Abstract
The wear tests of titanium matrix composites (TMCs) at the loads of 50, 100, 120, and 150 N were carried out with an MMW-1 vertical universal friction and wear tester to study the addition of multilayer graphene (MLG)/nano-Fe2O3 composites (0, 0.1, 0.2, 0.3, 0.4, and 0.5 g) on the dry sliding wear behavior of TMCs. TMCs presented a marked variation in wear loss as a function of the amount of MLG/Fe2O3 addition, and a significant decrease in the friction coefficient was obtained, reducing this parameter up to 50%. With the rise and fall of wear loss, TMCs underwent a transition from severe wear to mild wear. These phenomena were attributed to the existence of a protective lubricating film, which prevented the surface from coming in direct contact, and the lubricating film was 15–20 μm thick and made up of MLG/Fe2O3 (1:2) nanocomposites. Its structure was speculated to be similar to a rolling wood.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Graphene, a two-dimensional material discovered in 2004, has been investigated extensively in the recent decade and demonstrates great potential for a wide range of applications [1,2,3,4]. Multilayer graphene (MLG), a derivative of graphene, possesses similar properties to graphene, such as super charge carrier mobility, high fracture strength, high Young’s modulus, and extreme thermal conductivity [5,6,7]. Therein, its tribological properties had been investigated on numerous research works [8,9,10,11,12,13,14]. Because of having a high Mohs hardness of 5.5, Fe2O3 is usually considered with a good load bearing capacity, which has been reported elsewhere [15,16,17]. Iwabuchi et al. [18] investigated the effect of Fe2O3 particles on the severe–mild wear transition of a sliding steel interface, and it was confirmed that this transition occurred when a compacted oxide layer was formed. The formation of load bearing Fe2O3-containing tribolayers might be a prerequisite for the wear reduction.
Zhou et al. [19] investigated the additions of MLG/Fe2O3 (with mass proportions of 2:1, 1:1, and 1:2) nanocomposite on TC11 alloy, in order to improve the tribological properties, and results showed that MLG/Fe2O3 nanocomposite (1:2) drastically improved the wear resistance of TC11 alloy. Therefore, MLG/Fe2O3 (1:2) nanocomposite was chosen as additive on titanium matrix composites (TMCs) in this paper. TMCs have been widely investigated due to their superior properties, and with the development of TMCs, according to the practical application of various requirements, the study of the wear resistance for TMCs becomes more and more important. However, the wear test, reported by Zhou et al. [19], was conducted with a single load and did not explored how MLG/Fe2O3 nanocomposite (1:2) influenced the wear mechanism deeply. Based on these facts, sliding wear tests of TMCs against W6Mo5Cr4V2 high-speed steel at various loads, including 50, 100, 120, and 150 N, were performed, with the addition of MLG/Fe2O3 (1:2) nanocomposite on the sliding interface. The worn surfaces and subsurfaces were systematically characterized. Further, the effects of nanomaterial addition amount on the wear behavior were explored.
2 Experimental details
2.1 Material preparation
MLG/Fe2O3 (1:2) nanocomposite was prepared by a liquid-phase exfoliation method. The details were described as follows (Fig. 1): Firstly, a magnetic stirring apparatus was used to disperse the expanded graphite and Fe2O3 nanoparticles for 1 h. Then, an ultrasonic cell disruptor was applied to exfoliate the uniform mixture with the following details: 2 h for operating time, 25 s for ultrasonic time, 5 s for interval time, 30 min for stewing, 20 mm for shift lever diameter, and 540 W for the power. Subsequently, the remaining turbid liquid was centrifugally separated under a high velocity of 8000 r/min for 10 min. After that, the precipitate was washed with ethanol and dried at 343 K in a vacuum drying oven for 24 h. Finally, MLG/Fe2O3 nanocomposite (1:2) was obtained.
For wear test, TMCs and W6Mo5Cr4V2Co5 high-speed steel (American band: M35) were selected as pin and disk materials, respectively. Their chemical compositions are given in Table 1. Ti6Al4V bar (ϕ10 mm), the titanium sponge (purity 99.6%), and B4C powder (purity 99%) were chosen as raw materials for (TiC + TiB)/Ti6Al4V in situ composites and melted in a magnetron tungsten arc furnace. In order to ensure the uniformity of the composition of the sample, the preparation for each sample was repeated three times. (TiC + TiB) reinforcements with a theoretical volume fraction of 5% were obtained. Then, TMCs were machined into cylindrical pins with diameters of 5 mm and heights of 15 mm. M35 steel was machined into counterpart disks with diameters of 52 mm and thicknesses of 10 mm. After quenching at 1473.15 K for 20 min, then oil cooling, and tempering at 823.15 K for 2 h for three times, the hardness of the disks was adjusted to 64–66 HRC.
