Abstract
High magnetization materials are highly desirable for the development of advanced multifunctional magnetic applications. Co:TiO2 is a widely investigated diluted magnetic semiconductor (DMS) system which persists ferromagnetism to above room temperature. However, the magnetic moment observed in Co:TiO2 so far typically lays between 0.01 μB per Co atom to the Co bulk value, 1.7 μB/Co, while higher saturation magnetization (Ms) surpasses 2 μB/Co which has only occasionally been reported. The huge magnetization difference suggests formation of Co nanoclusters is poorly controlled and immensely dependent on growth parameters. In this work, 5 at% Co-doped TiO2 thin films on SrTiO3 substrate were deposited via pulsed laser deposition. By delicately choosing the deposition parameters, various sized Co nanoclusters are embedded into TiO2 thin films with unique surface nanostructures. The Co nanoclusters show metallic features, confirmed by X-ray photoelectron spectroscopy. Moreover, the thin films exhibit excellent cluster-dependent Ms, reaching 2.5 μB/Co under optimal parameters. This work provides an effective approach to design high magnetization thin films with various surface morphology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diluted magnetic semiconductors (DMSs) have drawn a great deal of research attention over the course of past few decades, in the hope of the realization of a technology that can integrate logic semiconductors into magnetic information-storage capability. As semiconductor devices continue to miniaturize, such multifunctional elements with combined semiconducting and magnetic properties are of considerable interest. Moreover, DMSs provide a platform to the observation and investigation of a series of fundamental physics phenomena, including tunneling anisotropic magnetoresistance, spin torque, and spin Seebeck effect [1,2,3].
Traditional II–VI and III–V DMSs have a typical Curie temperature (Tc) below 200 K [4,5,6,7,8]. Diluted magnetic nitrides and oxides (DMNs and DMOs) later have become a brand-new research highlight as Tc above room temperature was predicted in these materials by Dietl [9]. Co:TiO2 is a remarkable promising diluted magnetic system which has demonstrated room-temperature ferromagnetism (FM) in both rutile and anatase forms in a variety of morphologies, like thin films [10, 11], nanoparticles [12, 13], and nanorods [14]. However, even as researchers have struggled for years to clarify the origin of the FM in the system, they failed to achieve a fully convincible explanation. Segregation of Co nanoclusters is frequently observed in Co-doped TiO2 and proven to be ferromagnetic [15, 16]. On the other hand, a number of reports claim to find evidence linked to intrinsic FM using advanced techniques [17, 18]. However, it may need strict deposition parameters.
The magnetic moment observed in Co:TiO2 so far typically lays in the range of 0.01 μB/Co and 1.7 μB/Co (value of Co bulk), while higher saturation magnetization (Ms) surpasses 2 μB/Co which has only occasionally been reported. The huge magnetization fluctuation is consistent with immature understanding and control of ferromagnetism in heterogenous DMSs, in which magnetic properties are determined by nanoregions of highly concentrated magnetic clusters, rather than randomly distributed substitutional magnetic cations. For Co:TiO2, the relationship between local magnetic properties and structure of Co nanoclusters is still open to question. Therefore, methods to control Co aggregation meanwhile ensure large magnetic moments are desirable. In this work, 5 at% Co-doped TiO2 thin films on SrTiO3 substrate were deposited via pulsed laser deposition (PLD). By delicately adjusting the deposition parameters, purposefully designed Co nanoclusters are embedded into TiO2 thin films with magnetization as high as 2.49 μB/Co. This work provides a new strategy to design high magnetization for heterogenous DMSs with a nonrandom distribution of dopants.
2 Experiment
Five at% Co-doped TiO2 target was prepared by first mixing Co3O4 and TiO2 (99.99% in purity) powders in a mortar. Afterward, the mixed powders were pressed into pellet and then sintered in a furnace at 1300 °C for 10 h in air. A series of Co-TiO2 thin films were grown on (001) SrTiO3 (STO) single crystal substrates by PLD with a KrF (wavelength of 248 nm) excimer laser. The laser fluence and oxygen partial pressure were kept constant at ~ 3 J/cm2 and 10−5 Torr. Two deposition rates and a wide range of substrate temperatures were selected for the growth of four films, as shown in Table 1.
