membrane assisted by introducing TpPa covalent organic frameworks interlayer with nanorods for desalination and NaCl /dye separation. Sep Purif Technol 270, 118802 (2021)
Y.Y. Su, X. Yan, Y. Chen et al., Facile fabrication of COF-LZU1/PES composite membrane via interfacial polymerization on microfiltration substrate for dye/salt separation. J Memb Sci 618, 118706 (2021)
C. Yin, S. Fang, X. Shi et al., Pressure-modulated synthesis of self-repairing covalent organic frameworks (COFs) for high-flux nanofiltration. J Memb Sci 618, 118727 (2021)
L. Chen, W. Wang, Q. Fang et al., High performance hierarchically nanostructured graphene oxide/covalent organic framework hybrid membranes for stable organic solvent nanofiltration. Appl Mater Today 20, 100791 (2020)
X. Sui, Z. Yuan, C. Liu et al., Graphene oxide laminates intercalated with 2D covalent-organic frameworks as a robust nanofiltration membrane. J Mater Chem A 8, 9713–9725 (2020)
C. Mao, S. Zhao, P. He et al., Covalent organic framework membranes with limited channels filling through in-situ grown polyaniline for efficient dye nanofiltration. Chem Eng J 414, 128929 (2021)
Z. Wang, Z. Si, D. Cai et al., Synthesis of stable COF-300 nanofiltration membrane via in-situ growth with ultrahigh flux for selective dye separation. J Memb Sci 615, 118466 (2020)
S. Hao, T. Zhang, S. Fan et al., Preparation of COF-TpPa1 membranes by chemical vapor deposition method for separation of dyes. Chem Eng J 421, 129750 (2021)
K. Chen, P. Li, H. Zhang et al., Organic solvent nanofiltration membrane with improved permeability by in-situ growth of metal-organic frameworks interlayer on the surface of polyimide substrate. Sep Purif Technol 251, 117387 (2020)
Y. Xiao, W. Zhang, Y. Jiao et al., Metal-phenolic network as precursor for fabrication of metal-organic framework (MOF) nanofiltration membrane for efficient desalination. J Memb Sci 624, 119101 (2021)
C. Echaide-Górriz, J.A. Zapata, Etxeberría-Benavides M, et al Polyamide/MOF bilayered thin film composite hollow fiber membranes with tuned MOF thickness for water nanofiltration. Sep Purif Technol 236, 116265 (2020)
H. Liu, M. Zhang, H. Zhao et al., Enhanced dispersibility of metal-organic frameworks (mofs) in the organic phase: via surface modification for tfn nanofiltration membrane preparation. RSC Adv 10, 4045–4057 (2020)
S. Zhou, X. Feng, J. Zhu et al., Self-cleaning loose nanofiltration membranes enabled by photocatalytic Cu-triazolate MOFs for dye/salt separation. J Memb Sci 623, 119058 (2021)
Y.A.J. Al-Hamadani, B.M. Jun, M. Yoon et al., Applications of MXene-based membranes in water purification: a review. Chemosphere 254, 126821 (2020)
X. Yang, Y. Liu, S. Hu et al., Construction of Fe3O4@MXene composite nanofiltration membrane for heavy metal ions removal from wastewater. Polym Adv Technol 32, 1000–1010 (2021)
J.H. Kim, G.S. Park, Y. Kim, et al., Large-area Ti3C2Tx‑MXene coating: toward industrial-scale fabrication and molecular separation. ACS Nano 15, 8860–8869 (2021)
Y. Xu, H. Huang, G. Ying et al., Flexible, fouling-resistant and self-cleaning Ti3C2Tx-derivated hydrophilic nanofiltration membrane for highly efficient rejection of organic molecules from wastewater. J Mater Res Technol 9, 11675–11686 (2020)
X. Zhu, X. Zhang, J. Li et al., (2021) Crumple-textured polyamide membranes via MXene nanosheet-regulated interfacial polymerization for enhanced nanofiltration performance. J Memb Sci 635, 119536 (2021)
D.D. Shao, Q. Zhang, L. Wang et al., Enhancing interfacial adhesion of MXene nanofiltration membranes via pillaring carbon nanotubes for pressure and solvent stable molecular sieving. J Memb Sci 623, 119033 (2021)
C. Wang, Y. Feng, J. Chen et al., Nanofiltration membrane based on graphene oxide crosslinked with zwitterion-functionalized polydopamine for improved performances. J Taiwan Inst Chem Eng 110, 153–162 (2020)
K. Yang, L.J. Huang, Y.X. Wang et al., Graphene oxide nanofiltration membranes containing silver nanoparticles: tuning separation efficiency via nanoparticle size. Nanomaterials 10, 454 (2020)
S. Amiri, A. Asghari, V. Vatanpour, M. Rajabi, Fabrication and characterization of a novel polyvinyl alcohol-graphene oxide-sodium alginate nanocomposite hydrogel blended PES nanofiltration membrane for improved water purification. Sep Purif Technol 250, 117216 (2020)
Y. Liu, Z. Yu, Y. Peng et al., A novel photocatalytic self-cleaning TiO2 nanorods inserted graphene oxide-based nanofiltration membrane. Chem Phys Lett 749, 137424 (2020)
K. Eum, D.W. Kim, Y. Choi et al., Assembly of graphene oxide nanosheets on diamine-treated PVDF hollow fiber as nanofiltration membranes. ACS Appl Polym Mater 2, 3859–3866 (2020)
D. Wang, J. Lin, J. Huang et al., A chitosan/dopamine-TiO2 composite nanofiltration membrane for antifouling in water purification. Cellulose 28, 4959–4973 (2021)
J. Ding, H. Wu, P. Wu, Development of nanofiltration membranes using mussel-inspired sulfonated dopamine for interfacial polymerization. J Memb Sci 598, 117658 (2020)
G. Li, B. Liu, L. Bai et al., Improving the performance of loose nanofiltration membranes by poly-dopamine/zwitterionic polymer coating with hydroxyl radical activation. Sep Purif Technol 238, 116412 (2020)
Acknowledgements
We would like to show our gratitude to the National Elite Foundation (NEF), Tehran, I. R. Iran, and Center of Excellence in Sensors and Green Chemistry IUT.
Funding
We received financial support from the Research Affairs Division of Isfahan University of Technology (IUT), Isfahan. I. R. Iran, and Iran Nanotechnology Initiative Council (INIC) Tehran, I. R. Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Mallakpour, S., Azadi, E. Novel methodologies and materials for facile fabrication of nanofiltration membranes. emergent mater. 5, 1263–1288 (2022). https://doi.org/10.1007/s42247-021-00278-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-021-00278-3