2.2 Wear tests
Dry sliding wear tests of TMCs against M35 steel with the addition of MLG/Fe2O3 (1:2) nanocomposites were performed in air at room temperature using an MMW tribometer with a pin-on-disk contact geometry. Foam plastic and adhesive tape were adhered onto the center and periphery of the disk, respectively, to prevent the aggregation and spillover of additives as shown in Fig. 2. The normal loads during the wear tests were 50, 100, 120, and 150 N at a speed of 100 r/min (or 0.33 m/s). The sliding test durations were 5000 cycles or 990 m, and the amounts of adding nanocomposites were 0.1, 0.2, 0.3, 0.4, and 0.5 g, respectively. For comparison, the dry sliding wear test without any additive was also performed. Prior to each sliding, the pins and disks were initially cleaned by sonication in acetone and then in isopropanol alcohol to remove any organic contaminants that may have been left from the machining and polishing operations performed during sample preparation. An electronic balance with an accuracy of 10−4 g was used to measure the wear loss of the pins before and after tests.
2.3 Microscopic analysis
A JEM-2100F high-resolution transmission electron microscope (TEM) and a D/Max-2500/pc type X-ray diffractometer (XRD) with Cu Kα radiation were applied to characterize MLG/Fe2O3 nanocomposite samples. Phase constitutions of the worn surfaces were measured on an X-ray diffractometer and a DXR laser Raman spectrometer (Raman). A JSM-7001F scanning electron microscope (SEM) and an Inca Energy 350 energy dispersion spectrometer (EDS) were utilized for the measurement of the microstructures and compositions of the worn surfaces and subsurfaces. The roughness of the worn surfaces was measured using an OLS4000 Olympus laser confocal scanning microscope.
3 Results and analysis
3.1 Microstructure characterization
Figure 3 shows the microstructure of TMCs. It can be seen that TMCs were composed of α-Ti phase, β-Ti phase, TiB phase, and TiC phase. The matrix exhibited typical α + β dual-phase microstructure (whisker-like TiB and granular TiC).
Figure 4 shows TEM images of MLG (with an SEM inset), Fe2O3 and MLG/Fe2O3 nanocomposites. From Fig. 4a, the prepared MLG was observed to be a nanometer-thick lamellar structure, which was almost transparent. In Fig. 4b, Fe2O3 was noticeably agglomerated, and its form was difficult to distinguish. However, in MLG/Fe2O3 nanocomposite, shown in Fig. 4c, the degree of agglomeration of Fe2O3 nanoparticles was drastically decreased. Instead, the spindle-shaped Fe2O3 particles were randomly distributed on the surface of MLG. Clearly, Fe2O3 was successfully composited with MLG.
3.2 Wear loss
Figure 5 illustrates the wear loss of TMCs as a function of MLG/Fe2O3 addition amount under different loads, and it is found that TMCs presented a marked variation in wear loss as a function of MLG/Fe2O3 addition. In general, different varying trends of wear loss were demonstrated with an increase in MLG/Fe2O3 addition under different loads. At the load of 50 N, the wear loss varied in two stages, corresponding to MLG/Fe2O3 additions of 0.1 g and 0.2–0.5 g, respectively. Less MLG/Fe2O3 nanocomposites caused an obvious increase in the wear loss, but with more MLG/Fe2O3 nanocomposites addition, the wear loss reached an extremely low value. At the load of 100 N, when MLG/Fe2O3 addition increased from 0.1 to 0.5 g, the wear loss decreased to a lowest value at first, then approximately maintained around zero with MLG/Fe2O3 addition ranging from 0.2 to 0.4 g, and then eventually increased at 0.5 g. As for 120 N, when MLG/Fe2O3 addition increased from 0.1 to 0.3 g, the wear loss slightly increased to reach a peak at 0.3 g and then substantially decreased to a lowest point at 0.5 g. In order to further explore the influence of MLG/Fe2O3 addition, the test with an MLG/Fe2O3 addition of 0.6 g at 120 N was conducted, and the wear loss rapidly increased beyond 0.5 g. At the load of 150 N, comparing with that at the load of 120 N, almost the same wear loss varying trend was showed with MLG/Fe2O3 addition ranging from 0.1 to 0.5 g. Similarly, complementary experiments were conducted with MLG/Fe2O3 addition of 0.6–0.7 g at 150 N, and the wear loss remained low at 0.6 g but rapidly increased at 0.7 g. Therefore, there appeared to be a critical point of MLG/Fe2O3 addition on the wear loss versus MLG/Fe2O3 addition curves under different loads.