The phase characterization of the thin films was examined by X-ray diffraction (XRD) using Cu Kα radiation. Scanning electron microscopy (SEM) and atomic force microscopy (AFM) were used for evaluation of film surface structures. Transmission electron microscopy (TEM) was performed on a JEOL 2200FS equipped with energy dispersive X-ray spectrometer (EDX) for element mapping. The valence states were analyzed by X-ray photoelectron spectroscopy (XPS). All binding energies were referenced to the C 1-s peak at 284.85 eV. The acceleration voltage applied in Ar etching was 1 kV to avoid reduction of cobalt oxides [19]. The in-depth distribution analysis of elements was carried out by secondary ion mass spectrometry (SIMS). A superconducting quantum interference device (SQUID) was used for magnetic property measurements.
3 Result and discussion
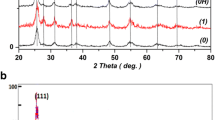
Figure 1 displays the XRD patterns of 5 at% Co-doped TiO2 thin films deposited under various temperatures and growth speed. Regardless of the difference in deposition temperature and rate, only diffraction peaks of anatase phase are observed in all the films. However, the intensity of (004) anatase peak of film 1 is almost 10 times lower than other three films, indicating high deposition rate can reduce the crystalline quality of TiO2 films. The peak becomes both higher and shaper with reduced growth rate and higher temperature, suggesting better degree of crystallinity under those conditions.
Although all four films share the same crystalline structure, there are huge discrepancies among their surface morphologies. Only film 1 has a rather smooth surface, as shown in Fig. 2. With lower deposition rate, small nanoparticles with typical size of 30~40 nm begin to appear in film 2. As growth temperature rises, not only does the particle size remarkably enlarge but also the distance between neighbor particles becomes notably larger. The densely packed nanostructure vanishes when deposition temperature reaches 800 °C as particles are separated by more than 1 μm and each particle is attached to a rectangular-shaped pyramid which should be the anatase grain. This sudden change of shape, from rectangular to irregular, is possibly resulted from the change of surface energy due to Co aggregation at grain boundaries. The surface condition of the films is further demonstrated by AFM, as shown in Fig. 3. The root mean square (RMS) roughness of film 1 is smaller than 1 nm. The height of nanoparticles on films 2 and 3 lays in the range of 20–50 nm and 100–250 nm, respectively, while the particles on film 4 can even reach larger than 800 nm.
Cross-section STEM images and SAED patterns of four thin films are presented in Fig. 4. In agreement with SEM and AFM, only film 1 has a rather smooth surface. Films 2, 3, and 4 are mainly composed with a continuous anatase film plus various particles on top. SAED pattern of film 1 shows a polycrystalline feature, while the epitaxy gets better crystallinity when higher temperature and slower growing rate are applied. From Fig. 4f, the epitaxial relationship between films 2, 3, and 4 and STO substrate are [100]Anatase||[100]STO, [010]Anatase||[010]STO (in-plain), and (001)Anatase||(001)STO (out-of-plain). We also observe that [020]Anatase and [020]STO do not overlap in SAED. The lengths a of unit cell in anatase and STO are 0.378 and 0.39 nm, respectively. Therefore, there is a − 3.1% lattice mismatch between anatase (001) and STO (001) planes. The difference of [020]Anatase and [020]STO in Fig. 4f is equal to this mismatch, indicating the epitaxy is fully relaxed. SAED obtained from mere particles in films 3 and 4, as shown in Fig. 4g, suggests the particles are polycrystals. The crystalline nature of films can be also obtained from the fast Fourier transform (FFT) of high-resolution TEM images (Figure S1).