Friction coefficient (μ) of TMCs against M35 steel as a function of MLG/Fe2O3 addition under different loads is presented in Fig. 6. It can be observed that the friction coefficient varied with the load and MLG/Fe2O3 addition. Obviously, the friction coefficient exhibited a similar varying trend as compared to the wear loss (Fig. 5). At 50 N, the friction coefficient was firstly increased and reached a friction coefficient value of 0.385. However, with a further increase in MLG/Fe2O3 addition from 0.2 to 0.5 g, the friction coefficient decreased to a certain platform with a range of 0.126–0.187. At the load of 100 N, the friction coefficient maintained at low stable values with MLG/Fe2O3 addition range of 0.2–0.4 g, and the coefficient increased with MLG/Fe2O3 addition of either 0.1 or 0.5 g. As the load varied between 120 and 150 N, the critical MLG/Fe2O3 addition value increased to 0.5 g and (0.5–0.6) g, respectively.
3.3 XRD analysis of worn surfaces
XRD patterns and Raman spectra are shown in Fig. 7. From Fig. 7a, at the load of 50 N, with MLG/Fe2O3 addition of 0.1 g, only Ti, TiC, and TiB were detected on the worn surface. However, MLG was detected in XRD patterns with the addition of 0.2 g of MLG/Fe2O3 (1:2) nanocomposites, but no Fe2O3 was observed in XRD pattern. Whether Fe2O3 was detected on the worn surface could be verified by the Raman spectrums, as shown in Fig. 7b. It is obvious that the peaks of Fe2O3 emerged except for the ones of MLG, indicating that there was Fe2O3 on worn surfaces, while minor amount of Fe2O3 could be hardly identified by XRD. At the load of 100 N, MLG appeared with 0.4 g MLG/Fe2O3 addition (but not observed with 0.5 g addition), which could also be verified by the Raman spectrum, as shown in Fig. 7b. As shown in Fig. 7c, d, both MLG and Fe2O3 emerged on the worn surfaces with 0.5 g addition of MLG/Fe2O3 (1:2) nanocomposites at the load of both 120 and 150 N. The absence of MLG and Fe2O3 in XRD patterns resulted from either a tiny quantity or nonexistence. Whether or not MLG and Fe2O3 were actually present was determined by Raman spectra, shown in Fig. 7d. It is worth mentioning that three main characteristic peaks, D mode (~ 1350 cm−1), G mode (~ 1580 cm−1), and 2D mode (~ 2700 cm−1), were adopted to examine the existence of MLG [20]. And D + D′ (at ~ 2940 cm−1) was activated by defects in the graphene layers. This finding supports the fact that sliding interfaces produce defected graphene. Therefore, it is clear that when both MLG and Fe2O3 emerged on the worn surfaces, the wear loss reached an extremely low value. On the other hand, when MLG/Fe2O3 addition amount was higher than critical value, both of MLG and Fe2O3 disappeared.