EDS mapping and SIMS were carried out to examine the spatial distribution of the Co in the films. From both results, it is noticeable that Co is uniform in none of the four films. Co tends to aggregate as nanoclusters near the interface in film 1, confirming by the intensity maximum near the interface in SIMS, as shown in Fig. 5a. In film 2, Co nanoclusters tend to grow bigger and migrate toward surface. Large clusters can be observed in the vicinity of the particles on film surface, while some nanoclusters still remain near the interface, as two peaks can be seen in the SIMS of film 2. Nearly all Co is blended into the surface particles in film 3, except for a minority of smaller clusters within the film near the particles. Thus, the SIMS intensity in film 3 quickly reaches the maximum near the surface. Co can be hardly detected through EDS in film 4 (the red dots in Fig. 4d are from the noises in gold and other elements). The absence of Co in EDS can be caused by the preparation of TEM sample Co which is entirely buried deep inside the micro particles. Since the thickness of FIB sample is less than 100 nm, much thinner than the particle size, it is reasonable to miss the Co clusters during sample cutting. Another possible reason is the evaporation of Co due to long-time exposure on the surface at high temperature, leaving a hole in the original Co position, as shown in Fig. 4d. In addition, the extremely low SIMS intensity of film 4 also suggests Co has been evaporated during deposition, at least to some extent. The schematic of film growth is illustrated in Fig. 5b. In an early work, Chambers et al. investigated oxygen plasma–assisted MBE grown Co-doped anatase TiO2 thin film at different growth rates and temperatures [20]. The epitaxial thin films with highest crystalline quality and most evenly dopant distribution were deposited at temperatures between 550 and 600 °C with a slow growth rate around 0.01 nm/s. However, similar parameters lead to Co segregation in our work, suggesting film growth is extremely sensitive to the equipment.
The valence state of Co is of vital importance to the film’s magnetic performance. Co 2p core-level XPS collected at the surface of film 2–4 show the characteristic peaks which can be ascribed to the 2+ valence state (Fig. 6a), with strong satellite peaks and a spin-orbit splitting of ~ 16.0 eV. The Co 2p peaks of film 1 shifts to lower binding energy. In addition, the spectrum has a smaller spin-orbit splitting (~ 15.3 eV) and decreased satellite to main peak intensity ratio. These features indicate the appearance of Co3+ ions in film 1 [21, 22]. As the cobalt precursor in the target is Co3O4, this suggests the growth condition of film 1 can prevent the reduction of Co3O4 at the surface. The peak intensity of film 4 is considerably lower than the other three, which is consistent with partial the evaporation assumption. However, XPS can only collect signals of electrons that escape from the top layer of the films, resulting in a quite shallow characterization depth. To unveil the valence state of Co in deeper layers, an argon ion beam was used to etch the films for 90 s to reach ~ 20 nm below the original surface. The XPS spectra, as presented in Fig. 6b, show totally different features compared with those taken at the surface. The sharp Co0 peak with binding energy of ~ 778.2 eV and significantly spin-orbit splitting (Δmetal = 14.99 eV) is highly consistent with past studies investigating Co metal nanoclusters in TiO2. Therefore, Co2+ can only be observed at the very surface, likely due to surface oxidation.
The hysteresis loops of film 1 measured at various temperatures are illustrated in Fig. 7a. The film shows obvious ferromagnetic features with a Ms of 40.5 emu/cm3 at 5 K. The ferromagnetic ordering is further confirmed by the coercivity of the M–H loops, as shown in the inset. Increasing the temperature does not lead to a dramatic drop in Ms, which remains mostly at the same level of 36.9 emu/cm3 above 200 K. The persisted Ms to 300 K suggests the Tc of the film is above room temperature. Considering the entire magnetic moment is contributed by Co, the dopant will have a magnetic moment of 2.49 μB/Co at room temperature. Figure 7b shows the hysteresis loops of different films at room temperature, with the Ms constantly decreasing from film 1 to 4. Despite the reduced Ms, films 2 and 3 have significantly enlarged coercivity compared with film 1, as shown in the inset of Fig. 7b. The coercivity of film 3 measured at low temperature is even larger, reaching ~ 1 k Oe.