3.4 Morphology of worn surfaces
The morphologies of worn surfaces with different amounts of MLG/Fe2O3 (1:2) nanocomposites addition are shown in Fig. 8. At the load of 50 N, with 0.1 g MLG/Fe2O3 addition, the worn surface of TMCs presented typical characteristics of adhesive and abrasive wear, including adhesive traces, furrows, and severe delaminated regions. Additionally, a small amount of wear debris was remained on the worn surfaces. Conversely, the worn surface with 0.2 g addition amount was found smoother, and furrows were narrower and shallower than those of 0.1 g MLG/Fe2O3 one. It is clear that the addition of MLG/Fe2O3 (1:2) nanocomposites improved the wear resistance effectively. As for 100 N, a well-protected worn surface appeared with 0.4 g MLG/Fe2O3 addition, while severe plastic tearing was shown on the worn surface with 0.5 g MLG/Fe2O3 addition, whose variation implied that TMCs might have undergone a severe wear owing to the excessive MLG/Fe2O3 addition amount. A similar transition of the morphologies of TMCs worn surfaces was shown at the load of both 120 and 150 N. When MLG/Fe2O3 addition amount was 0.4 g (Fig. 8e, g), the worn surfaces were far severer than those with 0.5 g addition (Fig. 8f, h), whose worn surfaces were smoother and neater than those with 0.4 g addition. A large amount of C, O, and Fe elements were detected on the worn surfaces as shown in EDS analysis (Fig. 8h), which indicates that MLG/Fe2O3 (1:2) nanocomposites remained on the worn surface, inducing a good protection on the surface and further resulting in the extremely low value of the wear loss.
3.5 Cross-sectional morphology of worn surfaces and subsurfaces
The cross-sectional morphologies of worn surfaces and subsurface with different amounts of MLG/Fe2O3 (1:2) nanocomposites addition are shown in Fig. 9. At the load of 50 N, a sporadic, discontinuous tribolayer was formed on the worn surfaces with 0.1 g MLG/Fe2O3 addition. Underneath the tribolayer, plastic deformation took place in the subsurface regions, but it was not very intensive (Fig. 9a). However, within 50 N–0.2 g sample, a ceramic-like tribolayer appeared and there was no plastically deformed layer underneath the worn surface, as shown in Fig. 9b, corresponding to the extremely low value of the wear loss in Fig. 5. As for the load of 100 N, the morphology of worn surface and subsurface with 0.4 g MLG/Fe2O3 addition, as shown in Fig. 9c, was similar to that of 50 N–0.2 g sample. When MLG/Fe2O3 addition increased to 0.5 g, the plastically deformed tribolayer appeared on the worn surface and at the subsurface, respectively (Fig. 9d). However, the tribolayer was thicker and more irregular. As for the cases of both 120 and 150 N, with 0.4 g addition, the tribolayer was characterized by cracks, discontinuity, and deformed regions, as shown in Fig. 9e, g. However, as the amount of MLG/Fe2O3 addition reached the critical value (0.5 g), the plastically deformed region thoroughly vanished, as observed in Fig. 9f, h.
4 Discussion
4.1 Formation and characteristics of lubricating film
During the wear process, a lubricating film was formed on both the pin and the disk, which accounted for the appearing excellent tribological properties. The microstructures of both the pin and the disk were investigated, and line profile measurements and Raman tests were conducted to characterize the lubricating film. The results show that the lubricating film formed on the disk was found more complete than that formed on the pin, as shown in Fig. 10. Along the sliding track, a thin, dense, silvery white lubricating film was formed (Fig. 10a). The destruction of the lubricating film initiated from the edges and expanded to the central region because the lubricating film suffered higher Hertz pressure effects on the edges than the central region. Thus, the lubricating film on the edges was readily destroyed and gradually resulted in the sandwich-like morphology (Fig. 10b, c). As the sliding distance surpassed a critical point, the lubricating film was completely consumed.
A further investigation was taken to characterize the lubricating film, as shown in Fig. 11. Figure 11a shows a 3D profilometer image of the lubricating film with MLG/Fe2O3 addition of 0.3 g at the load of 50 N, indicating that the height of the lubricating film is 15–20 μm. Whether the lubricating film was made up of MLG/Fe2O3 (1:2) nanocomposites could be verified by the Raman spectrums, as shown in Fig. 11b, and it is obvious that Fe2O3 peaks were detected while those of MLG were not, indicating that the lubricating film was composed of MLG/Fe2O3 (1:2) nanocomposites. It is also worth noting that D + D′ (at ~ 2940 cm−1) appeared on both curves, supporting the fact that defected graphene was produced on the sliding interfaces after a certain degree of sliding distance.
Generally, the formation of the lubricating film was affected by numerous factors, such as the environment temperature, normal load, sliding speed, additives, and mechanical, thermal and chemical properties of the materials. In our present work, the normal load and the amount of additives were the two important factors, which influenced the formation of the lubricating film directly. At the lower load (50 N), as the amount of MLG/Fe2O3 addition surpassed 0.1 g, the lubricating film started to form, and film formation remained until MLG/Fe2O3 addition reached 0.5 g. However, at the higher load (100, 120 and 150 N), there appeared to be a critical range of amount of MLG/Fe2O3 addition, and while the amount of addition fell below or above the critical value, the lubricating film could not form. It is presumed that the formation of the lubricating film involved physical or chemical reactions, and only while a critical condition was satisfied, the lubricating film could exist. Below the critical value of MLG/Fe2O3 amount, there were not enough MLG/Fe2O3 (1:2) nanocomposites to form the lubricating film. Above the critical value of the additive amount, the film formation was likely to be limited by free energy, which was dependent on an increase in temperature [17]. During sliding at room temperature, the ambient temperature is mainly dependent on the frictional heat (q).
where P is the applied load and v is the sliding velocity [21]. In the present work, since the sliding velocity was fixed, the friction coefficient was almost identical under a certain load, which resulted in the similar temperature and free energy. Therefore, when the additive amount was above the critical value, a favorable free energy was absent, discouraging the formation of a lubricating film.
4.2 Wear mechanism
Schematic illustration of the wear mechanism is given in Fig. 12. In the present work, the mechanism was mainly related to the lubricating film. As shown in Fig. 12a, during the wear sliding process, a lubricating film was formed due to the addition of MLG/Fe2O3 (1:2) nanocomposites, rendering the excellent anti-friction and wear-resistant properties of the surfaces of both the pin and the disk. Moreover, there was absolutely no plastic deformation at this time (Fig. 12b), and the film showed an excellent protection owing to MLG/Fe2O3 (1:2) nanocomposites, which resulted in the extremely low value of wear loss. The functions of the lubricating film could be summarized as follows: On the one hand, the lubricating film can prevent direct contact of the surfaces of the tribopair materials. On the other hand, it can reduce the shear strength of the contacting thin layer. As a result, the friction coefficient was significantly reduced. When the amount of MLG/Fe2O3 addition fell below or above the critical value, the lubricating film was not formed on the surface (Fig. 12c), and the frictional properties of TMCs were worse than those with a lubricating film. Moreover, the area underneath TMCs surface underwent a severe plastic deformation (Fig. 12d), indicating that there was no protection provided by MLG/Fe2O3 (1:2) nanocomposites. The characteristic and formation of the lubricating film on the surfaces of tribopair material are believed to be the predominant parts of the wear mechanism.
4.3 Structure of lubricating film
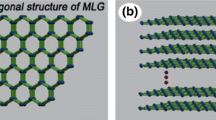
The structure of the lubricating film is schematically shown in Fig. 13. As we know, graphene is a two-dimensional material, presenting unique friction and wear properties (not typically seen in conventional materials), which is totally due to its lamellar structure (as shown in Fig. 13). Multilayer graphene possesses similar properties to graphene, and in order to illustrate the structure of the lubricating film clearly, the structure of MLG was needed to introduce in Fig. 13 as well. During sliding, MLG would be adsorbed onto the worn surface and the surface of wear debris, and its theoretical function is dependent on the movement of graphene layers. During this process, MLG possessed a low shear force, resulting in a lubricating function and a low frictional coefficient. In our present work, spindle-shaped Fe2O3 particles are vaguely shown in Fig. 3b. Thereby, a red bar is drawn in Fig. 13 to represent the structure of Fe2O3 particles.
Since the spindle-shaped Fe2O3 particles were randomly distributed on the surface of MLG (Fig. 4c), it could be speculated that the structure of MLG/Fe2O3 (1:2) nanocomposites unit was similar to a rolling wood structure, which is closely related to the structure of the lubricating film. When MLG/Fe2O3 nanocomposites were supplied onto the sliding interface, both the adsorption of MLG and the sintering of Fe2O3 are related to the formation of the lubricating film. According to the order from small to large size, MLG in the composites preferred to be adsorbed to the surface. Subsequently, the nomadic Fe2O3 particles and those distributed on the surface of small-sized composites were also sintered on MLG layer due to the high surface energy. Under the combined effect of pressure (caused by the normal force), temperature (cause by friction heat), and an appropriate amount of MLG/Fe2O3 addition, the formation of the lubricating film was initiated. The lubricating film was compacted every sliding cycle, and finally, a protective lubricating film was formed, whose structure was similar to a rolling wood as shown in Fig. 13.