It is well acknowledged that the presence of Co clusters gives rise to ferromagnetism persisted to above room temperature in inhomogeneous films. It is further confirmed with recent polarized neutron reflectometry results that magnetization merely originates from Co-rich regions and shares similar in-depth pattern to Co concentration [23]. However, magnetic moments induced by Co nanoclusters can hardly reach above 2.5 μB/Co regardless of the states of the clusters, including Co clusters embedded in Cu and SiO2 matrixes and free-standing clusters measured via molecular beam deflection [24, 25]. With very small sizes (fewer than 30 atoms), Co shows atom-like magnetic moments (~ 2.4 μB/Co), which decrease toward the bulk limit with increased cluster size. According to first principle calculation, magnetic moments of free Co clusters can vary based on different geometries, but all below 2.6 μB/Co [26, 27]. Other Co nanostructures, such as nanowire arrays, generate similar magnetic moments [28]. Magnetic moments between 3 and 4 μB/Co have only been reported in ultra-small free Co nanoclusters with less than 20 atoms at extremely low temperature, contributed from extra unquenched orbital moments [29,30,31]. However, such moments are significantly reduced with increased cluster size and temperature. Furthermore, whether the enhanced moments can persist after dispersing in a matrix remains doubtful as interactions between ultra-small magnetic particles frequently lead to superparamagnetic or spin-glass behaviors [32, 33]. The magnetic moments observed in our Co-doped TiO2 (2.49 μB/Co in film 1) is consistent with previous studies. Furthermore, although most Co clusters observed in film 2 have diameters in the range of 10–20 nm, the film still shows a Ms higher than 2 μB/Co, indicating the Ms may be more sensitive to the distribution of Co clusters than the mere size. The thin films can only maintain high magnetic moments when Co clusters are distributed inside the films, demonstrated by the sharp drop of Ms in film 4. Moreover, it is believed that a variety of defects, such as Ti3+ ions and oxygen vacancies, can also induce magnetic moments [34,35,36]. Thus, the high magnetization is likely attributed to the combination of Co nanoclusters and various defects.
4 Conclusion
In conclusion, controllable formation of Co nanoclusters embedded in TiO2 is achievable through pulsed laser deposition. With high deposition rate at 500 °C, Co aggregates into small clusters at the interface. The nanoclusters become increasingly bigger and move toward surface when growing slowly at high temperatures, leading to unique particle structures on the surface. Co-doped TiO2 films show enhanced magnetic moments of ~ 2.5 μB/atom. The magnetic properties are sensitive to the size and distribution of Co nanoclusters, especially the distribution. A part of the high moments may originate from defects. The control of dopant structures in semiconductor matrix is promising to design high magnetization components for spintronics.
References
A.M. Nazmul, S. Kobayashi, S. Sugahara, M. Tanaka, Electrical and optical control of ferromagnetism in III-V semiconductor heterostructures at high temperature (∼ 100 K). Jpn. J. Appl. Phys. 43(2A), L233–L236 (2004)
M. Ciorga, A. Einwanger, U. Wurstbauer, D. Schuh, W. Wegscheider, D. Weiss, Electrical spin injection and detection in lateral all-semiconductor devices. Phys. Rev. B 79(16), 165321 (2009)
H. Kizaki, Y. Morikawa, First-principles study of ZnSnAs2-based dilute magnetic semiconductors. Jpn. J. Appl. Phys. 57(2), 020306 (2018)