5 Conclusions
-
1.
The tribological properties of TMCs can be improved with the addition of MLG/nano-Fe2O3 composites, and a significant decrease in the friction coefficient was obtained, reducing this parameter up to 50%.
-
2.
There was a range of critical value of MLG/Fe2O3 addition, which made TMCs undergo a transition of severe wear to mild wear.
-
3.
The well-protected effect induced by MLG/nano-Fe2O3 composites was attributed to the formation of a protective lubricating film, which prevented the surface from coming in direct contact, resulting in improved tribological performance.
-
4.
The lubricating film was 15–20 μm thick and made up of MLG/Fe2O3 (1:2) nanocomposites. Its structure was speculated to be similar to a rolling wood.
References
J.X. Zhang, Z.Z. Zhuang, Y.T. Jiao, H.X. Yang, Y.Q. Li, J. Zhang, P. Gao, J. Power Sources 419 (2019) 99–105.
H. Zhou, J. Zhang, J. Zhang, X.F. Yan, X.P. Shen, A.H. Yuan, Inorg. Chem. Commun. 54 (2015) 54–56.
J.X. Shao, H. Zhou, J.H. Feng, M.Z. Zhu, A.H. Yuan, J. Alloy. Compd. 784 (2019) 869–876.
H. Zhou, X.Q. Liu, J. Zhang, X.F. Yan, Y.J. Liu, A.H. Yuan, Int. J. Hydrog. Energy 39 (2014) 2160–2167.
C. Lee, X. Wei, J.W. Kysar, J. Hone, Science 321 (2008) 385–388.
K.I. Bolotin, K.J. Sikes, Z. Jiang, M. Klima, G. Fudenberg, J. Hone, P. Kim, H.L. Stormer, Solid State Commun. 146 (2008) 351–355.
A.A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan, F. Miao, C.N. Lau, Nano Lett. 8 (2008) 902–907.
W.Z. Zhai, X.L. Shi, J. Yao, A.M.M. Ibrahim, Z.S. Xu, Q.S. Zhu, Y.C. Xiao, L. Chen, Q.X. Zhang, Compos. Part. B 70 (2015) 149–155.
Z.S. Xu, X.L. Shi, W.Z. Zhai, J. Yao, S.Y. Song, Q.X. Zhang, Carbon 67 (2014) 168–177.
C.F. Gutierrez-Gonzalez, A. Smirnov, A. Centeno, A. Fernández, B. Alonso, V.G. Rocha, R. Torrecillas, A. Zurutuza, J.F. Bartolome, Ceram. Int. 41 (2015) 7434–7438.
S.S. Kandanur, M.A. Rafiee, F. Yavari, M. Schrameyer, Z.Z. Yu, T.A. Blanchet, N. Koratkar, Carbon 50 (2012) 3178–3183.
H. Wang, G.Y. Xie, Z.G. Zhu, Z. Ying, Y. Zeng, Compos. Part. A 67 (2014) 268–273.
D. Berman, A. Erdemir, A.V. Sumant, Carbon 59 (2013) 167–175.
D. Berman, A. Erdemir, A.V. Sumant, Carbon 54 (2013) 454–459.
H. Kato, Tribol. Int. 41 (2008) 735–742.
H. Kato, Wear 255 (2003) 426–429.
H. Kato, K. Komai, Wear 262 (2007) 36–41.
A. Iwabuchi, H. Kubosawa, K. Hori, Wear 139 (1990) 319–333.
Y. Zhou, S.Q. Wang, W. Chen, Metall. Mater. Trans. A 48 (2017) 3287–3299.
A.C. Ferrari, J.C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K.S. Novoselov, S. Roth, Phys. Rev. Lett. 97 (2006) 187401.
W. Ames, A.T. Alpas, Metall. Mater. Trans. A 26 (1995) 85–98.
Acknowledgements
The authors would like to acknowledge the financial support of this work by National Natural Science Foundation of China (No. 51505199) and Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX19_1670).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, H., Jin, Yx., Niu, My. et al. Effect of multilayer graphene/nano-Fe2O3 composite additions on dry sliding wear behavior of titanium matrix composites. J. Iron Steel Res. Int. 27, 1117–1126 (2020). https://doi.org/10.1007/s42243-020-00460-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-020-00460-7