K. Olejník, M. Owen, V. Novák, J. Mašek, A. Irvine, J. Wunderlich, T. Jungwirth, Enhanced annealing, high Curie temperature, and low-voltage gating in (Ga, Mn)As: a surface oxide control study. Phys. Rev. B 78(5), 054403 (2008)
M. Wang, R. Campion, A. Rushforth, K. Edmonds, C. Foxon, B. Gallagher, Achieving high Curie temperature in (Ga, Mn)As. Appl. Phys. Lett. 93(13), 132103 (2008)
L. Chen, S. Yan, P. Xu, J. Lu, W. Wang, J. Deng, X. Qian, Y. Ji, J. Zhao, Low-temperature magnetotransport behaviors of heavily Mn-doped (Ga, Mn)As films with high ferromagnetic transition temperature. Appl. Phys. Lett. 95(18), 182505 (2009)
L. Tseng, A. Suter, Y. Wang, F. Xiang, P. Bian, X. Ding, A. Tseng, H. Hu, H. Fan, R. Zheng, Intrinsic and spatially nonuniform ferromagnetism in Co-doped ZnO films. Phys. Rev. B 96(10), 104423 (2017)
X. Luo, L.-T. Tseng, Y. Wang, N. Bao, Z. Lu, X. Ding, R. Zheng, Y. Du, K. Huang, L. Shu, Intrinsic or interface clustering-induced ferromagnetism in Fe-doped In2O3-diluted magnetic semiconductors. ACS Appl. Mater. Interfaces 10(26), 22372–22380 (2018)
T. Dietl, H. Ohno, F. Matsukura, J. Cibert, D. Ferrand, Zener model description of ferromagnetism in zinc-blende magnetic semiconductors. Science 287(5455), 1019–1022 (2000)
A. Saripudin, W. Purnama, D.L. Hakim, M. Somantri, Magnetic properties of co: TiO2 thin films with low cobalt concentration. Pertanika J. Soc. Sci. Hum. 2017, 55–66 (2017)
H. Saadaoui, X. Luo, Z. Salman, X.Y. Cui, N.N. Bao, P. Bao, R.K. Zheng, L. Tseng, Y.H. Du, T. Prokscha, A. Suter, T. Liu, Y.R. Wang, S. Li, J. Ding, S.P. Ringer, E. Morenzoni and J.B. Yi, Intrinsic Ferromagnetism in the Diluted Magnetic Semiconductor Co: TiO2. Physical Review Letters 117, 227202 (2016)
N. Guskos, J. Typek, G. Zolnierkiewicz, A. Guskos, P. Berczynski, D. Dolat, S. Mozia, K. Aidinis, K. Kruk, A.W. Morawski, Temperature study of magnetic resonance spectra of co-modified (Co, N)-TiO2 nanocomposites. Mater. Sci.-Pol. 34(2), 242–250 (2016)
V. Akshay, B. Arun, G. Mandal, G.R. Mutta, A. Chanda, M. Vasundhara, Observation of optical band-gap narrowing and enhanced magnetic moment in Co-doped sol–gel-derived anatase TiO2 nanocrystals. J. Phys. Chem. C 122(46), 26592–26604 (2018)
L.T. Tseng, X. Luo, N.N. Bao, J. Ding, S. Li, J.B. Yi, Structures and properties of transition-metal-doped TiO2 nanorods. Mater. Lett. 170, 142–146 (2016)
J.P. Xu, S.B. Shi, L. Li, X.S. Zhang, Y.X. Wang, X.M. Chen, J.F. Wang, L.Y. Lv, F.M. Zhang, W. Zhong, Structural, optical, and ferromagnetic properties of Co-doped TiO2 films annealed in vacuum. J. Appl. Phys. 107(5), 053910 (2010)
D. Kim, J. Yang, K. Lee, S. Bu, T. Noh, S.-J. Oh, Y.-W. Kim, J.-S. Chung, H. Tanaka, H. Lee, Formation of Co nanoclusters in epitaxial Ti0.96Co0.04O2 thin films and their ferromagnetism. Appl. Phys. Lett. 81(13), 2421–2423 (2002)
J.D. Bryan, S.M. Heald, S.A. Chambers, D.R. Gamelin, Strong room-temperature ferromagnetism in Co2+-doped TiO2 made from colloidal nanocrystals. J. Am. Chem. Soc. 126(37), 11640–11647 (2004)
Y.B. Lin, Y.M. Yang, B. Zhuang, S.L. Huang, L.P. Wu, Z.G. Huang, F.M. Zhang, Y.W. Du, Ferromagnetism of Co-doped TiO2 films prepared by plasma enhanced chemical vapour deposition (PECVD) method. J. Phys. D. Appl. Phys. 41(19), 195007 (2008)
H.A. Hagelin-Weaver, G.B. Hoflund, D.M. Minahan, G.N. Salaita, Electron energy loss spectroscopic investigation of Co metal, CoO, and Co3O4 before and after Ar+ bombardment. Appl. Surf. Sci. 235(4), 420–448 (2004)
S.A. Chambers, C.M. Wang, S. Thevuthasan, T. Droubay, D.E. McCready, A.S. Lea, V. Shutthanandan, C. Windisch Jr., Epitaxial growth and properties of MBE-grown ferromagnetic Co-doped TiO2 anatase films on SrTiO3 (001) and LaAlO3 (001). Thin Solid Films 418(2), 197–210 (2002)
D. Barreca, C. Massignan, S. Daolio, M. Fabrizio, C. Piccirillo, L. Armelao, E. Tondello, Composition and microstructure of cobalt oxide thin films obtained from a novel cobalt (II) precursor by chemical vapor deposition. Chem. Mater. 13(2), 588–593 (2001)
J.-S. Gwag, Y.-K. Sohn, Interfacial natures and controlling morphology of Co oxide nanocrystal structures by adding spectator Ni ions. Bull. Kor. Chem. Soc. 33(2), 505–510 (2012)
D.L. Cortie, Y. Khaydukov, T. Keller, D.J. Sprouster, J.S. Hughes, J.P. Sullivan, X.L. Wang, A.P. Le Brun, J. Bertinshaw, S.J. Callori, R. Aughterson, M. James, P.J. Evans, G. Triani, F. Klose, Enhanced magnetization of cobalt defect clusters embedded in TiO2-delta films. ACS Appl. Mater. Interfaces 9(10), 8783–8795 (2017)
Y. Qiang, R.F. Sabiryanov, S.S. Jaswal, Y. Liu, H. Haberland, D.J. Sellmyer, Magnetism of Co nanocluster films. Phys. Rev. B 66(6), 064404 (2002)
I.M.L. Billas, A. Chatelain, W.A. Deheer, Magnetism from the atom to the bulk in iron, cobalt, and nickel clusters. Science 265(5179), 1682–1684 (1994)
J. Guevara, A.M. Llois, F. Aguilera-Granja, J.M. Montejano-Carrizales, Structural evolution of free Co cluster magnetism. Solid State Commun. 111(6), 335–340 (1999)
J. Souto-Casares, M. Sakurai, J.R. Chelikowsky, Structural and magnetic properties of large cobalt clusters. Phys. Rev. B 93(17), 174418 (2016)
D. Sellmyer, M. Zheng, R. Skomski, Magnetism of Fe, Co and Ni nanowires in self-assembled arrays. J. Phys. Condens. Matter 13(25), R433–R460 (2001)
S. Peredkov, M. Neeb, W. Eberhardt, J. Meyer, M. Tombers, H. Kampschulte, G. Niedner-Schatteburg, Spin and orbital magnetic moments of free nanoparticles. Phys. Rev. Lett. 107(23), 233401 (2011)
F.W. Payne, W. Jiang, J.W. Emmert, J. Deng, L.A. Bloomfield, Magnetic structure of free cobalt clusters studied with Stern-Gerlach deflection experiments. Phys. Rev. B 75(9), 094431 (2007)
J. Meyer, M. Tombers, C. van Wullen, G. Niedner-Schatteburg, S. Peredkov, W. Eberhardt, M. Neeb, S. Palutke, M. Martins, W. Wurth, The spin and orbital contributions to the total magnetic moments of free Fe, Co, and Ni clusters. J. Chem. Phys. 143(10), 104302 (2015)
M. Klimenkov, J. von Borany, W. Matz, D. Eckert, M. Wolf, K.H. Muller, Structure and magnetic properties of Co nanoclusters fabricated by ion beam synthesis in SiO2 films. Appl. Phys. A Mater. Sci. Process. 74(4), 571–575 (2002)
S.A. Koch, G. Palasantzas, T. Vystavel, J.T.M. De Hosson, C. Binns, S. Louch, Magnetic and structural properties of Co nanocluster thin films. Phys. Rev. B 71(8), 085410 (2005)
M. Parras, A.U. Varela, R. Cortés-Gil, K. Boulahya, A. Hernando, J.M. González-Calbet, Room-temperature ferromagnetism in reduced rutile TiO2−δ nanoparticles. J. Phys. Chem. Lett. 4(13), 2171–2176 (2013)
B. Santara, B. Pal, P.K. Giri, Signature of strong ferromagnetism and optical properties of Co doped TiO2 nanoparticles. J. Appl. Phys. 110(11), 114322 (2011)
S. Zhou, E. Čižmár, K. Potzger, M. Krause, G. Talut, M. Helm, J. Fassbender, S. Zvyagin, J. Wosnitza, H. Schmidt, Origin of magnetic moments in defective TiO2 single crystals. Phys. Rev. B 79(11), 113201 (2009)
Funding
This work is funded by the Australia Research Council Future Fellowship FT160100205.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 18785 kb)
Rights and permissions
About this article
Cite this article
Ding, X., Ahmed, S., Bao, N. et al. Clustering-induced high magnetization in Co-doped TiO2. emergent mater. 2, 295–301 (2019). https://doi.org/10.1007/s42247-019-00056-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-019-00056-2